†These authors contributed equally.
Academic Editor: Pietro Gentile
Background: The defect of intervertebral disc (IVD) after discectomy may impair tissue healing and predispose patients to subsequent IVD degeneration, which is thought to be an important cause of recurrence. Cell-based approaches for the treatment of IVD degeneration have shown promise in preclinical studies. However, most of these therapies have not been approved for clinical use due to the risks of abnormal differentiation and microorganism contamination of the culture-expanded cells. Selective cell retention (SCR) technology is non-cultivation technique, which can avoid those preambles in cell expansion. In this study, we used a commercially available BONE GROWTH PROMOTER device (BGP, FUWOSI, Chongqing, China) to concentrate mesenchymal stromal cells (MSCs) from bone marrow aspirate (BMA) through SCR technology. Methods: A small incision was made on the L2/3, L3/4 and L4/5 discs of goats and part of nucleus pulposus (NP) was removed to construct IVD defect model. The L2/3 disc was subjected to discectomy only (DO group), the L3/4 disc was implanted with enriched BMA-matrix (CE group), and the L4/5 disc was implanted cultured autologous bone marrow MSCs matrix (CC group). And the intact L1/2 disc served as a non-injured control (NC group). The animals were followed up for 24 weeks after operation. Spine imaging was analysis performed at 4 and 24 weeks. Histology, immunohistochemistry, gene expression and biomechanical analysis were performed to investigate the IVD morphology, content and mechanical properties at 24 weeks. Results: The CE and CC groups showed a significantly smaller reduction in the disc height and T2-weighted signal intensity, and a better spinal segmental stability than DO group. Histological analysis demonstrated that CE and CC groups maintained a relatively well-preserved structure compared to the DO group. Furthermore, real-time PCR and immunohistochemistry demonstrated that aggrecan and type II collagen were up-regulated in CE and CC groups compared to DO group. Conclusions: The strategy of MSCs enrichment combined with gelatin sponge by SCR technology provides a rapid, simple, and effective method for cell concentration and cell-carrier combination. This reparative strategy can be used in clinical treatment of IVD defect after discectomy. Clinical Trial Registration: NCT03002207.
Intervertebral disc (IVD) degeneration (IVDD) is a common disease that can lead to low back pain, nerve compression and disability. Lumbar disc herniation (LDH) with radiculopathy is one of the most common IVDD-linked clinical diseases, which causes huge socioeconomic burden in the world. The treatment of severe LDH often requires surgical approach [1]. Discectomy remains the classic surgical procedure for treating LDH, which consists of removal of the herniated part of nucleus pulposus (NP) to relieve radicular pain. However, despite alleviating pain and improving function, 20%–25% of patients experience long-term unsatisfactory outcomes [2, 3]. The main reason is that the IVD defect after discectomy failed to spontaneous repair, which can result in progressive IVDD, a loss of disc height and even LDH recurrence. Decreased disc height can lead to reduced range of motion.
Several attempts have been made to seal IVD defects by sutures or rigid buttress devices. Bailey et al. [4] used an FDA-approved X-close device to suture open annulus fibrosus (AF) defect after discectomy. In addition to sutures there are currently commercial annular closure devices such as Barricaid implant and Inclose Mesh [5, 6], which can block off the IVD defect by forming a mechanical barrier. However, these repair techniques focus on closing the wound of defect without functional restoration of the IVD and some scholars found the additional AF repair had no significant superiority [4, 7]. Repair methods ideally need to provide both functional restoration and induce IVD regeneration. This makes biologically active tissue scaffold methods attractive options [8]. Therefore, a biological repairing method for IVD defect is needed.
Advances in cell-based therapy and tissue engineering have led to significant progress in the field of regenerative medicine for IVD regeneration. Various cell type and source, including IVD-derived cells and multipotent cells, have been proven to delay the progressive degenerative process [9, 10, 11]. Mesenchymal stromal cells (MSCs) are a convenient cell source with translational advantages [12]. Several reports have described the application of MSCs, including bone marrow-derived MSCs (BM-MSCs), adipose-derived MSCs (ASCs) and umbilical cord-derived MSCs (UC-MSCs), to repair and regenerate the damaged IVD in preclinical and clinical studies [13, 14, 15]. In recent years, many completed and ongoing clinical trials testing the efficacy of cell-based therapies for regeneration IVD [16, 17, 18, 19, 20, 21]. In clinical trials, Yoshikawa et al. [22] reported that using cultured autologous BMSCs combined with gelatin sponge repairing the degenerated IVD could significantly reduce the pain symptoms and increase the IVD water content. However, most of the preclinical and clinical studies used culture-expanded cells for transplantation, with the risk of differentiate abnormality and complex steps which is time consuming and difficult for clinical application. Bone marrow aspirate (BMA) is considered an important source of MSCs in both clinical and experimental studies [23]. Unfortunately, the MSCs represent a very small fraction of about 0.001–0.01% of nucleated cell count of the BMA [24, 25, 26], leading to a limited therapeutic effect. Selective cell retention (SCR) technology could concentrate target cells (including stem and progenitor cells) and effective components from BMA into a carrier material to facilitate the rapid attachment of nucleated cells, which holds great potential for cell preparation.
In terms of IVDD model, previous studies have established in small and large animal models, including rats, rabbits, sheep [27, 28], goat [29], mini-pigs [30] and canine [31, 32], which was induced mechanically, enzymatically or surgically by puncture or incision of the AF with or without nucleotomy, or by injection of biochemical agents for extracellular matrix degraded. Recently, larger sized AF defect or IVD defect model after annulotomy with or without nucleotomy was established and used to study the repair of IVD. To the authors knowledge discectomy-associated defects are more common clinically and need to be repaired. Therefore, it is necessary to construct a suitable animal model where discectomy has been performed to mimic the conditions in human IVDs after discectomy.
The gelatin sponge was widely used in spine surgery, with the advantage of high histocompatibility, high plasticity and absorbability. The gelatin sponge could be used safely as cell carrier for disc regeneration therapy and AF defect repair [22, 33]. In this study, we performed a preclinical study that gelatin sponge combine with autologous cultured BMSCs or enriched BMA were transplanted into NP defect, followed by suturing and sealing AF to repair IVD defect after discectomy in a goat model. We hypothesized that this treatment could repair the IVD defect and retard disc degeneration. Our secondary aim was to compare the repair efficacy between BMA concentrate and cultured BMSCs.
