†These authors contributed equally.
§On behalf of the Australian Respiratory Early Surveillance Team for Cystic Fibrosis (AREST CF).
Academic Editors: Massimo Conese and Lorenzo Guerra
Background: People with Cystic Fibrosis (CF) develop pulmonary
inflammation, chronic infection and structural lung damage early in life, with
these manifestations being prevalent among preschool children and infants. While
early immune events are believed to play critical roles in shaping the
progression, severity and disease burden later in life, T cells and their subsets
are poorly studied in the CF lung, particularly during the formative early stages
of disease. Methods: Using flow cytometry, we analyzed Mucosal
Associated Invariant T (MAIT) cells,
Although early life immune/inflammatory events are believed to play an important role in the progression and prognosis of cystic fibrosis disease [1], we are only now starting to examine immune cell phenotypes present in the CF lung in early childhood [2]. In healthy individuals, macrophages comprise the predominant population in bronchoalveolar lavage (BAL), with lymphocytes constituting the second most common immune cell population. In CF however, infections and inflammation alter this profile, most markedly by the recruitment of neutrophils, the main immune cell infiltrate associated with CF pathogenesis [3]. Most research on immunity in CF has therefore understandably focused on macrophages and neutrophils.
Individual lymphocyte populations will likely also be altered by infection and inflammation, potentially contributing to CF disease progression. While T cells are present in the CF airway where much of the severe structural damage occurs, the contribution of T cells in CF children are not well studied [4] and it is unclear whether they play protective or pathological roles in CF pathogenesis. Studies that have analyzed T cells in the CF lung have predominantly been performed in adults or adolescents, by which age considerable disease progression has already occurred, and most have only considered conventional T cells.
T cells comprise a complex range of immune cells with a multitude of roles which, depending on the cell type and circumstances, include coordinating and linking different aspects of immunity, regulating inflammation and fighting infection. An improved knowledge of T cell subsets in the lung during the early stages of CF would increase our understanding of the contribution such cells might make to long-term disease progression.
An important group of such cells that have thus far been almost unstudied in the
CF lung are the unconventional T cells. Little is known about the roles of
unconventional T cells in CF, especially during the formative early years of
disease progression. These unconventional cells are important components of the T
cell repertoire, providing a large source of inflammatory cytokines, including
IFN
By using FACS analysis to show the presence and frequencies of these cells, we here provide the first identification and analysis of these important immune cells in the lungs of children with CF. Comparing the frequencies of these cells with infection status, we found that the proportion of NKT-like cells increased during viral infections. These findings therefore identify potentially important interactions between mucosal cellular immune responses and pathogenic infection in early CF pathogenesis.
Bronchoalveolar lavage (BAL) samples were pooled aliquots of the 2nd and 3rd washings from the right middle lobe, collected from CF patients by bronchoscopy under AREST CF protocols at the Royal Children’s Hospital, Melbourne with approval of the hospital Human Research Ethics Committee (HREC #25054). In Australia, antibiotic prophylaxis with amoxicillin-clavulanic acid (15 mg/kg/day) is prescribed during the first two years of life to children with CF. Microbiological clinical status of patients was determined using standard clinical laboratory cultures at the Royal Children’s Hospital, Melbourne.
BAL samples were passed through a cell strainer to remove debris and any clumps
of tissue. After washing, cell pellets were resuspended in 1 mL of RPMI-1640
(Thermo Fisher Scientific) and manually counted using a hemocytometer. Each
sample was resuspended in FACS buffer (PBS plus 2% fetal bovine serum (Thermo
Fisher Scientific) plus 10% human serum for 20 minutes on ice, followed by
staining with LIVE/DEAD™ Fixable Near-IR Dead Cell Stain (Thermo
Fisher) for 15 minutes in the dark to allow the exclusion of dead cells from the
analysis. After washing, a cocktail of the antibodies listed below was added and
staining completed in the dark for 25 minutes before fixing cells in 2%
paraformaldehyde in PBS overnight. Samples were resuspended in FACS buffer, then
analyzed on a BD LSRFortessa™ X-20 using BD FACSDiva (BD
Biosciences). Antibody cocktail (all antibodies from BD Biosciences): anti-CD3
UCHT1 BUV737, anti-CD4 SK3 (aka: Leu3a) BV480, anti-CD8 RPA-T8 PE, anti-CD19
HIB19 PerCP-Cy5.5, anti-CD45 HI30 BUV395, anti-CD56 NCAM16.2 BV711,
anti-TCR
Single CD45+ leukocyte events were gated on a SSC-A vs FSC-A plot, followed by
dead cell exclusion. All subsequent analyses were performed on BAL total T cells
gated as CD45+CD3+CD19– cells. Bulk CD4 and CD8 T cells were defined by the
expression of CD4+CD8– and CD8+CD4– by BAL total T cells, respectively.
