1 PAPRSB Institute of Health Sciences, Universiti Brunei Darussalam, BE1410 Gadong, Brunei Darussalam
2 The Brunei Cancer Center, Pantai Jerudong Specialist Centre, BG3122 Jerudong, Brunei Darussalam
3 National Pathology Department, Raja Isteri Pengiran Anak Saleha Hospital, BA1712 Bandar Seri Begawan, Brunei Darussalam
†These authors contributed equally.
Academic Editor: Anastasios Koulaouzidis
Abstract
Introduction: Colorectal cancer (CRC) is one of the most common cancer types, with rising incidence due to imbalanced lifestyle and dietary habit. Association between CRC cases and KRAS mutation has been established recently. Brunei Darussalam, located within the Borneo island, is of diverse ethnicity which could represent the genome of Southeast Asia population. Our study, for the first time, determined the survival outcome of metastatic colorectal cancer (mCRC) and established the link with KRAS mutation by modelling the population in Brunei Darussalam. Methods: We collected data of 76 metastatic CRC (mCRC) patients undergoing treatment at The Brunei Cancer Centre, the national centre for cancer treatment in Brunei. These patients were diagnosed with Stage 4 CRC between 1 January 2013 and 31 December 2017. Age, gender, ethnicity, date of diagnosis, site of primary tumour, metastatic sites and molecular analysis of KRAS mutation status (either KRAS mutated or KRAS wild-type) of tumour were recorded. The survival outcomes of these mCRC patients were analysed. Results: The end of this study period recorded 73.1% deceased mutant KRAS mCRC patients and 46.0% deceased wild-type KRAS mCRC patients, contributing to death rates of 45.2% and 54.8%, correspondingly. Chi-squared analysis showed a significant difference between the survival outcomes of wild-type KRAS and mutant KRAS mCRC patients (p-value = 0.024). Conclusions: There is a significant difference between the survival outcomes of wild-type KRAS and mutant KRAS mCRC patients in the Brunei population. In addition, we found that mutations in codon 12 of KRAS gene on mutant KRAS mCRC patients have shorter survival median periods than those with mutations within codon 13 of KRAS gene. This is the first study in Brunei Darussalam to analyse both the survival outcomes of mCRC patients and those of mutant KRAS mCRC patients.
Keywords
- colorectal cancer
- survival
- KRAS
- median
- codon
- metastasis
- sided
- tumour
Globally, colorectal cancer (CRC) is the third most common cancer with 147,950 cases reported in United States alone [1]. The rising incidence of CRC cases is attributable to lifestyle, diet and obesity, while the reduction in mortality cases in developed nations is credited to cancer screening, management and treatment [2]. With increased incidence of CRC, national colorectal cancer screening programmes were encouraged and initiated in a number of Asian countries to ensure early detection and treatment of CRC, with a view to lower the CRC mortality rates [3]. According to a retrospective study spanning from 2007 to 2017, the median survival for CRC patients in Brunei Darussalam was 57.0 years [4].