A total of 15
goats (female, 24 months, 48.3
 Fig. 1.
Fig. 1.Schematic illustration of the study design of in vivo repair IVD defect after discectomy at goat model.
Five goats were anesthetized by intramuscular injection of atropine (0.01 mL/kg)
and intravenous injection of 3% pentobarbital sodium (1 mL/kg).
The
BMA was
aspirated from iliac crest of the goat
using a 16 G bone marrow puncture needle, which was rinsed
with 1000 U/mL heparin
to prevent coagulation (Fig. 2A). Two puncture holes were made at one side iliac
crest, and 10 mL BMA was
aspirated from each hole [37]. For
preparation of the enriched
BMA-matrix grafts, two pieces of
gelatin sponge (60 mm
 Fig. 2.
Fig. 2.Surgical approach. (A) The BMA was aspirated from the ilium crest of goat. (B) Exposure of the IVD. (C) Construction of IVD defect model after discectomy (arrow). (D) Graft implantation. (E) Sutured annulus fibrosus. (F) All animals recovered from the procedure without complication and retained full lumbar spine function.
The enriched BMA-matrix was taken out and fixed in 4% paraformaldehyde for 12 h. After gradient ethanol dehydration and critical point drying, the sample and gelatin sponge was cut into 1 mm slices and pasted on the carrier table with conductive tape, respectively. The surfaces were sprayed with gold and observed by scanning electron microscope (SEM, Quanta 200, Fei, Hillsboro, OR, USA).
To assess the enrichment effect, the
volume of BMA was measured before and after enrichment process, and the samples
were taken for flow cytometry
(FACS Aria III, BD Corporation, Franklin
Lakes, NJ, USA) to detect the number of nucleated cells and the number of the
target cells with negative expression of CD34 and CD45 and positive expression of
CD90. The number and concentration of cells in the BMA before and after BMA
enrichment using BGP were assessed by flow cytometry. The steps are as follows:
(1) Take three samples of BMA before and after enrichment, isotype and
corresponding isotype control, and put 300
Furthermore,
100
For isolation of BMSCs, 20 mL of BMA was collected from
the iliac crest and mixed with 2 mL heparin
(1000 U/mL). Bone marrow mononuclear cells
were isolated by density-gradient
centrifugation using
lymphoprep [38].
The
mononuclear cells were cultured for 4 days in a 100 cm
To facilitate analysis of the tracking of transplanted
BMSCs in IVD, the BMSCs were infected with
lentivirus-GFP
(catalog number: GV492,
Shanghai Genechem Co., Ltd, China), a lentivirus vector expressing the
green fluorescent protein
(GFP) gene. BMSCs were cultured and washed
with PBS, and infected with lentivirus-GFP
at optimal multiple of
infection (MOI = 1:10). After 3 days, GFP
expression was observed using a fluorescence microscope (Leica, DMI4000, Wetzlar,
Germany).
100
The goats were general anesthetized by intramuscular injection of atropine (0.01 mL/kg) and xylazine hydrochloride (0.02 mL/kg). All animals receive perioperative intravenous antibiotic (ceftiofur sodium, 25 mg/kg). Once anaesthetized, the goat is placed on the operating table in the lateral position, and the operation area was shaved and disinfected routinely with iodophor. A longitudinal 10 cm incision parallel and 1 cm anterior to the transverse processes is made according to the desired discs level using a left lateral retroperitoneal approach [39]. The subcutaneous layers were dissected using monopolar cautery. With the thoracolumbar fascia divided, the retroperitoneal space can be entered. The psoas and quadratus lumborum muscles are retracted posterolaterally, by the assistant, using a retractor, and blunt separation of the muscles attached to the disc, further exposing the target discs (Fig. 2B). A small incision was made at the left-anterior surfaces of the L2/3, L3/4 and L4/5 discs with a sharp knife, and approximately 50 mg NP tissues was removed from a single disc with small NP forceps to construct IVD defect model (Fig. 2C). The L2/3 disc was subjected to discectomy only (DO group), the L3/4 disc was implanted with enriched BMA-matrix (CE group), and the disc L4/5 was implanted cultured BMSCs-matrix (CC group) (Fig. 2D). And the intact L1/2 disc served as a non-injured control (NC group). The AF incision was closed using an absorbable suture (Fig. 2E). Then hemostasis is achieved, and muscle, fascia and skin were separately sutured layer by layer. Strict sterile precautions are maintained at all times. The goat was awakened by benzoxazole hydrochloride injection (0.01 mL/kg IM). After operation, all goats received intramuscular injection of flunixin meglumine (0.2 mL/kg) for analgesia for three days. Dressing change regularly until healing of the incision site was complete. The animals were observed at least once daily for general health and appearance by the veterinary staff and carefully monitored for signs of pain, discomfort, gait or posture. The animals were followed up for 24 weeks after the operation.
Lumbar X-ray imaging of goats was performed
under general anesthesia at
pre-operation
and 4, 24 weeks after operation. The change in IVD height was evaluated by the
disc height index
(DHI) [40, 41]. Disc height and the
adjacent vertebral body heights were measured on the midline and 25% of the
disc’s width from the midline on either side.
The DHI was expressed as
the mean of the 3 measurements from midline to the boundary of the central 50%
of disc width divided by the mean of the 2 adjacent vertebral body heights.
Changes in DHI were expressed as %DHI and were standardized to the preoperative
DHI as follows: %DHI = ((postoperative DHI/preoperative DHI)
MR scans were obtained in T2-weighted images in all groups at pre-operation and
4, 24 weeks after operation in the sagittal and axial planes using a 1.5 T
scanner (GE, Boston, MA, USA). T2-weighted
sections in the sagittal plane were obtained under the following settings: fast
spin echo sequence with time to repetition of 2500 seconds and time to echo of 85
seconds; 384 (h)
Goats were sacrificed by intravenous injection of overdosed pentobarbital at 24
weeks after surgery, and the ten lumbar spines were harvested. Biomechanical
testing was performed on the spinal motion segment with ligamentous attachments
and muscles removed.