Flow cytometry data were analyzed using FlowJo V10 software (Becton Dickinson, Ashland, USA). Statistical analyses were performed with GraphPad Prism Version 6 (GraphPad Software Inc., California, USA). Data were checked for normality using the Kolmogorov-Smirnov test and normally distributed data analyzed by unpaired t-test. Three groups of data were compared by Kruskal-Wallis test.
The aim of this study was to characterize the composition of unconventional T cells within BAL samples of young children with CF. BAL cells were analyzed from 17 children with CF. These comprised 12 females and 5 males aged between 2 and 6 years of age (characteristics and infection status are listed in Table 1).
| Child number | Age (Years) | Sex | Infection status |
| 1 | 2.9 | F | Haemophilus parainfluenzae (not type B), URTF |
| 2 | 5.2 | F | URTF, Respiratory syncytial virus |
| 3 | 4.6 | F | H. parainfluenzae, URTF |
| 4 | 4.9 | F | URTF, fungus |
| 5 | 5.0 | M | H. parainfluenzae, URTF, Haemophilus influenzae (not type B) |
| 6 | 5.9 | F | URTF |
| 7 | 6.0 | F | S. aureus, Stenotrophomonas maltophilia, Escherichia coli, H. influenzae (not type B), URTF, Parainfluenza virus |
| 8 | 6.0 | F | Pseudomonas aeruginosa (rough), URTF, Influenza virus |
| 9 | 3.0 | F | H. influenzae, Methicillin resistant Staphylococcus aureus, URTF |
| 10 | 6.1 | F | H. influenzae (not type B), S. aureus, URTF |
| 11 | 3.0 | M | URTF |
| 12 | 3.0 | M | Haemophilus haemolyticus, S. aureus, URTF |
| 13 | 3.0 | F | URTF, H. parainfluenzae, parainfluenza virus |
| 14 | 5.9 | F | URTF |
| 15 | 5.1 | F | Respiratory syncytial virus, Moraxella catarrhalis, H. parainfluenzae |
| 16 | 2.9 | M | Respiratory syncytial virus, URTF, Haemophilus species |
| 17 | 3.9 | M | H. parainfluenzae, Gram negative bacilli, H. influenzae (not type B), URTF |
Routine clinical assessment of BAL cells by light microscopy showed, as usual for such samples, that macrophages were the predominant cell population, with a typical neutrophil infiltration and only a low proportion of lymphocytes (0–1.7%) (Supplementary Fig. 2). T cells within the BAL fluid from young children with CF were identified by flow cytometric analysis as CD45+CD3+CD19– cells (gating strategy, Supplementary Fig. 2), and contained a mix of CD4+ and CD8+ T cells (Supplementary Fig. 3). These contained a higher proportion of CD8+ T cells (median 34% to 44% respectively) which is consistent with previous studies in healthy children, and children with CF [2, 7].
Further analyses quantified the proportions of the unconventional T cell
populations, specifically NKT-like cells,
 Fig. 1.
Fig. 1.Flow cytometric phenotyping of unconventional T cells in the BAL
of young children with CF. (a) The relative proportions of MAIT cells
(CD45+CD3+CD19–MR1-tetramer+),
MAIT cells and
To see if age-related changes in unconventional T cell frequencies were detectable in BAL during very early progression in CF, the frequencies of T cell subsets were compared with the age of the children. Correlating age against the frequencies of the unconventional T cell subtypes analyzed provided no evidence of any change in the proportions of these cells, as children with CF progressed from 2 to 6 years of age (Fig. 2a). Similarly, no significant difference was detected in the frequencies of unconventional T cell populations in BAL cells collected from young male (n = 5) and female (n = 12) children with CF (Fig. 2b).
