†These authors contributed equally.
Academic Editors: Jiang Pi and Deyu Kong
Background: The theory of free radical oxidative stress (ROS) is one of the leading theories of ageing, and antioxidants play an important role in antiaging. Dendrobium has always been popular as a natural antioxidant. Methods: This study investigated the effects of various polarity fractions of ethanol extracts from Dendrobium nobile Lindl. (D. nobile) on D-galactose-induced aging mice. D. nobile stems were extracted by ethanol to form the crude extract (EA), which was sequentially extracted by trichloromethane, ethyl acetate, and n-butanol to yield the secondary extracts, named TCM, EAC, and NBA, respectively. EA, TCM, EAC and NBA were intragastrically administered at a dose of 200 mg/kg b.w. to the aging mice induced by D-galactose for 8 weeks. Results: Compared with the aging control group (AC), D. nobile extracts reduced body weight and lipid accumulation and enhanced endurance and immunity by increasing the index of the spleens and thymus. Meanwhile, D. nobile extracts showed antioxidant properties by lowering Malondialdehyde (MDA) levels and increasing the activities of superoxide dismutase (SOD), catalase (CAT), and Glutathione peroxidase (GSH-Px) in the skin, blood, liver, and brain. Furthermore, D. nobile extracts had a good protective effect on the cell structure and function against lesions of the skin, liver, brain, kidney, and ovary of aging mice. In particular, EA and EAC had better antioxidant and antiaging effects, suggesting that the most effective components were flavonoids and polyphenols that existed in EAC. Both EA and EAC downregulated the expression of aging-related genes such as Il1a, Il1b, Il1rn, Ccl3, Ccl4, Fos and Gck in the brain at the transcriptome level. Both EA and EAC reversed the increase in the Firmicutes/Bacteroidota ratio in aging mice, increased the abundance of probiotic bacteria Lactobacillus and Muribaculum, and decreased the abundance of pathogenic bacteria such as Staphylococcus, Corynebacterium and Brevibacterium. Conclusions: The EA and EAC extracts of D. nobile have better effects on immunity improvement, antioxidation and antiaging by remodelling the intestinal microecosystem and downregulating the expression of age-promoting genes in the brain. D. nobile, especially EA and EAC extracts, could be used as an antiaging drug or functional food.
Aging is a complex and progressive biological process involving a wide range of chronic alterations in organs and tissues, including physiological and pathological changes, and is associated with multiple diseases, such as diabetes, cardiovascular disease, neurological diseases and cancer, and other geriatric syndromes [1, 2, 3, 4]. The free radical oxidative stress (ROS) doctrine has become one of the most convincing theories of aging [5]. The accumulation of ROS under oxidative stress conditions leads to the induction of lipid peroxidation, resulting in advanced lipid oxidation end products (ALEs). Elevated ALEs lead to protein cross-linking and aggregation, resulting in altered cell signalling and function, leading to cellular damage and death [6]. A number of cellular models have been used to study the biochemical changes that occur during ageing, and erythrocytes are superior among them [7, 8].
Antioxidants play an important antiaging role. Antioxidants include endogenous antioxidants, synthetic antioxidants, and antioxidants derived from natural products. Endogenous antioxidants are not enough to meet the needs of the body and need external replenishment. At present, synthetic antioxidants may have toxic side effects. Effective natural antioxidants with lower or no toxicity are favored and have become a research hot spot over the years. Natural product antioxidants are an important source of substances for the body to delay ageing, including a large number of phytochemicals, such as plant fibres, plant polysaccharides, plant sterols, phenols, flavonoids, terpenoids, and natural pigments [9]. Traditional Chinese medicine is an important source of natural products for antioxidants and delayed aging.
Dendrobium is a rare Chinese herbal medicine whose base plants mainly include Dendrobium nobile Lindle (D. nobile), Dendrobium officinale, Dendrobium drumstick, and so on. Most studies have shown that Dendrobium officinale polysaccharide has an antioxidant effect and delays aging by removing free radicals [10, 11]. Another study showed that the antioxidant effect of nonpolysaccharide components in Dendrobium officinale was better than that of polysaccharide components [12]. Ye Tianshi’s “Unengraved Ben Ye Medical Case” mainly records that D. nobile is nourishing Yin and clearing heat, and its nourishing Yin function is reflected in modern medicine as “delaying aging”. D. nobile, rich in flavonoids and polyphenols [13, 14], is antitumour and improves memory loss, the cardiovascular system and gastrointestinal function [15], which has attracted much attention and has become the focus of Dendrobium efficacy pharmacology. Although it has also been proven previously that the alcohol-soluble crude extractives from D. nobile showed antioxidant activity at the in vitro chemical and cell levels [16], we still do not know which polarity composition of the fat-soluble extract has better antioxidation and anti-aging activity.