The
denture base resin embedding sample was vertically installed on the biomechanical
machine (MTS 858 Bionix II, Maumee, IN, USA) for biomechanical testing.
Every vertebra of L1-5 was marked by markers in the same
position (the specific position of every vertebra), and the motion of the markers
was recorded by 3D Dynamic Capture System Camera (NDI Corporation, Canada) from
different direction (Fig. 3A). The fresh spinal specimens were kept wet during
the process of testing. Preconditioning was repeated three times (30
seconds/time) with 1 N preloading. The multidirectional bending moments (flexion
and extension (
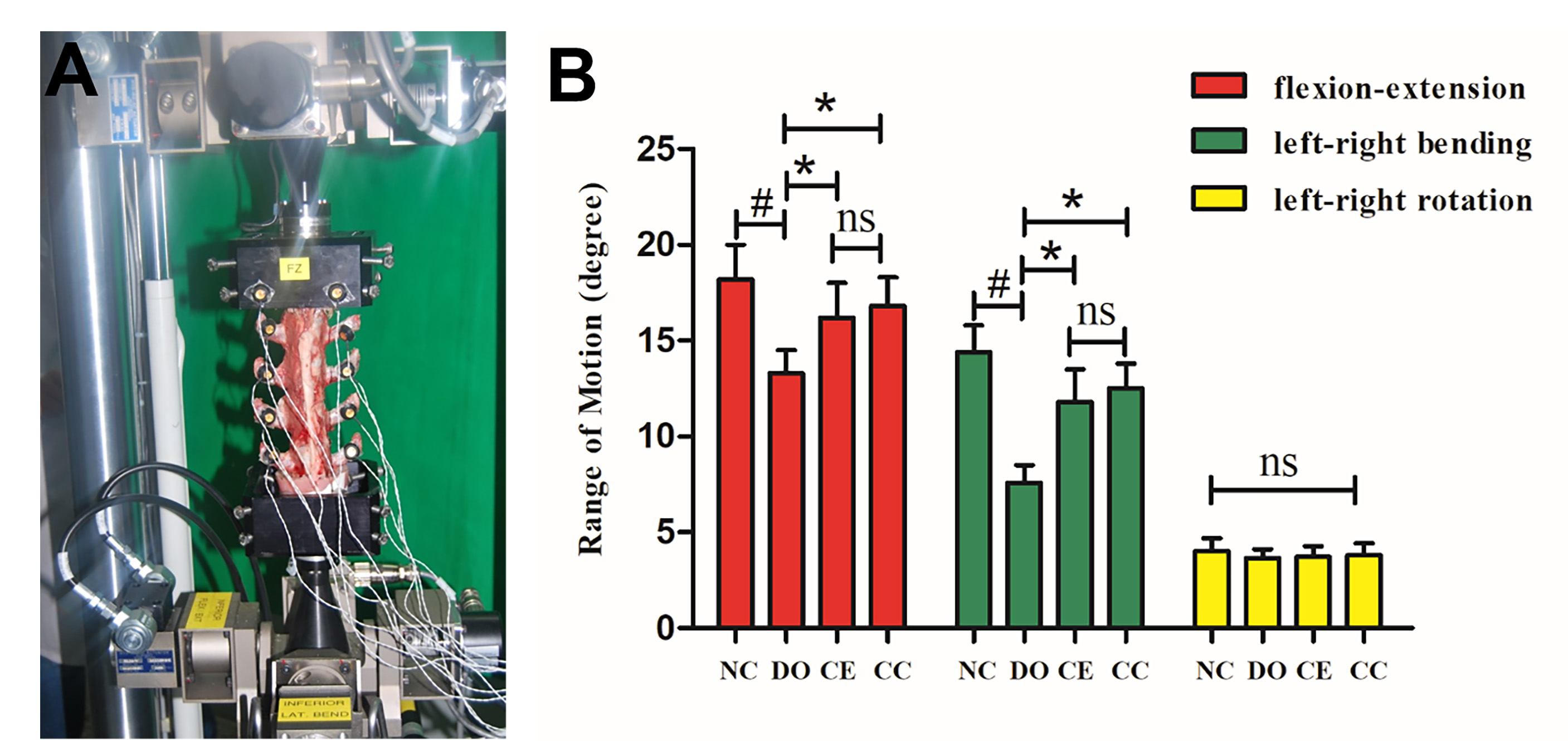 Fig. 3.
Fig. 3.Biomechanical analysis. (A) Spine specimens were tested in
biomechanical tester. (B) The ROM of flexion-extension and left-right bending in
the CE and CC groups were larger than that of the DO group at 24 weeks after
operation. However, there was no significant difference in ROM between the CE and
CC groups. ROM of left-right rotation was not statistically different in the four
groups. All the data represent mean
After the biomechanical testing, the L1/2, L2/3, L3/4, and L4/5 discs of nine goats selected randomly were cut transversally at the center of the NP for macroscopic evaluation. A previously published histological grading system was applied to assess disc degeneration [43, 44]. Two histologists who were blinded to the samples performed evaluation of these sections, with their intra-observer error being minimum. One half of every disc was used for histological studies and identify the tracking transplanted cells in the NP tissues, and the other half was stored in liquid nitrogen (–196 ℃) for realtime PCR analysis of gene expression.
The remaining discs of one goat were isolated consisting of the total IVD and
0.5 cm of adjacent vertebral body and fixed in 4% paraformaldehyde for 5 days.
Then the samples were decalcified in 15%
EDTA
for 6 months and subsequently embedded in
paraffin. To visualize intact disc, the
coronal sections of entire disc were cut
using microtome (Leitzl516, Leica, Wetzlar, Germany) and stained with
hematoxylin and eosin
(H&E).
To visualize histologic
changes in NP tissue, NP tissues were
fixed in
4% paraformaldehyde for 24 h and processed
for paraffin embedding and sectioning into
transversal sections (6
Immunohistochemistry was performed for Collagen II (II-II6B3, DSHB, Iowa City,
IA, USA) and Aggrecan (ABT1373, Millipore, Burlington, AL, USA) analysis.
