†These authors contributed equally.
Academic Editor: Graham Pawelec
Small cell lung cancer (SCLC) subtype classification, based on high-level expression of key transcriptional regulators; ASCL1 (SCLC-A), NEUROD1 (SCLC-N), POU2F3 (SCLC-P), and YAP1 (SCLC-Y), has recently been proposed. YAP1 (and POU2F3) has attracted attention as an important factor for non-neuroendocrine (non-NE) phenotypic subtyping of SCLC. However, subsequent studies reported that YAP1 expression alone cannot define a single group in primary SCLC, which makes it difficult to understand what SCLC-Y is by focusing only on SCLC. In this review, we concluded that YAP1 is an essential anti-neuroendocrine factor in both SCLC and non-small cell lung cancer (NSCLC) based on previous studies, including our own analysis of the cell lines and primary tumors of SCLC and NSCLC. The classification of SCLC-Y is a concept mainly established from the analysis of cell lines, and SCLC-Y cell lines correspond to “variant type” SCLC cell lines. Primary SCLC and large cell neuroendocrine carcinoma (LCNEC) are typically heterogeneous tumors composed mostly of NE-type cells, but they contain a small number of non-NE-type cells. Importantly, individual cells with NE features exhibit YAP1 loss, whereas the non-NE-type cells exhibit YAP1 expression. Although rare in primary SCLC, some cases of primary LCNEC have many YAP1-positive cells, which is correlated with chemotherapy resistance. YAP1 staining may be useful in the exclusion diagnosis of SCLC or in the selection of treatment for LCNEC.
The four major histological subtypes of lung cancer have been adenocarcinoma, squamous cell carcinoma, neuroendocrine tumor (NET), and large cell carcinoma since the publication of the 4th edition of the World Health Organization (WHO) classification of tumors of the lungs, pleura, thymus and heart [1]. Large cell neuroendocrine carcinoma (LCNEC), previously a subtype of large cell carcinoma, was incorporated into NET. In NET, high-grade neuroendocrine cancer (HGNEC), which includes small cell lung cancer (SCLC) and LCENC, is differentiated from atypical carcinoid tumor (intermediate-grade) and typical carcinoid tumor (low-grade), and diffuse idiopathic pulmonary neuroendocrine cell hyperplasia (DIPNECH) has been positioned as a pre-invasive lesion.
In recent years, molecular biological analysis of HGNEC has progressed rapidly, and it has become clear that SCLC and LCNEC can be divided into several subtypes according to gene mutation and expression patterns. YAP1 attracted attention as an important factor for non-neuroendocrine (non-NE) phenotypic subtyping of SCLC; however, several subsequent studies also suggested that YAP1 expression alone cannot define a single group in primary SCLC. At present, YAP1-positive SCLC remains unclear. We explain the meaning of YAP1 expression in SCLC and LCNEC from a pathological point of view based on recent literature and our own studies. Previous major studies on the classification of SCLC and LCNEC correlated with YAP1 expression are shown in Table 1 (Ref. [2, 3, 4, 5, 6, 7, 8, 9, 10]).
| SCLC Cell lines classification based on YAP1 expression levels | |||
| Authors | YAP1-high | YAP1-low | PMID |
| Ito, et al. [2] | YAP1-high SCLC cell lines: Adherent type, non-NE type, Resistant to cisplatin | YAP1-low SCLC cell lines: Floating type, NE-type, Sensitive to cisplatin | 27418196 |
| McColl, et al. [3] | YAP1-high SCLC cell lines: INSM1-low | YAP1-low SCLC cell lines: INSM1-high | 29088741 |
| Owonikoko, et al. [4] | YAP1-high SCLC: High expression of interferon- |
- | 33248321 |
| Immunohistochemical analysis of YAP1 in HGNEC cases | |||
| Authors | Results | PMID | |
| Ito, et al. [2] | In primary SCLC cases, YAP1 was negative in 40 of 41 (98%). | 27418196 | |
| In primary LCNEC cases, YAP1 was negative in 18 of 30 (60%). | |||
| In primary NSCLC cases except LCNEC cases, YAP1 was positive in 183 of 189 (97%). | |||
| YAP1 expression in HGNEC correlated with chemo-resistance. | |||
| Baine, et al. [5] | In primary SCLC cases, there was no predominantly YAP1-positive case (0/159: 0%). YAP1 was expressed at low levels, primarily in combined SCLC, and was not exclusive of other (ASCL1, NEUROD1, POU2F3) subtypes. | 30926931 | |