Mutations in the RAS gene family can be found in 20% to 25% of human cancers [5]; with a higher prevalence in colon, lung and pancreatic cancers [6]. Of the known mutations, 85% are associated with KRAS, whereas mutations in NRAS and HRAS constitute the remaining 12% and 3%, respectively [6, 7]. KRAS mutations account for approximately 40% of CRC cases, with approximately 80% of the mutations within codon 12 of the gene followed by 5% mutations in codon 13. Most detected KRAS mutations are missense mutations. These single nucleotide mutations give rise to different amino acid substitutions that result in various downstream pathways of the Ras proteins [8]. The Ras mutations in codon 12 give rise to either G12D, G12A, G12R, G12C, G12S, or G12V with the most common mutations being G12D and G12V. The most frequent mutation found in KRAS codon 13 results in G13D [8, 9, 10]. KRAS mutants G12D and G12V show different GTP hydrolysis rates, which can be translated to differential coupling with an activation of Ras effector [11].
In this study, we aimed to investigate the survival outcomes of mCRC patients in Brunei Darussalam with regards to the KRAS status of the patient, location of primary tumour and metastasis sites. We also attempt to correlate the various KRAS mutations to the patient survival outcomes in accordance to the patient’s gender, age, site of primary tumour and metastatic location. These data may grant insights to the prognosis of patients and influence the treatment guidelines for CRC patients, especially for advanced mCRC patients treated in Brunei Darussalam.
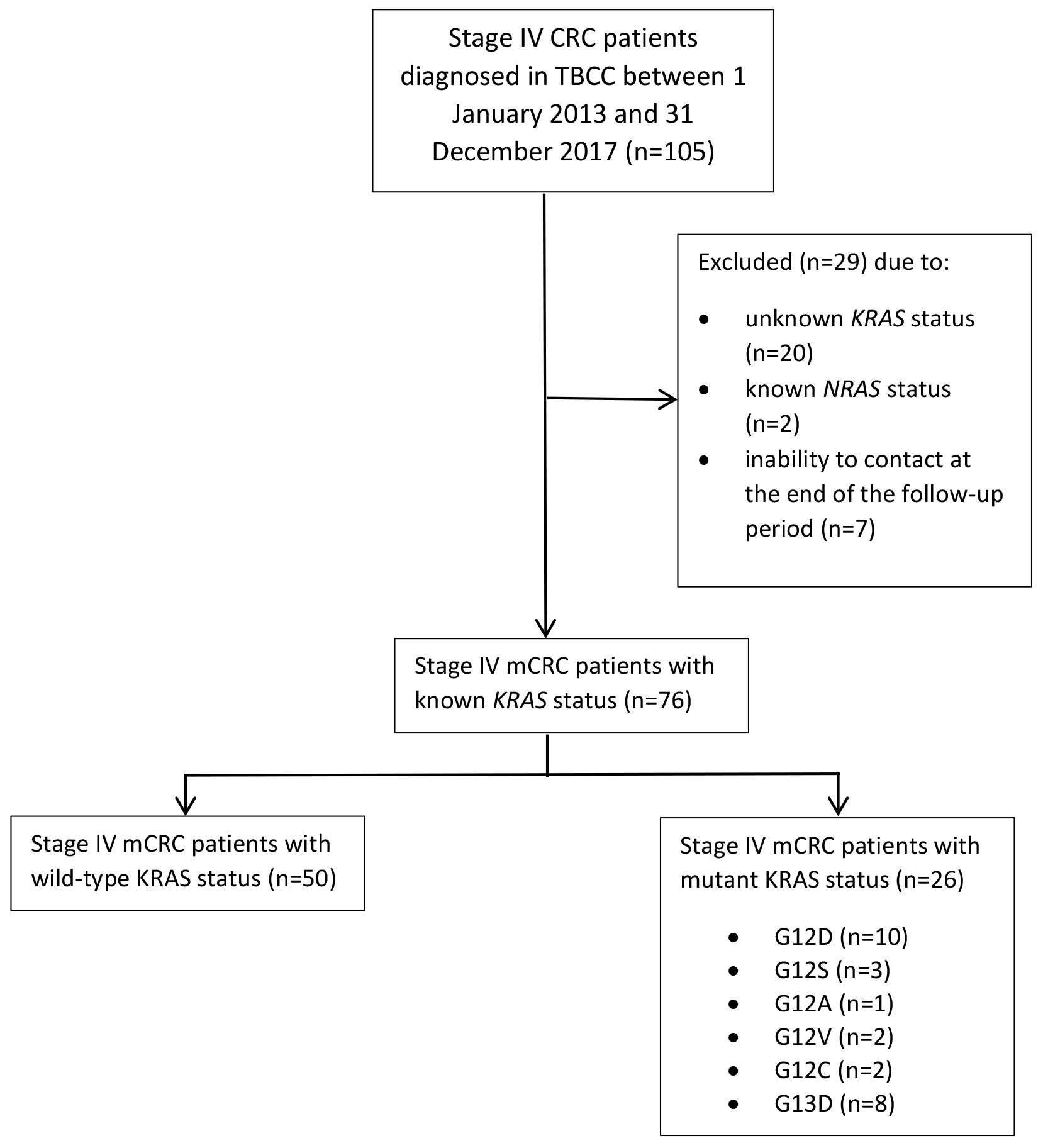
This is a retrospective study to investigate the survival outcome of metastatic CRC (mCRC) patients in Brunei Darussalam. The study was conducted at The Brunei Cancer Centre (TBCC), Pantai Jerudong Specialist Centre of Brunei Darussalam. We collected data of 76 mCRC patients diagnosed between 1 January 2013 and 31 December 2017 were collected and followed up until 30 April 2018. Age, gender, ethnicity, date of diagnosis, site of primary tumour, metastatic sites and tumour molecular analysis of KRAS mutation status (either KRAS mutated or KRAS wild-type) were recorded. KRAS mutation status was based on quantitative PCR results testing for missense mutations on codons 12 and 13 of KRAS from CAP accredited diagnostic laboratory in National University Hospital of Singapore. PCR testing for KRAS mutations is a validated method and a critical diagnostic tool for clinicians in prescription of anti-cancer drug type. KRAS testing includes testing for mutations in the following regions: both codons 12 and 13 of exon 2, codons 59 and 61 of exon 3 and codons 117 and 146 of exon 4. PCR testing is an inexpensive and simple method that provides good sensitivity, representing an excellent clinical molecular pathology diagnostic tool. A limitation of PCR methods is the restricted coverage of mutation hotspots defined by the designed primers. It should be noted that various PCR methods and tests may also have varying sensitivities [12]. The diagnostic date was the date of colonoscopy or sigmoidoscopy, or other diagnostic technique done. The metastatic sites were classified into either liver, lungs or others (inclusive of bone, brain, bladder and reproductive organs). Exclusion criteria were patients with known NRAS status, uncontactable patients and absence of metastasis. Known NRAS status was excluded to obtain a non-biased population of KRAS. Uncontactable patients refer to patients that were not followed up by the end of the follow up period. Fig. 1 illustrates the consort flow chart of sample patient categorisation.
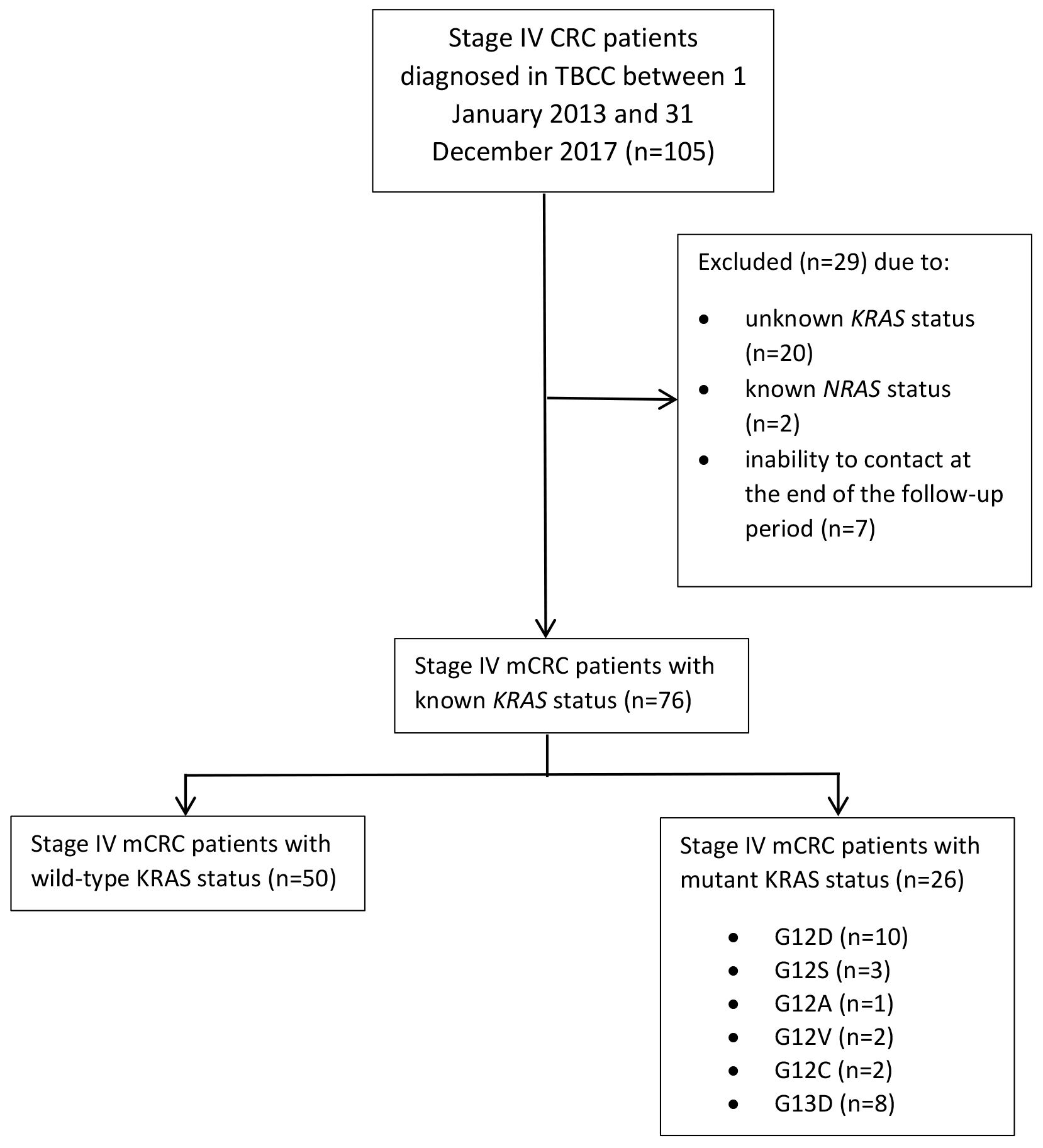 Fig. 1.