Rehydrated sections were serially incubated
at room temperature in proteinase K (Sigma, Hesse, Germany) for 5 min, 3%
hydrogen peroxide for 10 min, blocking reagent for 5 min (BD5001, Bioworld
Technology, Nanjing, China), and incubated with Collagen II (4
To
identify the existence of implanted cells in the NP tissues,
the NP tissues were embedded in
optimal
cutting temperature (OCT) compound (Fisher
Scientific, Pittsburgh, PA, USA) and sectioned at 6
Total RNA was extracted from the NP using
the TRIspin
[45].
After the cDNA had been obtained by reverse
transcription using
AMV
reverse transcriptase (Takara, Beijing, China), relative gene expressions of
Collagen I, Collagen II, Aggrecan and
SOX-9 were determined by
Real-time
PCR (RT-PCR). GAPDH were applied as
normalization control. The obtained cDNA was amplified by qPCR using SYBR Premix
Ex Taq
| Gene | Forward | Reverse |
| GAPDH | 5′-GGCGTGAACCACGAGAAGTA-3′ | 5′-GGCGTGGACAGTGGTCATAA-3′ |
| COL1A2 | 5′-CAAGGGAGATGCTGGTCCTG-3′ | 5′-TTCACCCTTAGCACCCACAG-3′ |
| COL2A1 | 5′-CAACCCTGGAACTGACGGAA-3′ | 5′-ATACCAGGCTCACCCGTTTG-3′ |
| ACAN | 5′-GCAAGCTCCAGAAGCAAGTG-3′ | 5′-TCCACCAATGTCGTATCCACC-3′ |
| SOX-9 | 5′-CACAAGAAGGACCACCCGGA-3′ | 5′-CACAAGAAGGACCACCCGGA-3′ |
The statistical analyses were performed
using SPSS version 24.0 software (IBM, Armonk, NY, USA).
Data was assessed for normality using the
Shapiro Wilks test. The radiograph measurements, biomechanical analyses, and
RT-PCR analyses data were all fitted with normal distribution, and statistical
analysis was performed using analysis of variance (ANOVA) and the Fisher’s least
significant difference post-hoc test. The MRI Pfirrmann grading and histological
grading data did not conform to a normal distribution, and Kruskal Wallis H test
was used for statistical analysis. Statistical significance was set at p
BMA was aspirated from 10 iliac crests of 5 goats for enrichment. After the BMA
was enriched by the BGP, the adhesion multiple of nucleated cells and target
cells was 6.40
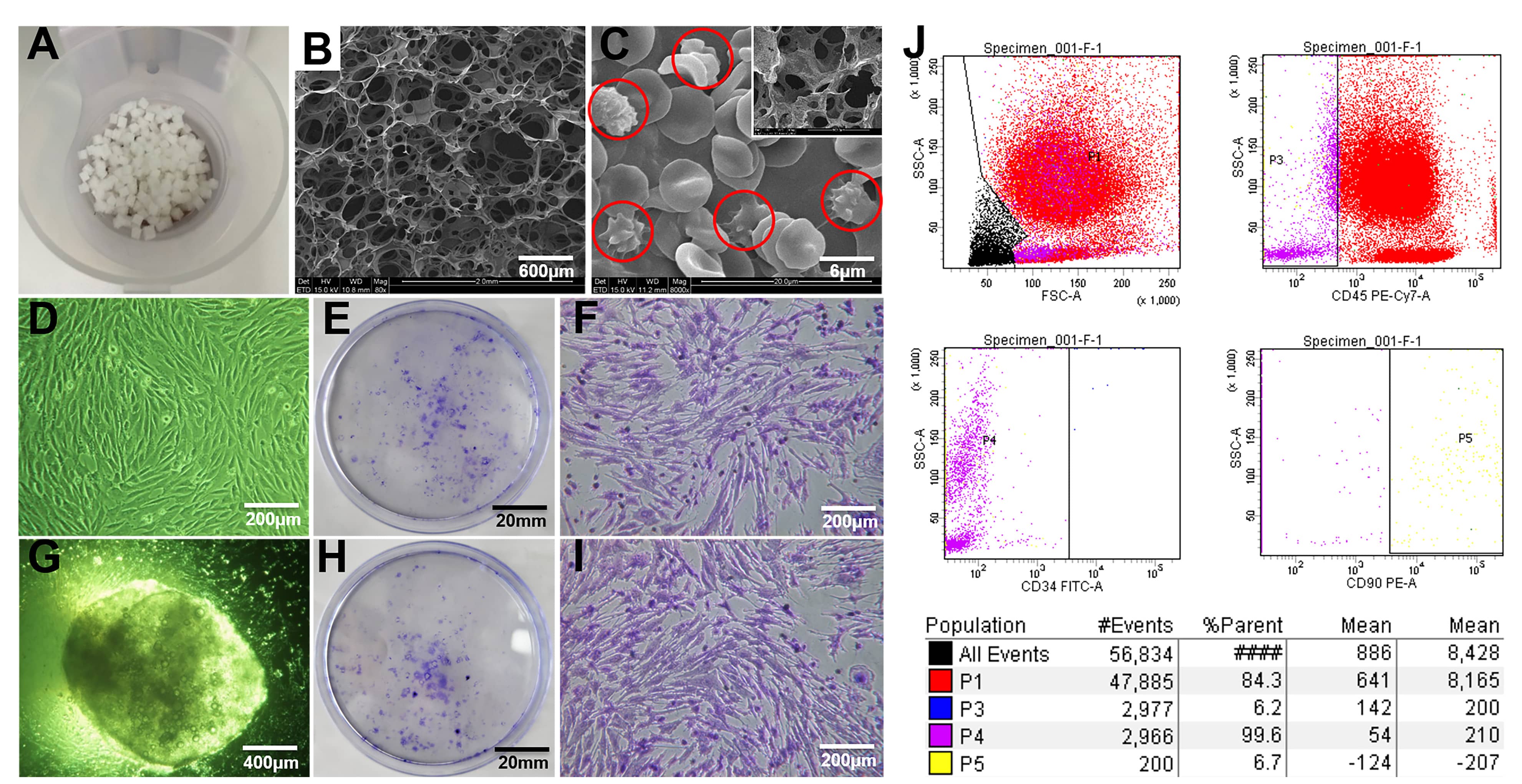 Fig. 4.