| Pearsall, et al. [6] | Although there was no predominantly YAP1-positive case among 39 CTC-derived SCLC tumors, more than 0.2% cancer cells were positive for YAP1 in 10 of 39, and more than 1% were positive for YAP1 in 2 of them. | 32721553 | |
| SCLC Cell lines classification possibly correlated with YAP1 expression levels | |||
| Authors | YAP1-high | YAP1-low | PMID |
| Carney, et al. [7], Gazdar, et al. [8] | Variant type: Adherent type, non-NE type, Resistant to radiation | Classic type: Floating type, NE-type | 2985257 |
| 2985258 | |||
| Calbo et al. [9] | Mesenchymal phenotype: Adherent type, non-NE type (mouse SCLC) | Neuroendocrine type: Floating type, NE-type (mouse SCLC) | 21316603 |
| LCNEC classification related to YAP1 expression levels | |||
| Authors | YAP1-high | YAP1-low | PMID |
| George et al. [10] | Type II: RB1 mutations, with ASCL1-low/DLL1-low/NOTCH-high/YAP1-high/immune related genes-high | Type I: STK11/KEAP1 mutations, with ASCL1-high/DLL1-high/NOTCH-low | 29535388 |
The Hippo pathway, a signaling pathways, plays an important role in controlling
the size of organs through the coordinated regulation of cell fate [11]. Two
serine/threonine kinases (MST1/2 and LATS1/2) that form the core components of
the Hippo pathway inactivate the transcriptional coactivator YAP1 by
phosphorylation and shut down cell proliferation signaling [12, 13].
MST1/2—LATS1/2—YAP1 signaling is activated as a result of the cellular
response to physical changes in the extracellular environment (cell adhesion,
extracellular matrix hardness) [14]. Several signaling pathways (PI3K,
TGF
YAP1 and the Hippo pathway are involved in the regulation of alveolar epithelial differentiation. Core hippo pathway molecules, such as MOB1A/1B or MST1/2, were previously reported to regulate the differentiation of alveolar epithelium through the regulation of YAP1 expression [20, 21]. In 2021, Little et al. [22] reported that TTF-1 promotes cell differentiation into alveolar type 1 or alveolar type 2 by binding chromatin in a cell-type-specific manner, and that YAP1/TAZ direct TTF-1 to its alveolar type 1-specific sites and prevent its binding to alveolar type 2-specific sites.
The strong expression of YAP1 is frequently observed in tumors such as hepatocellular carcinoma, ovarian cancer, and NSCLC [23, 24, 25, 26, 27]. The overexpression of YAP1 overcomes cell contact inhibition, induces epithelial–mesenchymal transition, and promotes cancer cell proliferation and invasion [23, 24, 28]. YAP1 is considered a key determinant of the resistance of tumors to platinum, including NSCLC, oral cancer, cervical cancer, thyroid cancer, and ovarian cancer [29, 30, 31]. Cheng et al. [32] reported that the downregulation of YAP1 by verteporfin (a YAP1 inhibitor) sensitized cells to DNA-damaging agents.
In addition to its oncogenic role, YAP1 functions as a key regulator of differentiation in lung tumors. Yijun et al. [33] reported that YAP1 inhibits the squamous differentiation of LKB1-deficient lung adenocarcinomas. Overexpression of nuclear YAP1 was reported to correlate with a poor prognosis in NSCLC [24]; however, nuclear YAP1 expression is also frequently found in non-mucinous adenocarcinoma in situ (non-mucinous AIS, a well differentiated subtype) and reactive alveolar epitheliums [34]. Ito et al. [34] reported that co-expression of CADM1 (tumor suppressor) and hippo pathway core kinases at the cell membrane, which is observed in non-mucinous AIS and normal alveolar epithelium, is important for suppressing the oncogenic role of nuclear YAP1.
In 2016, we first reported the importance of the loss of YAP1 in NE differentiation of lung tumors [2]. We demonstrated that SCLC cell lines can be classified into two groups: YAP1-low, NE marker-high, and floating type, and YAP1-high, NE marker-low, and adherent type. However, among primary tumors, YAP1 expression was rare in SCLC cases (2%), but common in NSCLC cases excluding LCNEC (97%). The typical pattern of YAP1 and ASCL1 expression in primary SCLC cases is shown in Fig. 1; most cells are YAP1-negative and ASLC1-positive. YAP1 was negative in carcinoid tumors in this report [2] and we concluded that the loss of YAP1 defines NE differentiation of lung tumors. Moreover, the VMRC-LCD cell line, established as an adenocarcinoma cell line, was confirmed to be an LCNEC cell line with loss of YAP1 expression in this study.