Fig. 1.Consort flow chart illustrating the numbers of Stage IV metastatic CRC patients and their known KRAS statuses.
Overall survival (OS) refers to total duration in months between patients’ date of diagnosis to the end of the follow up period or date of termination (date of death). The status of the patients (whether the patient was alive or not at the time of study), gender, age, site of primary tumour, metastasis location, RAS status and mutated codon were documented. Primary tumours located in the ileocecal valve, cecum, ascending colon to the transverse colon was classified as the right sided colon tumour (RCC), while the primary tumours found within the splenic flexure, descending colon, sigmoid and rectum were classified as left sided colon tumours (LCC). All duration of date are recorded in months. To correlate the survival impact of the metastatic site, we have categorised the patients in accordance to the two most common metastatic organs, that is, the liver and the lungs. In addition, the number of metastatic sites were taken into account and therefore, the OS based on either single or multiple metastatic site was studied. Survival analysis of mCRC with mutated KRAS was compared with that of wild-type KRAS. A further inspection on the survival outcome of different KRAS mutations, such as G12D, G12S, G12V and G12C in codon 12 and G13D in codon 13 was conducted.
Chi-squared test was used to compare the survival outcomes of mCRC patients, and
Mann-Whitney U test was used to compare the median ages of both groups.
Kaplan-Meier survival curves were drawn and logrank test was used to compare the
survival outcome between two groups (namely between gender, age groups, location
of primary tumour in the colon, and KRAS status). Statistical analysis
was conducted using SPSS Statistics for Windows, version 17.0 (SPSS Inc.,
Chicago, III., USA) and R (ver.3.6.0, R Core Team, Vienna, Austria). A
p-value of
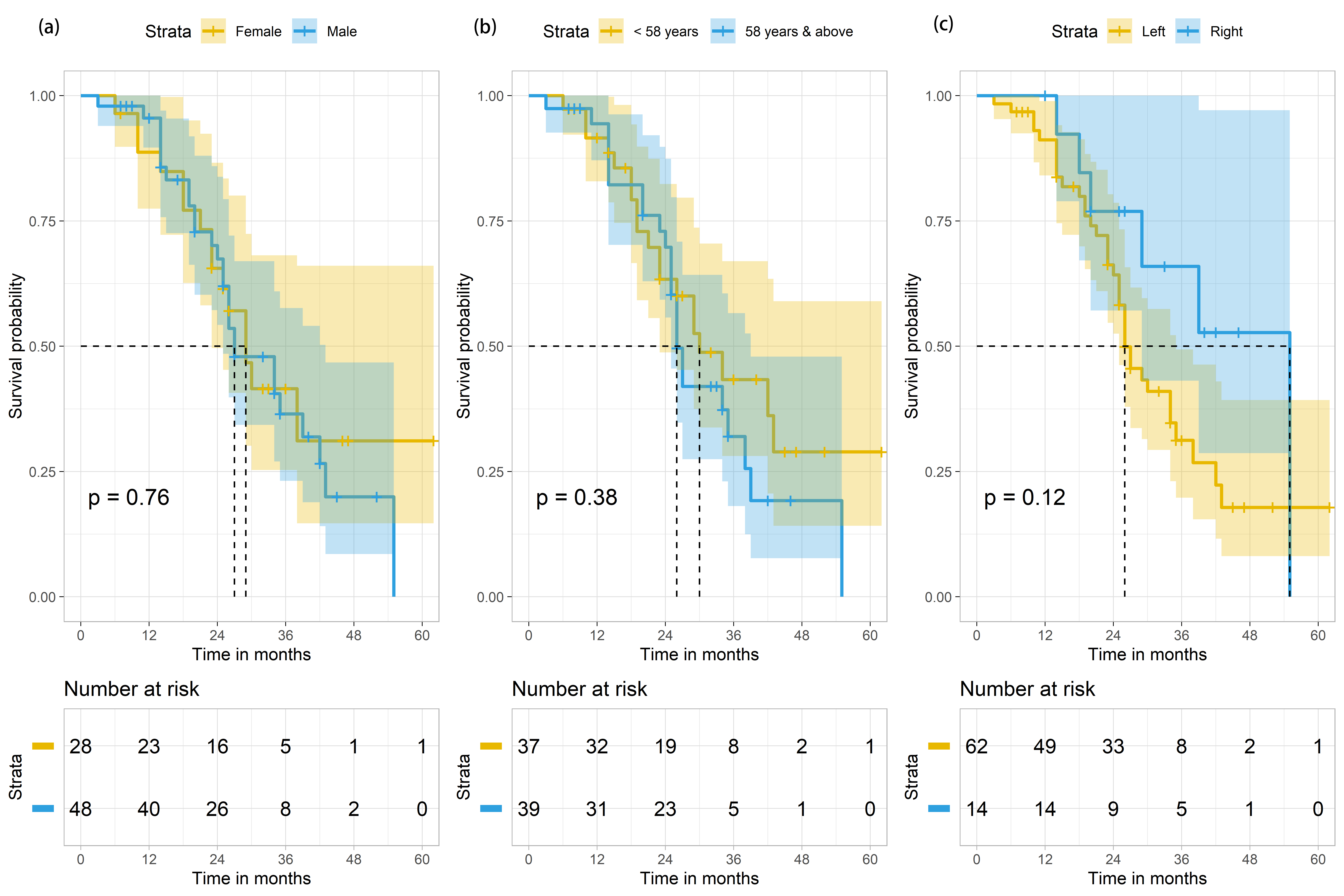
During the study period, a total of 105 patients with Stage IV CRC were treated in TBCC. These patients were initially diagnosed by either sigmoidoscopy or colonoscopy histopathologic biopsy. They were classified as stage IV patients due to presence of metastatic tumour(s). Of the 105 cases, 29 were excluded from the study due to the following reasons: unknown KRAS status (n = 20), known NRAS status (n = 2), and inability to contact at the end of the follow-up period (n = 7). Hence, 76 metastatic CRC (mCRC) patients were included in this study, and were followed up for a total of 1951 person-months. Their median age was 58 years (range = 20–79 years) (Table 1). At the end of the study period, only 21 (61.8%) of the surviving mCRC patients were male and 13 (38.2%) were female. There was no significant difference between survival outcome and gender (p = 0.821) (Table 1). The median OS was 29 months (95% CI 23.7–34.3), with males and females having median survival of 27 months (95% CI 20.3–33.7) and 29 months (95% CI 23.7–34.3), respectively. No significant difference (p = 0.764) in terms of median survival between the two genders (Fig. 2 left panel, Table 2) and in terms of the median survival based on median age distribution of 58 years old was found (Fig. 2 middle panel).