Fig. 4.Bone marrow enrichment. (A) Gelatin sponge cubes (5 mm
BMSCs differentiation assay demonstrated the autologous BMSCs possess the capability of osteogenic, chondrogenic and adipogenic differentiation. (Supplementary Fig. 1A–C) Meanwhile, flow cytometry analysis showed the CD90 was positively expressed, and CD34 and CD45 were negatively expressed on the surface of the target cells, which was consistent with the BMSCs surface markers, indicating that BMSCs was successfully isolated and identified (Supplementary Fig. 1D).
SEM images showed that the gelatin sponge was macroporous (Fig. 4B), and there were multiple round cells adhered to the porous wall of the enriched BMA matrix (Fig. 4C). After the BMA and the enriched BMA matrix were cultured for 11 days, crystal violet staining showed that there were a large number of colonies forming unit-fibroblast (CFU-F) at the bottom of the culture dish, and fusiform or polygonal cells were adherent to the wall. No bacterial growth was observed in all sample cultures (Fig. 4D,G). The results of flow cytometry detection and crystal violet staining showed that the MSCs in BMA could be effectively retained in gelatin sponge cubes by the BGP, and the cells viability were good.
The mean weight of the NP removed from the
DO, CE
and CC group was
50.18
Radiographic
analysis at 4 weeks after operation showed that the disc spaces in DO, CE and CC
groups narrowed significantly, and the DO group continued to
decrease at 24 weeks, while rate of
decrease in the CE and CC groups slowed down after 4 weeks. With a mean DHI for
NC group at beginning of the study expressed as 100%,
the mean DHI of the DO, CE and CC group was
89.3
 Fig. 5.
Fig. 5.Radiographic and MRI assessment. (A,C) Representative
radiographic and MRI images at 0 week before, 4, and 24 weeks after operation in
goats. (B,D) The change of disc height index (DHI) and relative grey index (RGI)
at 4 and 24 weeks after operation. All the data represent mean
According to the MRI T2-weighted results,
the mean signal intensity of NC group had barely changed throughout the study
(Fig. 5C,D). Signal
intensity in the DO group decreased significantly shortly and continued to
develop after the operation, while those of transplantation groups were
significantly delayed. The change of MRI T2-weighted signal intensity was
evaluated by RGI. For the DO, CE and CC groups the mean RGI were
0.56
The Pfirrmann classification analysis showed
that no obvious disc degeneration in all groups before the
operation (Fig. 5E). There was no
significant difference between the Pfirrmann classification of CE group and CC
group (p = 1.00), which was significantly lower than that of DO group
(p = 0.021, 0.01), but all of
them were significantly higher compared
with the NC group
at
24 weeks after operation (p
We further compared the biomechanical effect from the three operated discs (DO,
CE, and CC groups) and intact disc (NC group) at 24 weeks after
operation.
As
shown in Fig. 3, the mean ROM of
flexion-extension and left-right bending in the DO, CE and CC groups were
13.3
Gross observation of the lumbar spine showed that the fibrous connective tissue at the incision of AF in the CE and CC groups was rich, tough, and well repaired. However, the DO group had less connective tissue at the incision of the AF, and it was depressed and poorly repaired. The condition of NP and AF could be observed from the macroscopic view of freshly dissected IVD samples (Fig. 6A). In the DO group, in DO group, the NP and part of the AF tissue were black and yellow with severe degeneration. The CE and CC groups showed mild changes in NP tissue compared to the intact NC group. There are no NP protrusions in the discs of the CE and CC groups. Nine goats were assessed, showing that the histological grades of the discs in the CE and CC groups were mainly 2–3 grades, and that in DO group were mainly 4–5 grades. There was no significant difference in the grade of the discs between the CE and CC groups (p = 1.00), but both were lower than those of the DO group (p = 0.043, 0.024). Discs in NC group maintained grade 0 throughout the study (Fig. 6C).
 Fig. 6.
Fig. 6.Histological grading for assessment of IVD repair. (A)
Representative macroscopic view of discs in four groups at 24 weeks. (B) The
histological grading system for disc degeneration that focuses on morphological
change in the inner annulus fibrosus structure was used in this study. (C)
Histological grades were used to evaluate discs in the four groups at 24 weeks.
There was no significant difference in histological grade between CE and CC
groups (p = 1.0), but they were significantly lower than DO groups
(p
In this study, GFP-labeled BMSCs combined with gelatin sponge matrix were transplanted into the IVD in the CC group. The discs of three goats were used to detect the tracking of transplanted BMSCs by fluorescence microscopy. As shown in Supplementary Fig. 3D, the fluorescence microscopy confirmed the tracking of the GFP-labeled BMSCs in the discs of the CC group.
No GFP-positive cells were observed in the discs of the DO or CE group (Supplementary Fig. 3C). The results showed that the GFP-labeled BMSCs was successfully implanted into the IVD and grew in the CC group.
H&E, Safranin O and Sirius red staining revealed that compared with the DO group, the structure of NP tissue in CE and the CC group was more complete, the cell proliferation was more active, and the extracellular matrix was more abundant (Fig. 7A). HE staining of the mid-coronal sections showed that disc height was well maintained in NC, CE and CC groups, whereas disc narrowing was obvious in DO group (Fig. 7B). The immunohistochemical staining of NP tissue in transversal sections indicated that the staining intensity of NP in the CE and CC groups was strong positive in aggrecan and type II collagen, while the staining intensity in the DO group was weak or intermediate positive (Fig. 7C).