 Fig. 1.
Fig. 1.Typical immunohistochemical expression patterns of YAP1 and ASCL1 of SCLC. Most of the cells are (A) YAP1-negative and (B) ASCL1-positive.
In 2019, Rudin et al. [35] reported that SCLC can be subtyped into 4 groups by four molecules; ASCL1, NEUROD1, POU2F3, and YAP1, based on genomic data of primary SCLC samples and cell lines. ASCL1, NEUROD1, and POU2F3 are transcriptional factors. ASCL1 and NEUROD1 are implicated in the neuroendocrine differentiation (NE differentiation) of cells [36, 37, 38, 39, 40, 41]; therefore, ASCL1-positive SCLC (SCLC-A) and NEUROD1-positive SCLC (SCLC-N) strongly express NE markers. POU2F3 is an important master regulator for chemosensory cells [42, 43, 44, 45, 46], which are slightly present in the tongue, respiratory epithelium, trachea, urethra, and digestive organs. POU2F3-positive SCLC (SCLC-P) is considered a non-NE phenotype.
On the other hand, the triple-negative subtype (ASCI1-/NEUROD1-/POU2F3-) had higher expression of YAP1 and was classified as YAP1-positive SCLC (SCLC-Y). SCLC-Y is also considered a non-NE phenotype. SCLC-Y was reported to be characteristically sensitive to CDK4/6 inhibitors, HSP90 inhibitors, and Aurora kinase inhibitors, but insensitive to BCL2 inhibitors [3, 47, 48].
The classification of SCLC by Rudin et al. [35] is a perspective opinion model and requires further validation. In the subsequent reports, SCLC-A, N, and P were identified in primary SCLC cases, but not SCLC-Y. When Baine et al. [5] immunostained primary SCLC cases (n = 159) with these four markers, SCLC was divided into mainly two groups: one in which both or either of ASCL1 and NEUROD1 were positive, and another in which both ASCL1 and NEUROD1 were negative. The POU2F3-positive SCLC cases were included in the latter, but YAP1-positive SCLC cases were absent. Pearsall et al. [6] performed immunohistochemical analysis using 39 patients’ serum-derived circulating tumor cell (CTC)-derived xenograft (CDX: CTC-derived explants) models of SCLC. However, there were no cases in which YAP1 was predominantly positive. Thus, what is SCLC-Y?
Close observation of the figure of “Molecular subtypes of SCLC defined by expression of key transcription regulators” (https://www.nature.com/articles/s41568-019-0133-9/figures/2) by Rudin et al. [35] reveals that in the SCLC-Y group, there are only two cases of primary SCLC and they are mostly composed of SCLC cell lines.