| Variables | Total population | Dead (n = 42) | Alive (n = 34) | p-value | |
| n (%) | n (%) | n (%) | |||
| Gender | Male | 48 (63.2) | 27 (64.3) | 21 (61.8) | 0.821 |
| Female | 28 (36.8) | 15 (35.7) | 13 (38.2) | ||
| Age in years | Median (IQR, min–max) | 58.0 (16.0, 20–79) | 58.0 (13.0, 26–79) | 56.0 (22.0, 20–78) | 0.507 |
| Location of primary tumour | Right-sided | 14 (18.4) | 6 (14.3) | 8 (23.5) | 0.301 |
| Left-sided | 62 (81.6) | 36 (85.7) | 26 (76.5) | ||
| KRAS status | Wild-type | 50 (65.8) | 23 (54.8) | 27 (79.4) | 0.024 |
| Mutated | 26 (34.2) | 19 (45.2) | 7 (20.6) | ||
| Site | Colon | 19 (25.0) | 8 (19.0) | 11 (32.4) | 0.289 |
| Sigmoid colon | 36 (47.4) | 23 (54.8) | 13 (38.2) | ||
| Rectum | 21 (27.6) | 11 (26.2) | 10 (29.4) | ||
| Number of metastatic site(s) | Single | 23 (30.3) | 7 (16.7) | 16 (47.1) | 0.004 |
| Multiple | 53 (69.7) | 35 (83.3) | 18 (52.9) | ||
| Liver metastasis | Yes | 60 (78.9) | 32 (76.2) | 28 (82.4) | 0.512 |
| No | 16 (21.1) | 10 (23.8) | 6 (17.6) | ||
| Lung metastasis | Yes | 42 (55.3) | 29 (69.0) | 13 (38.2) | 0.007 |
| No | 34 (44.7) | 13 (31.0) | 21 (61.8) | ||
| *Other metastasis | Yes | 32 (42.1) | 21 (50.0) | 11 (32.4) | 0.121 |
| No | 44 (57.9) | 21 (50.0) | 23 (67.6) | ||
| Note: The whole numbers denote the data while the percentages are in
parentheses. IQR, Interquartile range. | |||||
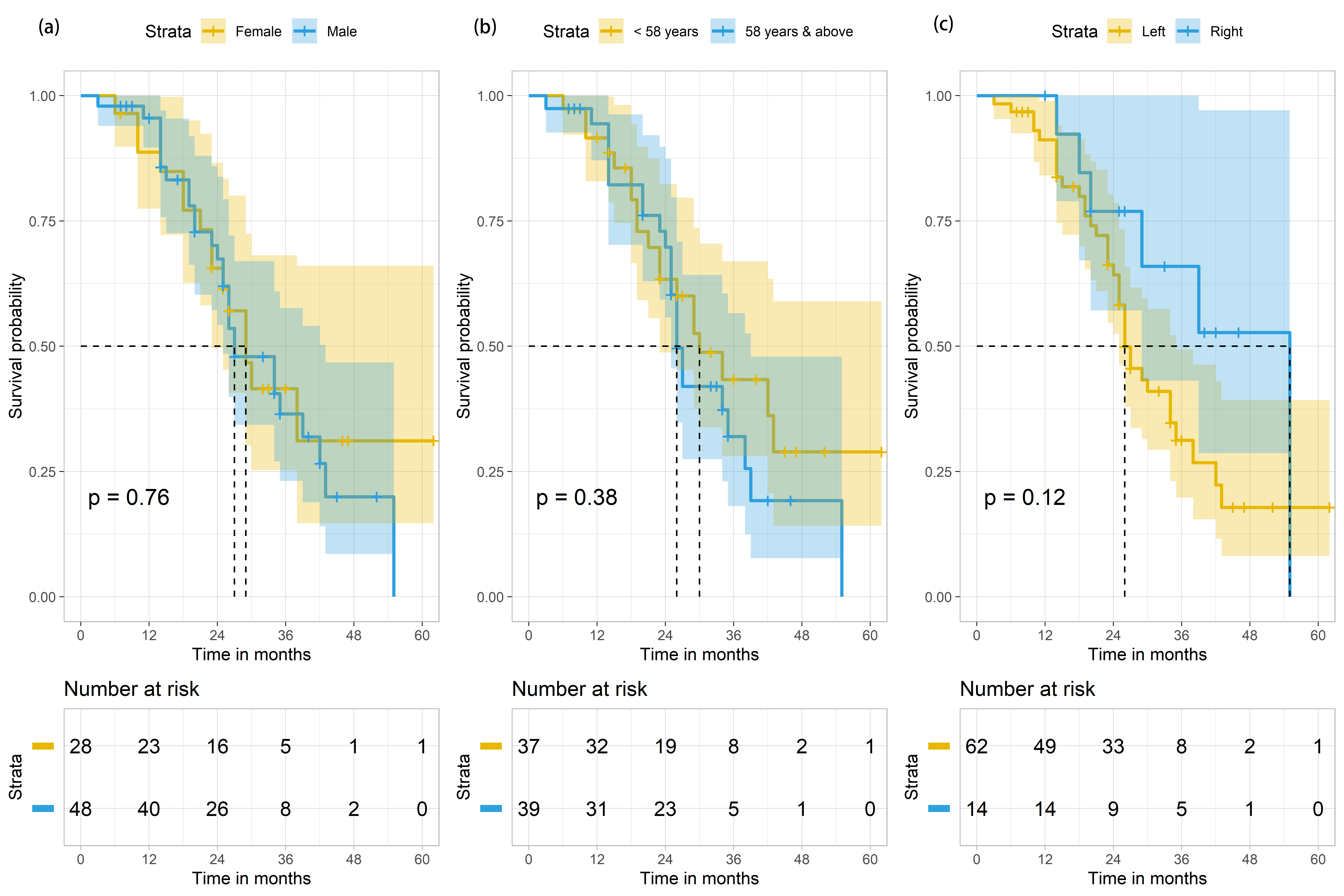 Fig. 2.
Fig. 2.Survival outcomes of mCRC patients (n = 76) based on
gender, age and site of primary tumour in colon. (a) Survival outcomes of mCRC
patients (n = 76) based on gender. p-value is derived from the log-rank
test comparing the 2 Kaplan Meier curves for females (in yellow) and males (in
blue). The shaded areas indicate the 95% confidence intervals for each group.