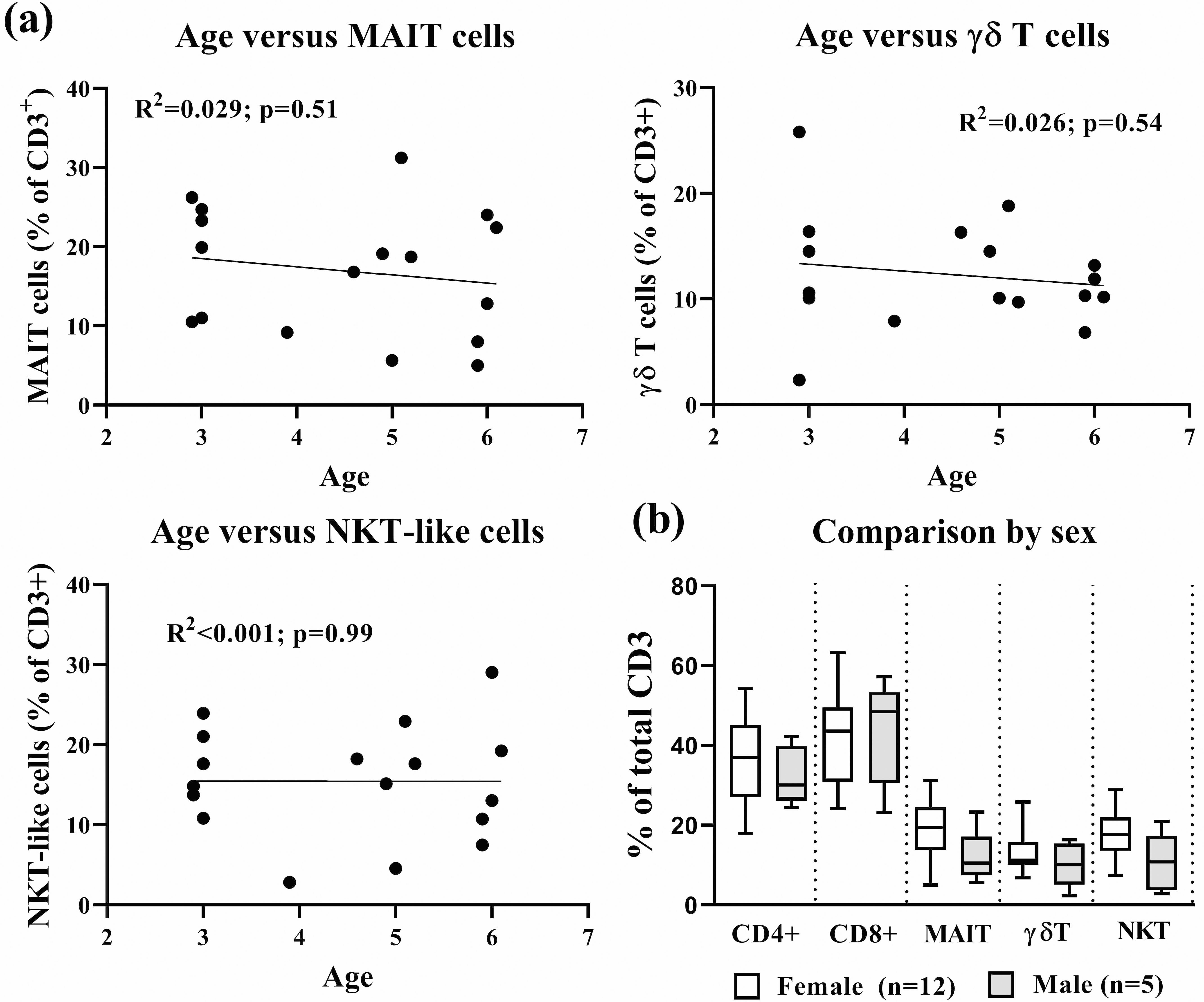 Fig. 2.
Fig. 2.Effect of age and sex on unconventional T cells subsets in BAL from young children with CF. (a) Correlation of age versus frequencies of unconventional T cells in children with CF. (b) Comparison analysis of different T cells subtypes between female (n = 12) and male (n = 5) CF patients. There were no statistically significant differences between the two sexes (unpaired t test) or any correlation of cell frequency with age (linear regression analysis). Data are single values pooled from children analyzed individually over an 18-month period (a) presented as individual patients or (b) as groups in boxplots, which present the middle 50% of values (box), the median (line) and range (error bars).