Cluster analysis of 51 SCLC cell lines in Cancer Cell Line Encyclopedia (CCLE) (https://sites.broadinstitute.org/ccle/) based on gene expression data of ASCL1, NEUROD1, POU2F3, YAP1, and INSM1 is shown in Fig. 2, and at least 18% (9/51) of SCLC cell lines highly express the YAP1 gene.
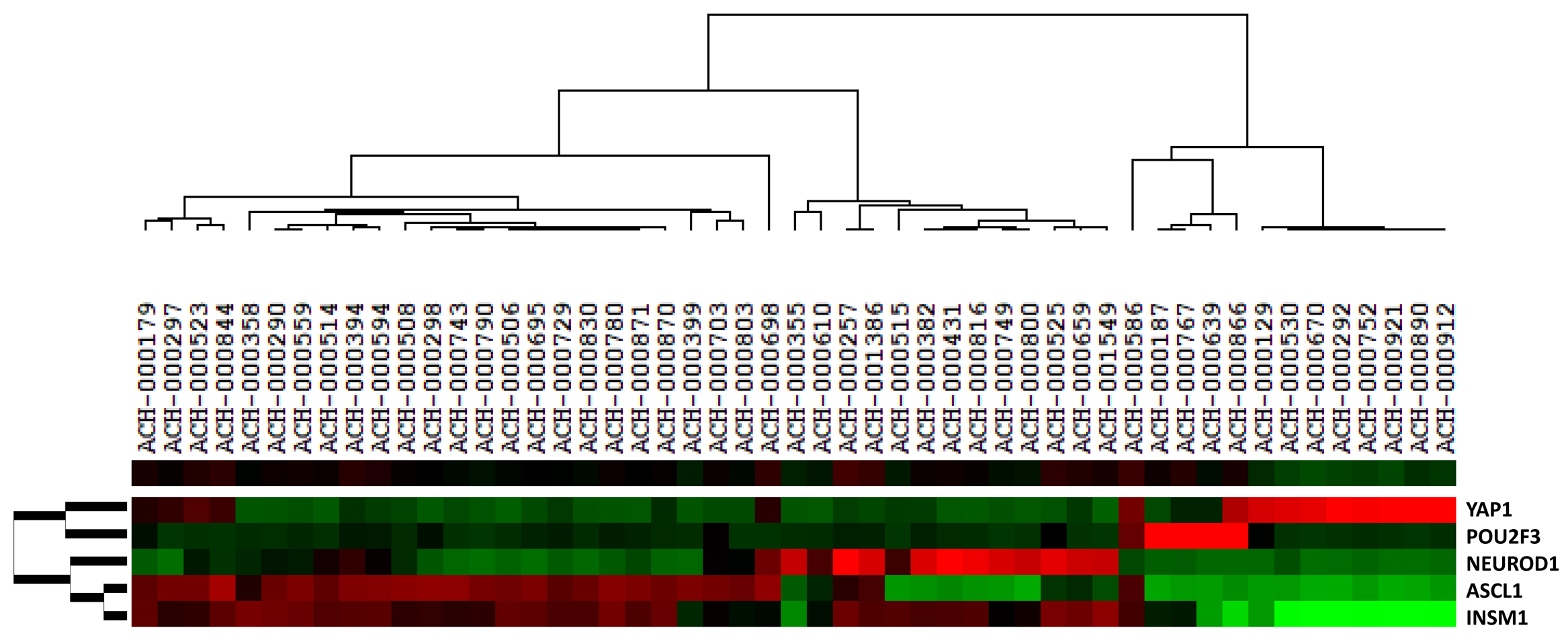 Fig. 2.
Fig. 2.Cluster analysis of 51 SCLC cell lines in Cancer Cell Line Encyclopedia (CCLE) (https://sites.broadinstitute.org/ccle/) based on gene expression levels of YAP1, INSM1, POU2F3, ASCL1 and NEUROD1. We used the cluster program (http://rana.lbl.gov/EisenSoftware.htm, accessed March 19, 2008) for a cluster analysis of the gene expression data of cell lines. In brief, we carried out average linkage hierarchical clustering of the 51 SCLC cell lines using the mean centering and normalization of genes. We then displayed the results obtained with the aid of TreeView software (Eisen Lab in Stanford University, Stanford, CA, USA) (http://rana.lbl.gov/EisenSoftware.htm, accessed March 21, 2008). The image used a color code to represent relative expression levels. Red represents expression levels greater than the mean for a given gene across all samples. Green represents expression levels less than the mean across samples. Fig. 2 shows that at least 18% (9/51) SCLC cell lines highly express the YAP1 gene, and that none of them show high-expressions of INSM1 gene, while high expressions of INSM1 gene can be found in the cells highly expressing ASCL1, NEUROD1, or POU2F3 genes.
As such, it can be concluded that the classification of SCLC-Y is a concept mainly established from the analysis of cell lines and should not be applied to primary SCLC cases.
In 1985, Carney, Gazdar et al. [7, 8] reported that there are two types of SCLC cell lines: “classic type”, which is floating with the NE phenotype, and “variant type”, which is adherent with a non-NE phenotype. Of note, “variant type” SCLC cell lines frequently originated from post-therapy tumors that recurred and were more resistant to radiation than the “classic type”.
The expression levels of YAP1, INSM1, POU2F3, NEUROD1, and ASCL1 of 14 SCLC cell lines (11 floating type and 3 adherent type) and 3 NSCLC cell lines (3 adherent type) in our lab are shown in Fig. 3. YAP1-low SCLC cell lines are the floating type and NE marker-positive, thus they correspond to “classic type”. On the other hand, as YAP1-high SCLC cell lines are the adherent type and NE marker-negative, they correspond to “variant type” (Fig. 3).