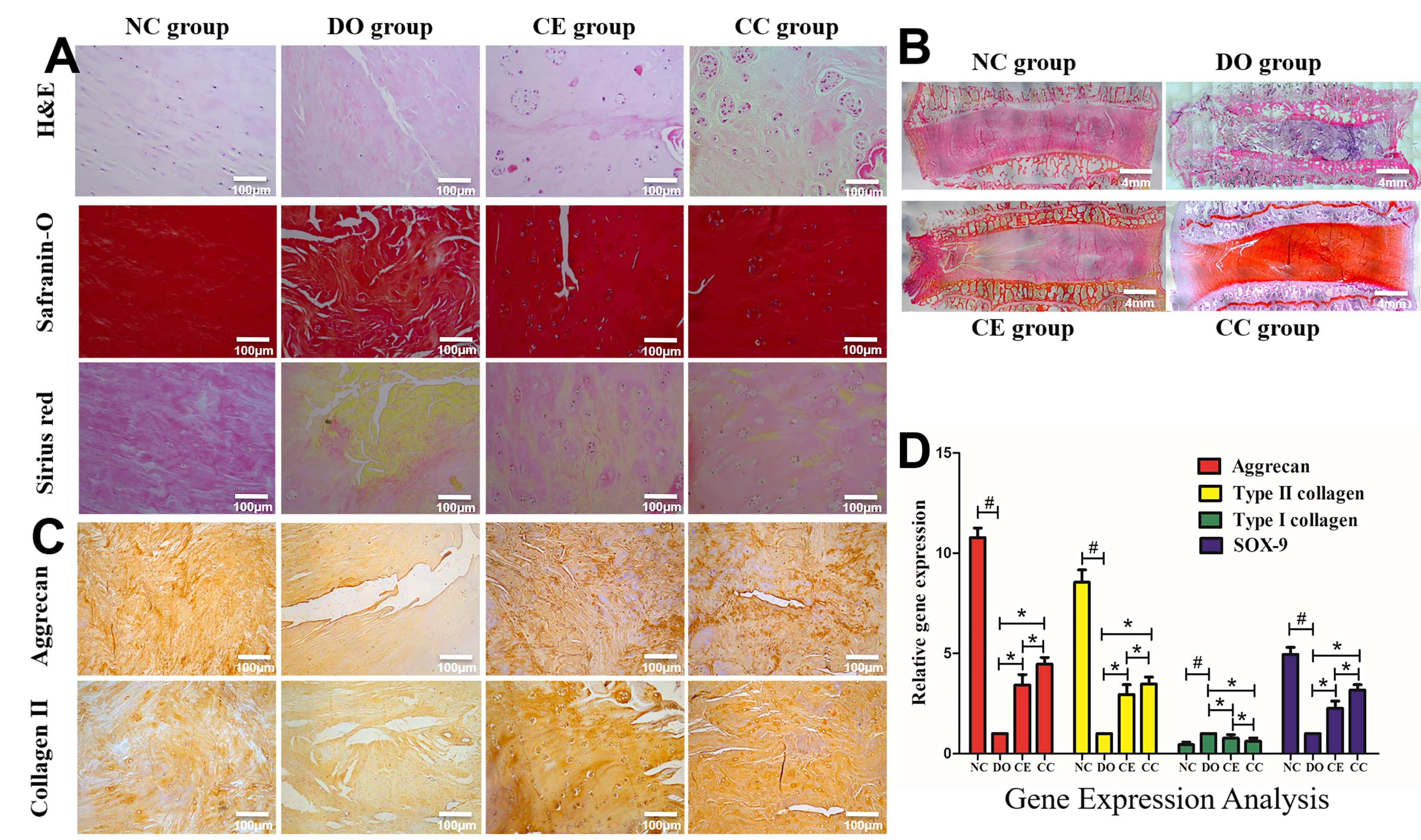 Fig. 7.
Fig. 7.Histological and real-time PCR analysis. (A) H&E, Safranin O
and Sirius red staining of NP in the transversal plane. (B) the intact IVD of
mid-coronal sections were stained with HE staining. (C) The immunohistochemical
staining for aggrecan and type II collagen. (D) Real-time PCR analysis. The
results showed that aggrecan, type II collagen, and SOX-9 mRNA expression
markedly increased in discs from the CE and CC group compared with the DO groups.
Bar plots are shown as means
For quantitative analysis the NP matrix,
RT-PCR was used to measure the levels of aggrecan, type II collagen, type I
collagen, and SOX-9 in the NC, DO, CE, and CC groups.
As shown in Fig. 7D, the levels of
aggrecan, type II collagen, and SOX-9 mRNAs
were decreased in the DO, CE, and CC groups as compared with the NC group, while
the type I collagen expression was significantly increased in the DO, CE, and CC
groups (p
IVD are avascular structures and their lack of oxygen and nutrient supply presents a substantial challenge for self-directed tissue repair [47]. IVD defects produced by discectomy may result in progressive degeneration, recurrence of disc herniation, and chronic pain. In the present study, we developed a biological approach to repair IVD defects following lumbar discectomy in goat models by implanting gelatin sponges seeded with autologous bone-marrow derived cells into the defect site before suturing and sealing. This preclinical proof-of-concept study demonstrated the feasibility and effectiveness of biomaterial and cell-based therapies for the repair of IVD defects.
Hydrogel materials are widely used in tissue repair due to the characteristics of high hydrophilicity, biocompatibility, and adjustable three-dimensional network mimicking extracellular matrix. The injectable hydrogel is of particular interest from a clinical point of view, as they can be injected as a liquid together with stem cells directly into the disc site in a minimally invasive way followed by gelation [44, 48, 49, 50, 51]. However, the hydrogel materials are generally not porous matrix structures, which were not suitable for cell enrichment in this study. The gelatin sponge is a three-dimensional porous structure, which is conducive to the smooth passage of BMA and the retention of target cells on the gelatin sponge. But, they are very soft when exposed to water and cannot provide mechanical support by themselves. However, through this experiment, the transplantation of enriched BMA-matrix or cultured BMSCs-matrix to repair the disc defect after discectomy can delay the degeneration of IVD and maintain a good height and mobility of IVD. The hydrogel has fluid properties, and the stem cell impregnated hydrogels is easily injected into the degenerated NP through a fine needle, with little effect on the AF, which could restore disc height, thus providing immediate pain relief, whilst delivery of MSCs provides gradual regeneration. The hydrogel appears to be more suitable for the repair of early to midstages of disc degeneration, with intact AF [49]. The main role of the gelatin sponge in this experiment is to retain the target cells during the enrichment process, and act as a carrier to reduce the leakage of the cells. However, the mechanical strength of gelatin sponge is insufficient. In the future, gelatin sponge with higher mechanical strength is developed for intervertebral disc repair, which can provide better space for the growth and differentiation of transplanted cells, and the effect may be better.