To determine if the frequencies of T cell subsets present in the lungs of children with CF might be influenced by their infection status, we analyzed the proportions of BAL T cell subsets, in relation to clinically prevalent CF bacterial and viral pathogenic infections present in BAL from our cohort of CF children. As the majority of children with CF in the present cohort had multiple infections (Table 1), T cell subset proportions were compared in terms of commonly presented CF viral and bacterial pathogens (Fig. 3).
 Fig. 3.
Fig. 3.Effect of infections on unconventional T cells subsets in BAL
from young children with CF. Flow cytometric comparisons of MAIT cells
(CD45+CD3+CD19–MR1-tetramer+),
Infection with Haemophilus species bacteria (including both H.
influenzae and H. parainfluenzae), while highly prevalent in the
patients studied (11 out of 17 children), had no significant effect on the
proportions of MAIT cells,
Some children included in this study were infected with respiratory syncytial virus (n = 3), parainfluenza (n = 2) and influenza (n = 1). As there were insufficient cases of any virus to analyze individually, viral infections were studied as a group. While the proportions of most T cell subsets were similar in all children regardless of viral infection status, there was a significant increase in the proportion of NKT-like cells in BAL cells from young children with CF who were infected with a virus (Fig. 3c).
The principal cause of death in people with CF remains loss of lung function due to pulmonary disease arising from chronic infection, with sustained inflammation and interspersed acute exacerbations. As for any infection-driven inflammation, T cells would be expected to play vital roles in the CF lung in fighting infections and modulating inflammation. There are two key ways that T cells could impact and interact with CF disease. First, T cell populations already present in the lung or recruited by infection could either increase or decrease the severity of the resulting inflammation and thus enhance disease progression. In addition, T cells themselves express CFTR which, when functionally impaired, can exhibit reduced ion channel activity with effects on the secretion of cytokines including the anti-inflammatory IL-10 [8]. Little is known about the precise role of T cell immunity in CF, particularly in early childhood, although regarding conventional T cell responses, increases in Th17 and Th2 responses in the adult CF lung have been identified, with potential negative effects on disease progression [9, 10].
Importantly, T cells are comprised of heterogeneous cell populations, each with
specific and varied functions, and the potential of either reducing or
exacerbating the pathological process in chronic disease. With the paucity of
information available regarding many important cell types in early CF, this study
set out to perform the first analysis of unconventional T cell composition in the
BAL of young children with this disease. Unconventional T cells comprising MAIT
cells, NKT cells and
MAIT cells are a subset of unconventional T cells, often highly abundant in humans, which due to their responsiveness to riboflavin-derived antigens, respond to a range of yeast and bacterial pathogens, including at mucosal surfaces such as the lung [13, 14]. Recent studies in mice indicated a role for these cells as early innate immune responders in lung defense against an array of pathogens, including Mycobacterium bovis bacillus Calmette-Guérin, Legionella longbeachae and influenza viruses [15, 16, 17]. MAIT cells have also been implicated in the pathogenesis of non-infectious chronic pulmonary disorders such as asthma [18]. Despite the potential importance of these cells, little is known about their frequency and potential role in the CF lung.
In the current study, we observed that MAIT cells comprised the largest proportion of the unconventional T cells in BAL fluid and that their frequency was relatively independent of the infection status. A previous study which examined MAIT cells in the peripheral blood of adults with CF with lung bacterial infections (particularly P. aeruginosa) found the frequency of these cells to be significantly lower in CF blood as compared to healthy controls and noted that reduced circulating MAIT cell numbers positively correlated with the severity of the lung disease [19]. Adults with CF typically have a more advanced disease state as well as different pathogen infection profiles compared to children with CF, and so it is unknown whether this inverse association would also be present during childhood. It also remains to be determined if this reflects a causative link between MAIT cells and pathogenesis or simply that the chronic inflammation in CF might deplete these cells in the circulation. However, it is interesting to note the report of one individual with CF who succumbed to bacterial infections at only 22 years of age and who was found to have a profound deficiency in circulating MAIT cells [20].