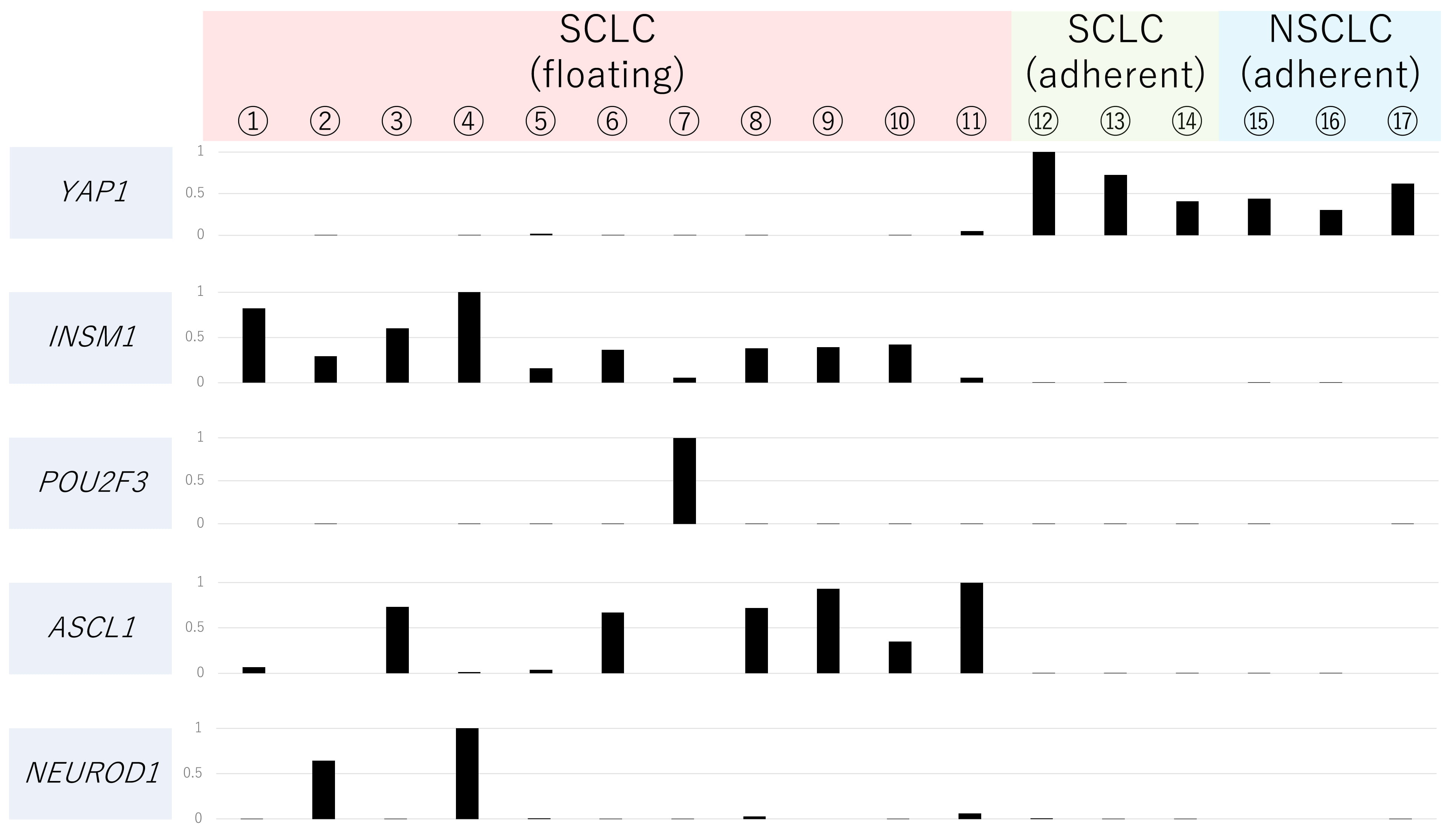 Fig. 3.
Fig. 3.Relative gene expression levels of YAP1, INSM1, POU2F3, ASCL1 and NEUROD1 of 17 cell lines. Gene expression analysis of the 17 cell lines (①–⑪: floating type SCLC cell lines, ⑫–⑭: adherent type SCLC cell lines, ⑮–⑰: adherent type NSCLC cell lines) was carried out per mRNA-Seq using an Illumina GAIIx sequencer (Illumina, San Diego, CA, USA). Details were shown in our previous report (PMID: 27418196). Names of cell lines are as follows: ① H69 ② N417 ③ H146 ④ Lu135 ⑤ Lu139 ⑥ 510A ⑦ 526 ⑧ 2081 ⑨ H889 ⑩ Lu130 ⑪ H446 ⑫ SBC3 ⑬ SBC5 ⑭ LCMA ⑮ Lu99 ⑯ H460 ⑰ Lu65A.
There are two possibilities for the establishment of SCLC-Y cell lines, as shown in Fig. 4.
 Fig. 4.
Fig. 4.Two possibilities for the establishment of “SCLC-Y” cell line. (A) SCLC contains minor component of YAP1 positive cells. “SCLC-Y” cell line might have been established from these minor component of YAP1-positive cells. (B) “SCLC-Y” cell line may have been established from NSCLC mimicking SCLC.