(b) Survival outcomes of mCRC patients (n = 76) based on age distribution, with
the median survival age of 58 years old as the cut-off point (
| Variables | Total population | Median survival in months | |
| N (%) | (95% confidence interval) | ||
| Overall | 76 (100) | 29 (23.7–34.3) | |
| Gender | Male | 48 (63.2) | 27 (20.3–33.7) |
| Female | 28 (36.8) | 29 (23.7–34.3) | |
| Age | 41 (53.9) | 29 (22.0–35.0) | |
| 35 (46.1) | 29 (22.3–35.7) | ||
| Location of primary tumour | ^Right-sided | 14 (18.4) | - |
| Left-sided | 62 (81.6) | 26 (22.3–29.7) | |
| KRAS status | Wild-type | 50 (65.8) | 35 (22.1–47.9) |
| Mutated | 26 (34.2) | 25 (18.3–31.7) | |
| Site | ^Colon | 19 (25.0) | - |
| Sigmoid colon | 36 (47.4) | 25 (20.8–29.2) | |
| Rectum | 21 (27.6) | 34 (28.4–39.6) | |
| Number of metastatic site(s) | Single | 23 (30.3) | - |
| Multiple | 53 (69.7) | 27 (23.3–30.7) | |
| Metastasis | Liver | 29 (20.1–37.9) | |
| Lungs | 25 (22.3–27.7) | ||
81.6% (n = 62) of mCRC patients were found to have the primary tumours on the left side of the colon (Table 1). Their median OS period was 26 months (95% CI 22.3–29.7) (Table 2, Fig. 2 right panel). Only 18.4% (n = 14) mCRC cases with primary tumours found in the right side of the colon (abbreviated here as RCC for right-sided colorectal cancer) were recorded, of which 6 died by the end of the study period, amounting to 14.3% of the deaths (Table 1). We were unable to calculate the median survival for mCRC patients with RCC because more than 50% of the patients were alive at the end of the study period (Table 2). Intriguingly, 19 of 26 (73.1%) mutant KRAS mCRC patients were found to have primary tumours on the left-side of the colon (abbreviated here as LCC to refer to as left-sided colorectal cancer), while 7 of 26 (26.9%) had RCC (Table 3). There were significantly more deaths among those with LCC, when compared to RCC (p-value = 0.035). 34.2% (n = 26) of the samples were mutant KRAS, generating a significant p-value of 0.024.
| Variable | Total population | Dead (n = 19) | Alive (n = 7) | p-value | |
| N (%) | n (%) | n (%) | |||
| Gender | Male | 15 (57.7) | 11 (57.9) | 4 (57.1) | 0.973 |
| Female | 11 (42.3) | 8 (42.1) | 3 (42.9) | ||
| Age in years | Median (IQR, min–max) | 60.0 (12.0, 39–78) | 58.0 (10.0, 39–73) | 67.0 (9.0, 52–78) | 0.035 |
| Location | Right-sided | 7 (26.9) | 3 (15.8) | 4 (57.1) | 0.035 |
| Left-sided | 19 (73.1) | 16 (84.2) | 3 (42.9) | ||
| Site of primary tumour | Colon | 7 (26.9) | 3 (15.8) | 4 (57.1) | 0.080 |
| Sigmoid colon | 11 (42.3) | 10 (52.6) | 1 (14.3) | ||
| Rectum | 8 (30.8) | 6 (31.6) | 2 (28.6) | ||
| Number of metastatic site(s) | Single | 5 (19.2) | 1 (5.3) | 4 (57.1) | 0.003 |
| Multiple | 21 (80.8) | 18 (94.7) | 3 (42.9) | ||
| Liver metastasis | Yes | 20 (76.9) | 13 (68.4) | 7 (100.0) | 0.090 |
| No | 6 (23.1) | 6 (31.6) | 0 (0.0) | ||
| Lung Metastasis | Yes | 18 (69.2) | 15 (78.9) | 3 (42.9) | 0.077 |
| No | 8 (30.8) | 4 (21.1) | 4 (57.1) | ||
| Other metastasis* | Yes | 1 (3.8) | 1 (5.3) | 0 (0.0) | 0.536 |
| No | 25 (96.2) | 18 (94.7) | 7 (100.0) | ||
| Codon 12 | Yes | 18 (69.2) | 14 (73.7) | 4 (57.1) | 0.418 |
| No | 8 (30.8) | 5 (26.3) | 3 (42.9) | ||
| Codon 13 | Yes | 8 (30.8) | 5 (26.3) | 3 (42.9) | 0.418 |
| No | 18 (69.2) | 14 (73.7) | 4 (57.1) | ||
| Note: The whole numbers denote the data while the percentages are in
parentheses. IQR, Interquartile range.
| |||||
Additionally, the site of primary tumour was also classified either as in the colon, sigmoid colon or rectum. The primary tumours of most mCRC were found in the sigmoid colon (with n = 36) constituting 47.4%, followed by rectum (n = 21, 27.6%) and colon (n = 19, 25.0%). No significant difference (p-value = 0.289) with regards to the survival outcomes and sites of primary tumours was found (Table 1). The median survival of mCRC patients with primary tumours found in the rectum (34 months [95% CI: 28.4–39.6]) was significantly longer (p-value = 0.016) than mCRC patients with primary tumours in the sigmoid colon (25 months [95% CI: 20.8–29.2]). The median survival of mCRC patients with primary tumours in the colon was non-calculable (Table 2), as more than half of the mCRC patients with tumours in colon were alive at the end of follow-up period. Correspondingly, for mutant KRAS mCRC patients, there was no significant difference between these patients with primary tumours located in either colon, sigmoid colon or rectum (p-value = 0.080, Table 3). No significant difference was found in the median survival between the mutant KRAS mCRC patients with primary tumours in the colon, sigmoid colon or rectum (p-value = 0.348) (Table 4).