Cell-based therapy is a promising reparative strategy for IVD regeneration. Given the insufficient stem cell or IVD cell number and sources, cells culture-expanded method was widely adopted for cell transplantation. However, cell culture in vitro is controversy in clinical application, which may increase the cost of the patients and the risk of differentiate abnormality and microorganism contamination [52, 53]. SCR is a non-cultivation technique, which can help avoid some ethical and physico-chemical influences of in vitro cell expansion. Muschler et al. [25, 34] first reported a SCR method that used a composite matrix preparation loaded with bone-marrow derived cells for lumbar fusion surgery, which indicated that cell-enriched matrix grafts were capable of delivering a mean of 2.3-fold more cells and approximately 5.6-fold more progenitor cells than matrix mixed directly with bone marrow. In the present study, we showed that the adhesion number of multiple nucleated cells and the target cells to gelatin sponges was approximately 6.4-fold and 4.2-fold, respectively, when compared to BMA (Fig. 4J). Additionally, MSCs can be concentrated and rapidly combined with scaffolds using SCR techniques. SEM images showed the enriched cells could adhere to the porous walls of gelatin sponges. Plastic-adherence is an important feature of MSCs [54]. After enriched BMA-matrices were cultured for 11 days, a large number of CFU-F grew adherently, which indirectly reflects the number of progenitor cells [37, 55, 56]. Moreover, MSC surface markers were detected by flow cytometry [55], which showed that BGP was more conducive to enrichment ability. These results indicated that BGP effectively retained MSCs within the gelatin sponges and maintained their stemness activity. Furthermore, the enrichment process totaled 5 min and this simplistic and timesaving procedure was appropriate for critical operation times. Our results indicated that SCR technology was a valid approach for concentrating cell numbers whilst cell activity could be preserved by using cell-carrier combinations.
Cell enrichment technology combined with implant materials to concentrate bone marrow has been used in spinal fusion [25, 34] and bone defect [35] treatments. In an open label pilot study, 26 patients with chronic back pain due to degenerative disc disease (median age 40 years; range 18–61) received autologous bone marrow concentrated intra-disc injections and all subjects presented a substantial reduction in pain [57]. However, few previous researches have attempted to use enriched BMA to repair surgical IVD defects. Our results, imaging analysis, and histological assessment showed that both enriched MSCs and cultured BMSCs combined with gelatin sponge cubes have the potential to repair IVD defects, maintain IVD height for 24 weeks, and may delayed IVD degeneration. Although SCR techniques do not provide the same number of MSCs as cell culture expansion techniques, they still produced efficacious therapeutic effects. We speculate that cytokines/chemokines within the bone marrow play a contributing role to this outcome. Compared with in vitro culture of BMSCs, MSCs enrichment by BGP is facile, faster, and more convenient for clinical point-of-use. Our study showed the feasibility and safety of enriched MSCs combined with gelatin sponge cubes for repairing IVD defects in large animal models, demonstrating the strong potential of this method for clinical use in patients with IVD defects.
MSCs have the capacity for multidirectional differentiation. A two-way communication has been observed wherein MSCs interact with nucleus pulposus cells (NPCs) [58, 59]. The paracrine environmental signalling from NPCs instructs MSCs to differentiate toward NPC-like cell lineages [60, 61, 62]. Concurrently, MSC signalling can influence the activity of NPCs [11, 63]. However, when MSCs leak from the IVD defect or injection site due to high intra-disc pressure, considerable ossification can occur [64, 65]. This suggests the significance of developing appropriate cell carriers and effective approaches to prevent cell leakage and the associated osteophyte formation before MSCs-based therapies can be applied in clinical settings. In this study, we sought to circumvent cell leakage issues by transplanting gelatin sponges seeded with autologous MSCs, and this was followed by AF suture to close the breach. Our results showed that there was no obvious indications of osteophyte or heterotopic ossification formation. Potential reasons for preventing osteophyte formation may be: (i) enhanced cell retention in situ by using clinically used gelatin sponges as cell-carriers, providing enhanced cell adhesion; (ii) physical closure of AF defects by suturing to prevent leakage of the transplanted cell-carrier; (iii) closure of disc defects reduced immuno-inflammatory responses otherwise invoked by the exposed disc; (iv) a reduced foreign body reaction and inflammatory cell interferences through use of autologous MSCs transplantation. Therefore, our strategy developed for IVD repair demonstrated safe and effective applicability of cell-carrier therapies for intra-disc delivery. Furthermore, some preclinical and clinical studies demonstrated that MSCs injection therapy is an effective treatment to retard disc degeneration and relieve pain. Despite tremendous research efforts, MSCs therapy to repair the IVD still has controversy and considerable challenges. The hostility of the microenvironment in IVDD niches may limits the viability and functionality of the transplanted MSCs. And the safety and efficacy of cell-based therapy is still controversial [66, 67].
In order to evaluate the effectiveness of the developed strategies for IVD tissue repair and regeneration, it is important to use appropriate animal models. Recent advancements in biological repair strategies have shown successful preclinical outcomes in vitro and in vivo [27, 28, 29, 30, 31, 68, 69]. Long et al. [28] established a biopsy-type AF defect in an ovine cervical model to evaluate the in vivo response of a composite AF repair strategy. Shu et al. [70] group utilized an ovine IVDD model and intradiscal heterologous MSCs to determine therapeutic efficacy at early, late acute and chronic stages of IVDD. MSCs were intradiscal administered into the NP from the contralateral AF away from the annular lesion site. Results showed that the administered MSCs can interact with resident disc cells to initiate and coordinate the repair processes and prevented the extension of the AF defect and promoted the healing of outer AF defect. However, there are a limited number of studies that have established animal models that mimic the clinical post-surgery conditions in humans with LDH. Until now, only two studies have reported the use of injectable defect-filing hydrogels [71] and modified hyaluronic acid [72] to repair post-discectomy IVD defects in sheep models. In this context, we successfully established a discectomy-associated defect model in goats, which simulated the clinical circumstances. Indeed, in artiodactyls, the anatomical structure, size, biomechanical properties, and biochemical components of goat IVDs are relatively close to human IVDs, which make the animal better model to recapitulate human IVD pathophysiology [29, 73]. Although goats are quadrupeds and do not share the upright characteristics of the human spine, the musculature of quadruped provides high muscle tension such that the pressure within lumbar IVDs is similar to the human intradiscal pressure [72, 74]. This experimental was testing in a quadraped animal and human IVD loading is different. The animal model of upright walking needs further research.