Histologically, SCLC looks uniform at first glance, but it is actually composed of a heterogeneous cell population.
In 2011, Calbo et al. [9] established two types of cell lines from the
same SCLC tumor from mice: an adhesive cell line with the EMT phenotype
(
In 2020, Pearsall et al. [6] reported that many of the CTC-derived tumors contained a small number of YAP1-positive and NE marker-negative cells as minor components. Furthermore, Pearsall et al. [6] established two types of cell lines, a YAP1-positive and NE marker-negative cell line, and a YAP1-negative and NE marker-positive cell line, from partially YAP1-positive CTC-derived tumors.
Ireland et al. [49] reported that MYC activates NOTCH signaling and transiently reprograms ASCL1-positive cells into NEUROD1-positive cells, and further into YAP1-positive and non-NE-type cells.
Based on the above reports, the following can be inferred: SCLC is essentially YAP1-negative and exhibits NE differentiation, but a few cells in the tumor mass are transiently reprogrammed into YAP1-positive and non-NE-type cells. This reprogramming may be promoted through the process of the acquisition of resistance to chemotherapy and radiation therapy. SCLC-Y cell lines may be irreversibly established from these minor components in SCLC (Fig. 4A).
The other possibility is that cell lines derived from NSCLC were misdiagnosed as YAP1-positive SCLC cell lines (Fig. 4B). The pathological diagnosis of SCLC only requires histological morphology, not immunostaining of NE markers. However, it is well known that some NSCLC (basaloid squamous cell carcinoma) histologically mimic SCLC. If we focus only on SCLC, YAP1-positive cases are rare, but if we extend the scope to NSCLC, many cases are YAP1-positive. In our study, 97% of NSCLC cases, excluding LCNEC, were YAP1 positive. As shown in Fig. 3, the characteristics of YAP1-high SCLC cell lines were more similar to those of NSCLC cell lines than to those of YAP1-low SCLC cell lines. Thus, whether SCLC-Y cell lines are NSCLC cell lines cannot be concluded.
Recently, Gay et al. [50, 51] demonstrated that according to the gene
signature analysis of SCLC cases that were triple-negative for ASCL1,
NEUROD1, and POU2F3, the triple-negative subtype uniquely
expressed numerous immune-related genes and was designated as SCLC-inflamed
(SCLC-I). SCLC-I highly expresses genes associated with the EMT phenotype and
immune-related genes such as HLA, IFN activity, and immune checkpoint
genes. Of note, SCLC-I is highly sensitive to immunotherapy compared with the
other three subtypes. Moreover, administration of cisplatin to xenografts derived
from SCLC-A patients results in tissue migration to SCLC-I. This suggests that
resistance to platinum drugs is acquired by switching subtypes. They also
reported that the expression of YAP1 was higher in both SCLC-P and
SCLC-I than in SCLC-A and SCLC-N, although YAP1 expression did not
exclusively define a subtype [51]. Owonikoko et al. [4] reported that
SCLC-Y is associated with the high expression of interferon-
Thus, there is significant phenotypic overlap between SCLC-I and SCLC-Y, and it is possible that we are looking at different aspects of the same tumor. However, as mentioned above, SCLC-Y is a concept established from cell lines and does not necessarily represent the full nature of the primary tumor. Further analysis of the relationship between SCLC-I and SCLC-Y is needed.
In SCLC, inactivating mutations in TP53 and RB1 are almost inevitable. Comprehensive next-generation sequence analysis of LCNEC in recent years revealed that inactivating mutations in RB1 and TP53 are also frequent in LCNEC. According to the genome profile, LCNEC was mainly divided into two groups: “SCLC-type” and “NSCLC-type” or “type I” and “type II”.
In 2016, Rekhtman et al. [52] reported next-generation sequencing analysis of 241 cancer-associated genes (oncogene and tumor suppressor genes) for 45 cases of pure-LCNEC and compared the results with those of lung adenocarcinoma (151 cases), squamous cell carcinoma (36 cases), small cell carcinoma (42 cases), and carcinoid (13 cases). In this report, LCNEC was mainly classified into two types: “SCLC-type” (18 cases) with joint inactivation of RB1 and TP53, and “NSCLC-type” (25 cases) with genetic abnormalities or decreased immunohistochemical expression of STK/KRAS/KEAP1/NFE2L2, which are both characteristic of NSCLC. However, they noted that genetic abnormalities of KEAP1 and NFE2L2 in “SCLC-type” LCNEC were more frequent than in SCLC.