| Variable | Total population | Median survival in months | |
| N (%) | (95% confidence interval) | ||
| KRAS mutation | 26 | 25 (18.3–31.7) | |
| Gender | Male | 15 (57.7) | 29 (17.0–33.0) |
| Female | 11 (42.3) | 25 (13.5–44.5) | |
| Age | Below 58 | 7 (26.9) | 18 (9.8–26.2) |
| 58 and above | 19 (73.1) | 26 (20.1–31.9) | |
| Location of tumour in colon | Right-sided | 7 (26.9) | 25 (19.0–31.0) |
| ^Left-sided | 19 (73.1) | - | |
| Primary tumour Site | ^Colon | 7 (26.9) | - |
| Sigmoid colon | 11 (42.3) | 20 (10.3–29.7) | |
| Rectum | 8 (30.8) | 29 (18.9–39.1) | |
| Metastatic site | 20 (76.9) | 29 (22.1–35.9) | |
| 18 (69.2) | 25 (18.4–31.6) | ||
| Number of metastatic site(s) | ^Single | 5 (19.2) | - |
| Multiple | 21 (80.8) | 25 (18.0–32.0) | |
| Codon 12 | G12D | 10 (38.5) | 25 (15.4–36.2) |
| G12S | 3 (11.5) | 25 (13.8–36.2) | |
| ^G12A | 1 (3.8) | - | |
| ^G12V | 2 (7.7) | - | |
| ^G12C | 2 (7.7) | - | |
| Codon 13 | G13D | 8 (30.8) | 29 (24.4–33.6) |
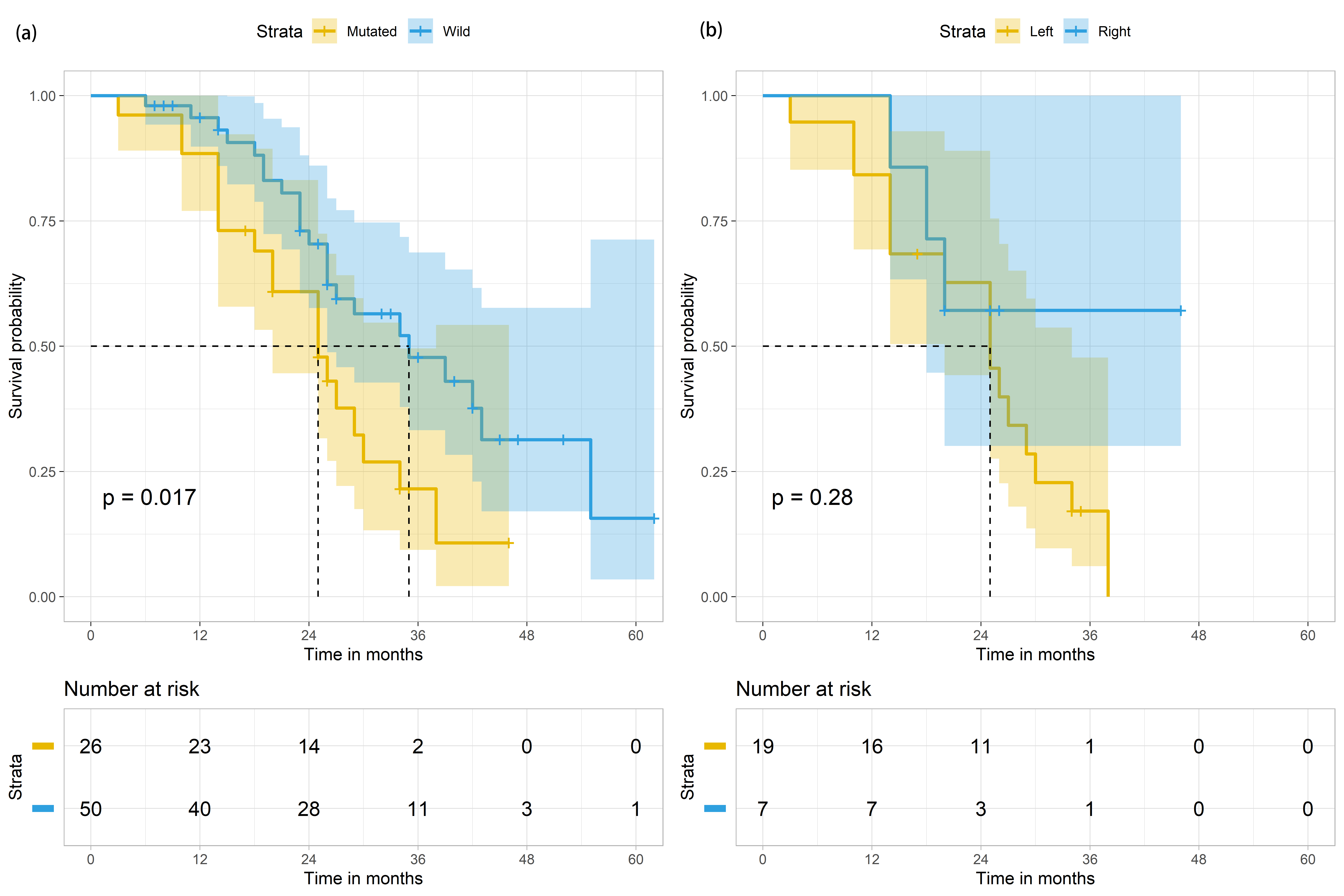
The median survival of mCRC patients with mutant and wild-type KRAS was 25 months (95% CI: 18.3–31.7) and 35 months (95% CI: 22.1–47.9), individually (Table 2, Fig. 3 left panel). Metastatic CRC patients with mutant KRAS were significantly more likely to have poorer median survival than mCRC patients with wild-type KRAS (p-value = 0.017, Table 2, Fig. 3 left panel). Further analysis was conducted specifically on the mCRC patients with mutated KRAS status (n = 26, Tables 3,4). The median survival of male and female mutant KRAS mCRC patients were 29 months (95% CI: 17.0–33.0) and 25 months (95% CI: 13.5–44.5), accordingly (Table 4). Among the mutant KRAS mCRC patients, 73.1% (n = 19) had LCC. Of these 19 patients, 84.2% (n = 16) died at the end of the study period (Table 3). The median survival of these 26 patients was 25 months (95% CI: 18.3–31.7) (Table 4, Fig. 3 right panel).
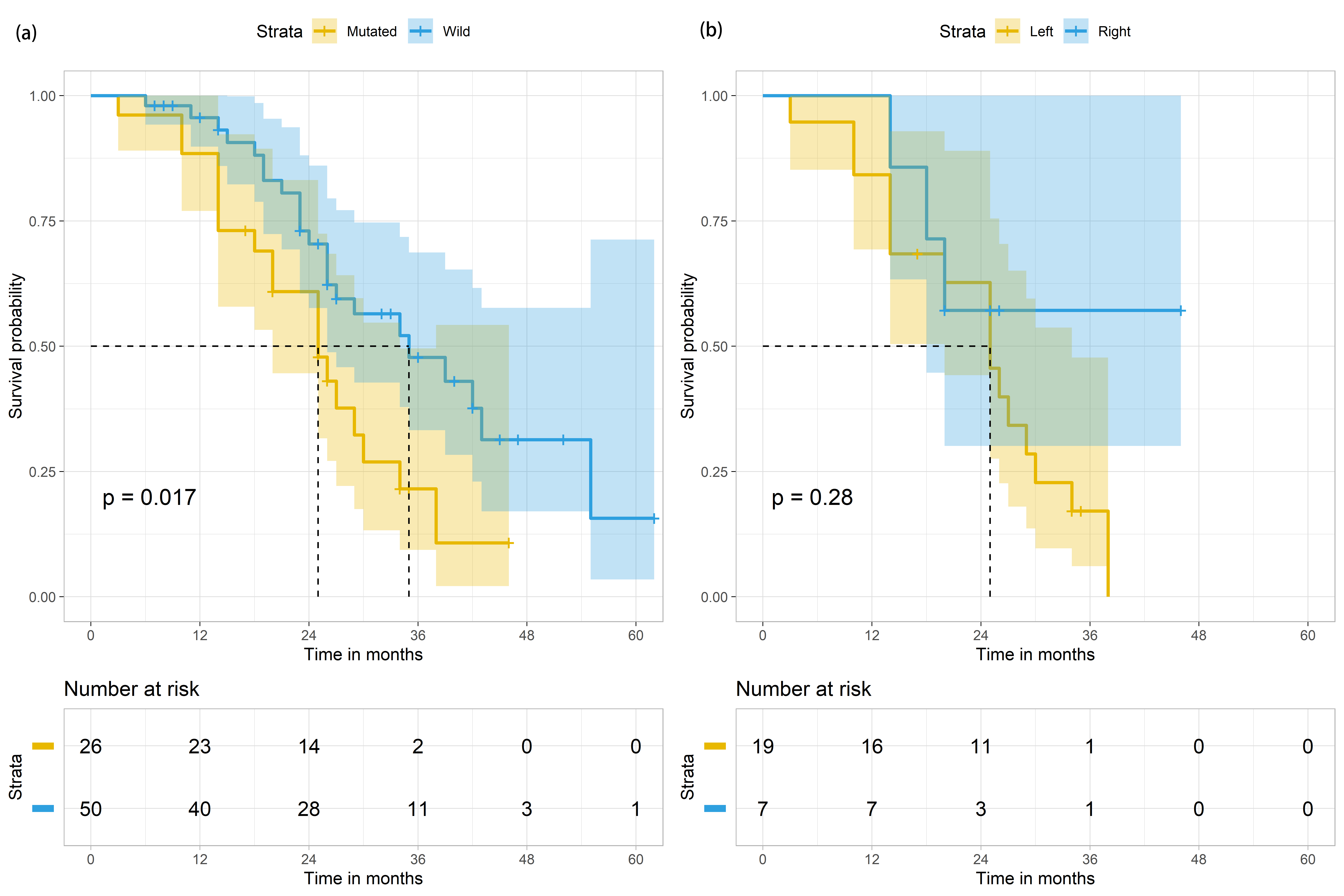 Fig. 3.