Mechanical integrity is important for IVD function. IVD defects can cause loading disorders, resulting in progressive degeneration [75]. Therefore, repairing the IVD defect may restore mechanical compliance. In addition, IVDs load bearing is complex and dynamic. Dynamic biomechanical testing should be conducted to evaluate IVD repair. This study tested biomechanical functions of whole lumbar kinematics after repairing IVD defect in vivo, including the range of motion in flexion, extension, bending and rotation tests. Mechanical testing results demonstrated that the ROM of flexion-extension and left–right bending in defect repairing group were better than that in discectomy alone group. However, there were no statistical differences calculated in ROM of rotation due to the long distance from the rotation center to the edge of AF defect, and this is a limitation of utilizing quadruped animal models such as goats, which rarely exert spinal rotation motions. Thorpe et al. [49] reported that a thermally triggered hydrogel was injected into bovine NP tissue explants in a statically cultured model. Mechanical analysis showed that MSC and hydrogel injected NP tissue explants displayed similar mechanical properties to control group with no significant difference following 6 weeks in culture. Our study indirectly assessed the effect of treatment measures on IVD biomechanical function by detecting the ROM in the motion segments of spine, which is not as accurate as direct mechanical testing of the disc, which is a limitation of the study.
The anatomy and mechanical property of the 4 level IVDs may have some differences. It would have been better to systematically rotate the groups among the different level IVDs. The lumbar IVDs in each spine should be randomized to receive different treatment strategies to reduce the impact of differences in anatomical and mechanical properties at different levels. An additional limitation of our study was that the cells were harvested from normal healthy goats, and the goats themselves were in a healthy and active state. In clinical settings, patients have a variety of compromising health conditions that can induce IVD degenerative states. An example setting wherein our approach is likely to be unsuitable is in elderly patients with severe degeneration of AF, resulting in heightened difficulty of suture and defect closure. Furthermore, MSCs fate after implantation was not a focus of our investigation. Lineage tracing and phenotypic evaluation are required in future investigations. This experimental only proved that the concentration of the target cells on the gelatin sponge increased after the BMA passed through the BGP. We currently have no direct evidence that cells adhere more readily to gelatin sponge scaffolds. This is a limitation of our study and requires further investigation.
This preclinical proof-of-concept study shows the feasibility and effectiveness of transplanting gelatin sponge seeded with autologous enriched MSCs into the disc defect after discectomy, and then suturing and sealing AF defect. To the authors knowledge, this in vivo study is the first to demonstrate the feasibility and effectiveness of IVD defect repair in goat lumbar model using enriched MSCs seeded gelatin sponge combined with AF suture. The present results indicate this novel reparative strategy could delay the progress of IVD degeneration and restore the extracellular matrix of IVD, imperative for maintaining disc height and biomechanical properties. Compared with culturing BMSCs, the enrichment of MSCs with SCR technology at the point of operation, is a simpler, safer, and more time- and cost-economical approach. In light of the promising evidence from this investigation, the authors have commenced a human clinical trial using the same reparative strategy in patients with LDH by endoscopic discectomy (ClinicalTrials.gov NCT03002207). Overall, the combinatory approach of SCR technology with autologous MSC adhesion to gelatin sponges facilitated enhanced repair of IVD defects, restored biomechanical function, and minimized risk of cell-leakage and ossification. The results from this study showing a potential avenue toward clinical translation for IVD defect repair and prevention of disc degeneration in post-discectomy settings.
IVD, intervertebral disc; SCR, selective cell retention; MSCs, mesenchymal stromal cells; BMA, bone marrow aspirate; NP, nucleus pulposus; IVDD, intervertebral disc degeneration; LDH, lumbar disc herniation; AF, annulus fibrosus; BM-MSCs, bone marrow-derived MSCs; ASCs, adipose-derived MSCs; UC-MSCs, umbilical cord-derived MSCs; MRI, magnetic resonance imaging; BGP, BONE GROWTH PROMOTER; PBS, phosphate buffered saline; GFP, green fluorescent protein; DHI, disc height index; EDTA, ethylenediamine tetraacetic acid; H&E, hematoxylin and eosin; DAB, diamino-benzidine; OCT, optimal cutting temperature; CFU-F, colonies forming unit-fibroblast; PCR, polymerase chain reaction; RT-PCR, real-time PCR; ROM, range of motion; SEM, scanning electron microscope; NPCs, nucleus pulposus cells.
QY, LD and HX contributed equally to this work, participated in the design of the study, performed enrichment testing, cell culture and animal experiments, imaging detection, biomechanical testing and analysis, (immuno)histochemistry detection, microscopy analyses, acquisition, analysis and interpretation of results, and drafted the manuscript. BX conceptualized, designed the study and provided financial supports. QY, LD, HX, KZ, QL, HZ and YL helped with implement of animal surgery. KZ carried out the gene detection. QY, LD, HX, HZ and BX helped with statistical analysis. XM and BX provided technical, administrative and material support. BX supervised the study and helped to revise the manuscript. All authors read and approved the final manuscript.
Animal experiments were approved by the Animal Experiments Ethical Committee of Institute of Radiation Medicine Chinese Academy of Medical Sciences, Tianjin, China (approval number: IRM-DWLL-2020142).
We want to thank all research personnel for their contributory works. Thanks to Yeda Wan, Yi Cao and Nan Wang of the Radiology Department of Tianjin Hospital for their help in image detection and analysis.
This study was supported by the National Natural Science Foundation of China (82072491, 31900967, 31670983) and Natural Science Foundation of Tianjin (19JCQNJC09300).
The authors declare no conflict of interest.