In 2018, George et al. [10] analyzed 75 cases of LCNEC by whole-exome sequencing (WES) and whole-genome sequencing (WGS). TP53, RB1, STK11, KEAP1, ADAMTS12, ADAMTS2, GAS7, and NTM8 were detected in LCNEC with a high mutation rate. TP53-inactivating mutation was detected in 92% of LCNEC cases and mutations in STK11/KEAP1/RB1 were detected in 82%. Mutations in RB1 and STK11/KEAP1 were mutually exclusive, and LCNEC was mainly divided into two groups: LCNEC with STK11/KEAP1 alteration (“type I” LCNEC) exhibiting high expression of NE genes (ASCL1 and DLL1) and low expression of NOTCH signaling-related genes (ASCL1-high/DLL1-high/NOTCH-low), whereas LCNEC with RB1 loss (“type II” LCNEC) exhibited the low expression of NE genes and upregulation of NOTCH signaling-related genes (ASCL1-low/DLL1-low/NOTCH-high).
Recently, Derks et al. [53] reported that overall survival is superior if RB1-positive (RB1 wild type) LCNEC is treated using NSCLC-type chemotherapy (platinum-gemcitabine or -taxanes) instead of SCLC-type chemotherapy (platinum-etoposide), but there was no difference in outcome for RB1-negative (RB1-mutated) LCNEC. The genetic status of RB1 may be an important factor in LCNEC classification and treatment selection. Focusing on RB1 mutations, “type II” in George’s report corresponds to “SCLC-type” in Rehktoman’s report, and “type I” corresponds to “NSCLC-type”, although the expression patterns of NE markers and NOTCHsignaling-related genes in “type I” and “type II” are different from those in typical NSCLC and SCLC, respectively.
George et al. [10] also reported that “type II” LCNEC characteristically highly expresses YAP1 and immune-related genes; therefore, “type II” LCNEC may be the counterpart of YAP1-positive SCLC. However, there are few studies that immunohistochemically examined the expression of YAP1 in LCNEC cases, except for our previous report.
We previously examined the staining patterns of YAP1 and NE markers in 30 LCNEC cases [2]. Of 30 LCNEC cases, 60% (18/30) were YAP1-negative and exhibited a similar pattern to typical SCLC cases, in which most cells were YAP1-negative (as shown in Fig. 5A,B), whereas 40% (12/30) were YAP1-positive LCNEC. YAP1-positive LCNEC exhibited a conspicuous mixture of YAP1-positive and YAP1-negative cells (Fig. 5C,D) or diffusely YAP1-positive pattern (Fig. 5E,F), in which YAP1-positive cells were NE marker-negative or weakly positive (Fig. 5C–F). This suggested that YAP1 staining is useful for detecting cell components with the loss or suppression of NE differentiation in high-grade neuroendocrine tumors. In addition, our previous study revealed that YAP1 positivity can predict resistance to platinum-based chemotherapy in LCNEC cases [2].
 Fig. 5.
Fig. 5.Immunohistochemical expression patterns of YAP1 and ASCL1 of LCNEC, using Serial sections. Top figures: YAP1-negative case, in which most of the cells are (A) YAP1-negative and (B) ASLC-1-positve. Middle figures: YAP1-positive case, which contains (C) conspicuous mixture of YAP1-positive and YAP1-negative cells. (D) YAP1-positive cell components are negative for ASCL1, and YAP1-negative cell components are positive for ASCL1. Bottom figures: YAP1-positive case, which is (E) diffusely positive for YAP1 and (F) weakly positive for ASCL1. Details of immunohistochemistry and evaluation are shown in our previous report (PMID: 27418196).
Several studies reported that YAP1 expression in SCLC cell lines is significantly correlated with resistance to chemotherapy and radiation therapy [2, 54, 55]; however, the mechanism remained unclear. In 2021, Qingzhe et al. [56] demonstrated that YAP1 is not only a predictive marker, but also a key molecule that causes loss of NE differentiation and determines chemotherapy resistance. Thus, YAP1 signaling plays an essential role in the establishment of intratumoral heterogeneity, promoting the fate conversion of SCLC from NE to non-NE tumor cells by inducing REST expression, and YAP1 suppresses GSDME expression in SCLC cells and is associated with acquired resistance to chemotherapy in SCLC [56].
In 2021, Pearson et al. [57] reported the possibility that the role of YAP1 is different between tumors characterized by RB1-inactivating mutations, such as SCLC and retinoblastoma, and solid tumors characterized by wild type RB1 such as adenocarcinoma and squamous cell carcinoma. In the former, YAP1 may act as a tumor suppressor, whereas in the latter, it may act as an oncogene. Focusing only on SCLC, it is difficult to understand the true role of YAP1 or other markers. However, by expanding the field of view to NSCLC or other tumors, such as retinoblastoma, the significance of these molecules may become clearer.