Human MAIT cells are heterogeneous, with the most commonly described subpopulations being CD4–CD8– (DN) or CD4–CD8+, which are developmentally related but functionally distinct subtypes [21, 22]. Our analyses found that DN MAIT cells were the dominant subpopulation in the CF lung, with smaller but fairly equivalent levels of CD4+ and CD8+ subsets. This distribution is different from human blood, where the majority of MAIT cells are CD4–CD8+ [13]. Potentially explaining this difference, DN MAITs from the peripheral blood have been suggested to be functionally more mature, expressing increased levels of cytokine receptors yet have also been shown to have a generally reduced proinflammatory cytokine response to bacterial (Escherichia coli) stimulation [22]. The increased proportions of DN MAIT cells in these BAL samples might therefore represent an increased activation or maturation state caused by the chronic inflammatory environment of the CF lung. Another important point is that the frequency of blood MAIT cells increases from childhood into adulthood, peaking at about 30 years of age before declining [23, 24]. As this study analyzed BAL samples from young children, it is possible the results for MAIT cells from older people with CF might be different, not only due to changes as the disease progresses, but also due to age-related increases in MAIT cells.
We found an average 18% of BAL T cells from children with CF aged 2–6 years of age were MAIT cells (Fig. 1), which is considerably higher than recently reported in control children aged 0–8 years of age, where MAIT cells comprised an average of only 1% of CD3+ BAL T cells [25]. In this latter study, the proportion of MAIT cells in BAL increased to an average of 3% in age and sex matched children with community acquired pneumonia [25]. Interestingly, MAIT cell populations in the lungs of mice dramatically expand in infection models, leading to the suggestion that laboratory mice have markedly lower levels of MAIT cells than humans due to their lack of exposure to infections [13, 17, 26]. The high levels of MAIT cells observed in the lungs of children with CF in this study is therefore likely to be at least partially the effect of their chronic and commonly multiple pathogenic infections.
This study also examined
As for other unconventional T cells, there are several
Finally, we evaluated CD56+ NKT-like cells. NKT cells have multiple anti-microbial effector functions and are capable of cytotoxic T cell activation [35, 36]. As such they potentially provide an important innate defensive role in combatting infection in CF, especially against viruses. Consistent with this, it is interesting that we observed a significant increase in the frequencies of NKT-like cells in samples from virally infected children with CF. The anti-viral activity of NKT cells is well established, so this increase in NKT-like cell frequency supports an important role for these cells in fighting viral infection in the lungs of children with CF.
While the status of NKT cells in CF is largely unknown, mutations in the
Cftr gene significantly increased invariant NKT (iNKT) cell accumulation
in the lungs of mice in a model of ceramide-induced autoimmune disease, with the
presence of these cells associated with increased chronic inflammation in CF
lungs [37]. While only limited data are available on the frequency of NKT cells
in BAL from healthy children, comparing our results with those available suggests
the frequency of NKT-like cells in children with CF might be increased. For
example, Hodge et al. [38] reported that NKT-like cells constituted a
median average of
An important limitation of this study was that, as for
This study provides the first identification and evaluation of unconventional T cells in the BAL of children with CF. This provides valuable insights into the potential roles these cells might play during the critical early formative years of this disease. Significantly, this study identified changes in the frequency of one T cell subtype that was linked to viral infections, indicating potentially important interactions between these cells and individual pathogens. Improving our understanding of these T cell subsets during the early stages of the disease process is essential for determining whether the role of these cells in CF is only to combat infection, or if they also contribute to the pathogenic process, and thereby provide new therapeutic targets for immunomodulatory approaches aimed at regulating inflammation and improving disease prognosis.
CF, Cystic Fibrosis; MAIT, Mucosal Associated Invariant T; NKT, Natural Killer T; BAL, Bronchoalveolar Lavage; FACS, Fluorescence Activated Cell Sorting.
PS designed the research study. RM and GAT performed the research. CMH, AJC and DGP provided help and advice on flow cytometric analysis of immune cells. RC and SR provided samples, help and advice relating to cystic fibrosis. RM, GAT and PS analyzed the data. RM, GAT and PS wrote the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript.
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Human Research Ethics Committee of The Royal Children’s Hospital Melbourne (protocol code HREC #25054).
Not applicable.
This research received no external funding. PS was supported by a DHB Foundation Fellowship. The institute where this work was performed was supported by the Victorian Government’s Operational Infrastructure Support Program.
The authors declare no conflict of interest.