Fig. 3.Survival outcomes of mCRC patients (n = 76) based on KRAS status and based on both KRAS status and primary tumour in the colon. (a) Survival outcomes of mCRC patients (n = 76) based on KRAS status (mutated in yellow; wild in blue). (b) Survival outcomes of mCRC patients with KRAS mutation (n = 26), based on the site of primary tumour in the colon (Left colon in yellow; Right colon in blue). The shaded areas indicate the 95% confidence intervals for each group. Metastatic sites of mCRC patients and their effects on survival periods.
We further investigated the number of metastatic sites detected in these mCRC patients. 30.3% (n = 23) of the study population had a single metastatic site detected (either liver, or lung or any other organs), while 69.7% (n = 53) of the 76 mCRC patients had two (double) or more metastatic sites (Table 1). mCRC patients with two or more metastatic sites were classified as multiple, regardless of the site of metastasis. There was a statistical significant difference between the number of metastatic sites and survival outcome (p-value = 0.004, Table 1). There was a statistically significance value of p = 0.047 between the survival outcomes of mCRC patients with a single metastatic site and mCRC patients with multiple metastatic sites (Table 2). At the end of the study period, 7 of the 23 single metastatic site CRC patients died while 35 of the 53 multiple (two or more) metastatic sites CRC patients died, yielding deaths rates of 30.4% and 66.0%, correspondingly (p-value = 0.004, Table 1).
Two metastatic target organs (liver and lungs) were also examined. 78.9% (n = 60) of the mCRC patients had liver metastasis, while 55.3% (n = 42) had metastasis to the lungs. It should be noted that a mCRC patient may have metastasis to both the liver and the lungs. The survival rate of mCRC patients with liver metastasis was 46.7% (n = 28), whilst the survival rate of mCRC patients with lung metastasis was 30.9% (n = 13). Chi-squared test showed no significant difference between the number of deaths and survival of mCRC patients due to liver metastasis (p-value of 0.512), but a significant difference with p-value of 0.007 was observed between the number of deaths and survival of mCRC patients due to lung metastasis (Table 1). The median survival for mCRC patients with liver metastasis and lung metastasis were at 29 months (95% CI: 20.1–37.9) and 25 (95% CI: 22.2–27.8), respectively. Survival analysis revealed a significant difference in the median survival times for mCRC patients with lung metastasis only (p = 0.011, Table 2).
In order to examine the survival outcomes of mutant KRAS mCRC patients (n = 26) with varying number of metastatic sites, mutant KRAS mCRC patients were categorised in accordance to the number of metastatic sites. Of the seven mutant KRAS mCRC patients who survived till the end of the study period, 4 (57.1%) have one metastatic site while 3 (42.9%) have two or more metastatic sites (Table 3). Mutant KRAS mCRC patients with multiple metastatic sites were significantly more likely to die by the end of the study period (p = 0.003, Table 3), as compared to mCRC patients with wild-type KRAS. The median survival of mutant KRAS mCRC patients with one metastatic site was non-calculable, however, the median survival for mutant mCRC patients with two or more metastatic sites was 25 (95% CI: 18.0–32.0) (Table 4). Survival analysis revealed a significant difference between these two mutant KRAS patient groups (p = 0.043).
Similarly, mutant KRAS mCRC patients were further analysed based on either liver or lung metastatic sites. Although majority of mutant KRAS mCRC patients with either metastatic site died, that is, 13 mutant KRAS mCRC patients with liver metastasis (68.4%) and 15 mutant KRAS mCRC patients with lung metastasis (78.9%) died at the end of the study period (Table 3).
The specific KRAS mutations detected in the mutant KRAS mCRC patients were studied. 69.2% (n = 18) and 30.8% (n = 8) of the mutant KRAS mCRC patients had mutations within codons 12 and 13, respectively (Tables 3 and 4). There was no significant difference between the survival outcomes of mutant KRAS patients with mutations in either codon 12 or 13 of the KRAS gene (Table 3). However, there is a significant difference in the median survival between the mutant KRAS mCRC patients with mutations in codon 12 and those with mutation in codon 13 of the KRAS gene (p-value = 0.003, Table 4). In our study population, the highest KRAS mutation was G12D (n = 10) (38.5%), followed by G13D (n = 8) (30.8%). There were 11.5% (n = 3) of G12S, followed by 7.7% each of G12V and G12C with 3.8% of G12A. Median survival for mutant KRAS mCRC patients possessing G12A, G12V and G12C missense mutations were non-calculable. Mutant KRAS mCRC patients with G12D, G12S and G13D had median survival of 23 months (95% CI 15.4–34.6), 25 months (95% CI 13.8–36.2) and 29 months (95% CI 24.4–33.6) (Table 4), correspondingly.
To our knowledge, this is the first study in Brunei Darussalam to analyse both the survival outcomes of metastatic CRC patients and those of mutant KRAS mCRC patients. Although a recent paper published on the survival rates and associated factors of CRC patients in Brunei Darussalam [4], investigation on survival outcomes of mutant KRAS mCRC patients and exploration on the various KRAS mutations in these patients are still in its infancy in this country. The reported median survival was 57.0 months for CRC [4], while for mCRC patients, we estimated that the median survival was 29.0 months. The survival rate of mCRC patients found within this study was 44.7% while the reported five year survival rate for CRC reported by Leong et al was 49.6% [4]. The differences in survival rates of these patients could be due to different population sizes and samples as well as different medical care conditions. There are more cases of male mCRC patients than female mCRC patients and a slightly shorter survival period of the male mCRC patients in our study (Table 1). Prior research has indicated that gender contributes to survival outcomes and females were deemed to have better OS [13], possibly due to the protective effects of female sex hormones against CRC.