We present the cluster analysis of 188 lung cancer cell lines, including 137 NSCLC cell lines and 51 SCLC cell lines, based on the gene expression of NE markers (CHGA, SYP, NCAM1, INSM1, TTF-1, and POU2F3) and Hippo pathway effectors (YAP1 and WWTR1) (shown in Fig. 6). Cells are divided into two groups: NE type (right side) and non-NE type (left side), and all of the non-NE-type cells exhibit high expression of YAP1 and all of the NE-type cells exhibit low expression of YAP1. On the other hand, high expression of POU2F3 was observed in both NE and non-NE types. The high expression of POU2F3 is not specific to NE tumors (like TTF-1). We can therefore conclude that the loss of YAP1 is the essence of NE differentiation of cancer cell lines regardless of whether they originated from primary SCLC or NSCLC.
 Fig. 6.
Fig. 6.Cluster analysis of lung cancer cell lines including 137 NSCLC and 51 SCLC cell lines in Cancer Cell Line Encyclopedia (CCLE) (https://sites.broadinstitute.org/ccle/) based on gene expression levels of WWTR1, TTF-1, YAP1, INSM1, POU2F3, ASCL1 and NEUROD1. We used the cluster program (http://rana.lbl.gov/EisenSoftware.htm, accessed March 19, 2008) for a cluster analysis of the gene expression data of cell lines, and displayed the results obtained with the aid of TreeView software (http://rana.lbl.gov/EisenSoftware.htm, accessed March 21, 2008) (Eisen Lab in Stanford University, Stanford, CA, USA), as mentioned in Fig. 2 legends. Fig. 6 shows that cell lines can be classified into two groups: NE type (right side) and non-NE type (left side).
We briefly explained the meaning of YAP1 expression in HGNEC based on recent literature and our own studies. Focusing only on SCLC, it is difficult to understand the true meaning of YAP1. Only by expanding the field of view to NSCLC can we see that the loss of YAP1 is the essence of neuroendocrine differentiation of individual cells.
SCLC-Y is a concept mainly established from SCLC cell lines. SCLC is characterized by NE features, but it is a heterogeneous tumor in which most cells exhibit NE features and lack YAP1 expression. However, a small number of cells that have lost the characteristics of NE features exhibit YAP1 expression. As SCLC-Y is thought to have been established from these YAP1-positive cells, it should not be applied to the classification of primary SCLC. Conversely, if we encounter a case diagnosed as pure SCLC with diffuse and strong YAP1 positivity, we should doubt the diagnosis and consider the possibility of NSCLC.
In our study, more than half of the LCNEC cases were YAP1-negative, but the remaining exhibited a mixture of YAP1-positive and NE-marker negative (or weak) cells, and YAP1 expression correlated with chemoresistance. At present where there is no gold-standard chemotherapy for advanced or metastatic LCNEC, immunostaining for YAP1 may help predict susceptibility to platinum-based chemotherapy. For researchers, focusing on this heterogeneity of LCNEC and analyzing YAP1-positive and -negative components may help elucidate the mechanism of NE differentiation or loss of NE features. Furthermore, new therapeutic methods may be developed based on the control of NE differentiation. “Type II” LCNEC with RB1 loss (RB1 mutations) and low expression of NE genes may be the counterpart of SCLC-Y, but there is no conclusive evidence. Further analysis of the relationship between the YAP1 expression pattern in LCNEC and its genetic background and susceptibility to therapies, including immunotherapy, is required.
HK—contributed to the manuscript preparation of Chapters 1, 2, and 3 and to the analysis of gene expression data. RM—contributed to the manuscript preparation of Chapters 4, 5, and 6 and to preparing the images of cancer tissue specimens. TI—contributed to the manuscript preparation of Chapters 7, 8, 9, and 10 and to the extraction of gene expression data of cancer cells in CCLE. DM—interpreted the data, designed the outline of this paper, and provided instructions to HK, RM, and TI.
In this review article, we used pictures of histology of SCLC and LCNEC cases in our previous study. Informed consent was obtained from all patients, and the study was approved by the Institutional Ethics Review Committee (R03-258 in the university of Tsukuba).
Not applicable.
This work was supported by JSPS KAKENHI (Grant Nos. 21K15381 to HK, 20K16165 to RM, 21K07091 to TI, and 19K07441 to DM) and SRF.
The authors declare no conflict of interest.