Despite the small population of Brunei Darussalam, Chi-squared analysis showed a significant difference between the survival outcomes of wild-type KRAS and mutant KRAS mCRC patients (p-value of 0.024). This is further corroborated by Kaplan-Meier survival analysis shown in Table 2, whereby there is a statistical significant difference (p-value = 0.017). The poorer survival outcome of mutant KRAS mCRC patients as compared to those of wild-type KRAS has been established [14], as KRAS has been identified as one of the six driver genes from the TCGA database that drives metastatic CRC [14]. Large cohort studies have consistently illustrated that mutant KRAS was associated with metastasis in CRC patients, including lymphatic and distant metastases [15].
The primary tumour of CRC has been classified as either on the right side of the colon (ileocecal valve, cecum, ascending colon to the transverse colon) or the left side of the colon (the splenic flexure, descending colon, sigmoid and rectum). A further categorisation of the primary tumour location on the left side of the colon refers to the sigmoid colon and rectum. In agreement with Buchwald et al. (2018) [16], we found that mCRC patients with primary tumours found in the rectum had better OS than those with primary tumours within the colon (Table 2). A limitation of our study is the lack of further sorting of the primary tumour, especially for the right side of the colon. There is an unusually high number of left-sided primary tumours (n = 62) as compared to right-sided primary tumours (n = 14) in these mCRC patients. Although most reports stated that KRAS mutation is associated with right-sided colon primary tumours in CRC [17, 18], we found that most of the KRAS mutations in our study population are found within the left-sided colon primary tumours (data not shown). Nevertheless, Table 3 illustrated that there was a significant difference in the survival outcome between the mutant KRAS mCRC patients with RCC and mutant KRAS mCRC with LCC patients. The poor survival outcome of mutant KRAS left-sided tumour mCRC patients was congruent to the findings of Charlton et al. (2017) [18] and Xie et al. (2019) [19]. Xie et al. (2019) [19] concluded that although KRAS mutations were in high occurrence in RCC than LCC, there was an association of KRAS mutations and poor prognosis in LCC, which was absent in RCC.
Increasing number of metastatic sites correlates with poorer survival outcomes in this study (Tables 1, 2 and 3). In Stage IV M1b Non-Small Cell Lung cancer, the number of metastases but not the location has been found to have an impact on the survival outcomes of the patients [20]. On the contrary, the number of metastases has no significant effect on survival in prostate cancer patients [21]. There are currently a few studies debating whether the impact of survival is dependent on the site of metastasis or the number of metastasis [22]. However, the primary tumour site of cancer may affect the survival of patients [23]. In this study, as for median survival, 78.9% of mCRC patients were found to have metastasis to the liver, while 55.3% of mCRC patients were found to have metastasis to the lungs. A higher proportion of mCRC patients was found to have metastasis to the liver due to the colon’s anatomical situation with regards to the portal circulation [24]. In addition, 81.6% of mCRC patients in this study were found to have LCC, and LCC was associated with higher incidence of liver metastasis [25]. Table 2 demonstrated that mCRC patients with metastasis to the lungs had significantly poorer survival outcome compared to mCRC patients with metastasis to the liver. Survival analysis estimated that mCRC patients with liver metastasis had an average of four months more survival period (Table 2). Majority of the mCRC patients with lung metastasis (n = 42) had metastasis to the liver (n = 60) too. Therefore, the number of metastases most likely determines the survival outcome, with metastasis to the lungs being an indicator of worse OS. On the other hand, there were more cases of metastasis to the lungs in mutant KRAS mCRC patients (69.2%), instead of 55.3% in the overall study population of mCRC patients. This is in agreement with the work of Ghidini et al. (2016) [26]. Compared to wild-type KRAS mCRC patients, mutant KRAS mCRC patients were found to have higher incidence of lung and brain metastases [26]. Taken together, in this study population, the number of metastatic sites accompany worsened OS, with metastasis to the lungs being a predictor of poor OS. Mutation in KRAS gene further worsened the prognosis.
85% of KRAS mutation occurs in codons 12 and 13. Codons G12V and G12D are the most common mutated gene found in KRAS gene in codon 12 [27], accounting for 70% of all KRAS mutation [28]. Various studies have associated specific KRAS mutations with different likelihood of survival. Bai et al. (2017) [27] determined that G12V and G12D have been associated with an increased risk of CRC associated death, while Jones et al. (2017) [29] established that G12C and G12V were predictors of worse OS. Between G12D, G12S and G13D KRAS mutations, our study substantiates that mutant KRAS mCRC patients with G12D have the lowest survival median at 23 months (95% CI 15.4–34.6). There is a lack of data for the median survival of mutant KRAS mCRC patients with G12A, G12V and G12C mutations due to the small population. In codon 13, G13D is the most common KRAS mutation [16] and this was the second most common specific KRAS mutation after G12D in our study with a survival median period of 29 (95% CI 24.2–33.6) months (Table 4). Similar to most studies, we obtained that mutant KRAS mCRC patient mutation in codon 13 had a comparably better OS than those with mutation in codon 12 [8, 27, 29]. Mutations at both codons 12 and 13 are close to the nucleotide binding pocket of K-Ras protein and different mutation affects the biochemistry of K-Ras differently. Mutations of KRAS within codon 13 have been found to escalate the nucleotide exchange by ten-fold while mutations within codon 12 affect hydrolysis but not rate of nucleotide exchange [30]. Intuitively, the escalation of enzymatic activity due to mutation within 13 in KRAS should result in a more aggressive cancer type and therefore worse OS. Nevertheless, counterintuitively, the accelerated nucleotide exchange due to mutation in KRAS codon 13 may actually result in the cancerous cells undergoing apoptosis due to depletion of GTP levels [31] and thus, resulting in less aggressive cancer type and better OS.
In conclusion, our data appear to show that mutant KRAS mCRC patients had a significantly poorer OS, which was shown to be worse when the primary tumours were found at the left side of the colon. Mutant KRAS mCRC patients with mutations in codon 12 were found to have shorter survival median periods than those with mutations within codon 13. This conclusion was reached while considering that this is a small study despite being representative of a country’s population. Although our conclusion explains the relationship of the genotype of the tumours and survival outcomes of mCRC patients within the country, caution has to be practised for it to be extrapolated in another population.
RP conceived the work. DD, RP, PUT and SKL acquired data. RP, DD, LLC and YCL analysed the data. DD and YCL wrote the manuscript. DD, RP, LLC, SKL, ZHL, KK, LCM and YCL contributed towards the discussion and revision of the manuscript. All authors read and agreed to the final version of the manuscript.
Ethical approval for the study was obtained from PAPRSB Institute of Health Sciences Medical and Research Ethics Committee (Reference: UBD/IHS/B3/8 dated 19 April 2018).
We would like to thank the nurses of The Brunei Cancer Centre, Pantai Jerudong Specialist Centre, for kind assistance during data collection, and Universiti Brunei Darussalam for support.
This work was funded by grant numbered CRGWG(020)/180101 supported by Universiti Brunei Darussalam.
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.