Furthermore, a variety of herbal drugs delay aging by improving gut microbiota disorders [17, 18, 19]. In this study, we extracted various polarity fractions of the alcoholic extract from D. nobile to screen which one has stronger antioxidant and anti-aging activities in D-galactose-induced ageing mice and to identify thether it works by remodelling the gut microecosystem. This study makes beneficial exploration to reveal the scientific connotation of delaying the aging of D. nobile and provides a new entry point for the development of new antiaging drugs.
D. nobile was purchased from Sichuan Gentle Orchid Agricultural Science and Technology Co., LTD (Ziyang, China). The stems of D. nobile were dried in an oven at 60 °C. The dried stems were ground into powder by a pulveriser, which was then sieved through an 80-mesh sieve. The powdered substance was steeped in petroleum ether for 30 min with ultrasonic oscillation to defeat it. After the solid‒liquid mixture was filtered, the residues were extracted by 80%, 75%, and 70% ethyl alcohol (1:50 g/mL w/v) by ultrasonication for 30 min at 50 °C and filtered. After mixing all of the ethanol extracts and evaporation, the crude extract was produced (labelled EA). The crude EA extract was dissolved in deionized water and then extracted with solvents of increasing polarity, trichloromethane (labelled TCM), ethyl acetate (labelled EAC), and water-saturated n-butanol (labelled NBA). All the extracts were evaporated and stored at –20 °C in the dark.
As previously published, aluminum chloride colorimetry was employed to examine
the total flavonoid concentration in the four D. nobile preparations,
with minor modifications [20]. The D. nobile extract (324
As previously stated, the Folin-Ciocalteu technique was used to determine the
total polyphenol content in the four D. nobile extracts, with minor
modifications [21]. The D. nobile extract (60
Female Kunming mice, 8–10 weeks old and weighing 40–45 g, were procured from Chongqing Medical University’s experimental animal center (Reg. No. SCXK 2018-0003). The mice were kept in a polypropylene cage with an ambient temperature of 25 °C, relative humidity of 40–70%, and a 12-hour dark/12-hour light cycle. For a one-week acclimation period, all mice were given free access to common rodlike meals and water.
Except for the animals in the negative control group (NC), D-galactose was administered subcutaneously at a dose of 125 mg/kg b.w. for creating subacute ageing models. After 10 days, the D-galactose-treated mice were randomly separated into six groups using a completely random design. The NC group and the aging control group (AC) were orally administered solvent every day. The positive control group (PC) was orally administered resveratrol (200 mg/kg b.w.) every day. Mice in the EA, TCM, EAC and NBA groups were orally dosed daily with D. nobile extracts (200 mg/kg b.w) extracted using ethanol, chloroform, ethyl acetate and n-butanol, respectively. All the animals except for the NC group were injected subcutaneously with D-galactose (125 mg/kg b.w.) every day. All treatments lasted for 8 weeks. After collecting blood samples from the tail vein, the mice were euthanized by isoflurane overdose. Tissue samples were obtained for physiological index measurement and histopathological observation.
The wrinkle grade of the back skin of the mice was rated by three observers (single-blind) before and every two weeks after the initial treatment, and the scoring details were adjusted properly based on the literature [22]. Meanwhile, the mice were weighed, and we observed whether their hair was smooth, dry, yellow, or lacking luster and whether they showed flexible or slow behavior and quick or slow response.
The running test was measured by a level treadmill (FT-200, Chengdu Taimeng Software Co. Ltd., Chengdu, China). Running training was given twice on nonconsecutive days (Days 1 and 3), with a fixed speed run of 20 m/min for 20 min. On Day 5, there were two stages to the treadmill exam. The first stage consisted of a five-minute run at a speed of 10 m/min. After 5 min of running, the speed is increased to 20 m/min until exhaustion is reached. The same treadmill level was used for all of the training and testing. Any mouse that resisted probing for 10 seconds and remained on the platform was declared fatigued, and the time was recorded.
After blood collection from the tail vein of mice, they were separated by
centrifugation at 3000 r/min for 10 min. After euthanasia of the mice, the skin,
liver and brain were quickly removed and homogenized in ice-cold 0.9% NaCl
solution, followed by centrifugation at 3000
Tissues taken from the euthanized mice were washed with ice-cold normal saline
before being immobilized for 24 hours in a 4% paraformaldehyde solution. The
tissues were then fixed in molten paraffin and cut into 3
Total RNA was extracted from the brain samples by the TRIzol (Invitrogen, Carlsbad, CA, USA) method, and genomic DNA was removed using DNase I (Takara, Tokyo, Japan). The quality of the RNA samples was determined by a 2100 Bioanalyzer (Agilent Technologies, Inc., Santa Clara, CA, USA) and ND-2000 (Nanodrop Technologies, Wilmington, DE, USA). A TruSeqTM RNA Sample Preparation Kit (Illumina, San Diego, CA, USA) was used to establish the RNA library. After quantification using the TBS380 microfluorometer (Yuanpinghao Biotechnology Co., Beijing, China) with Picogreen (Invitrogen, Carlsbad, CA, USA), high-throughput sequencing was performed on an Illumina HiSeq XTEN/NovaSeq 6000 sequencing platform (Illumina, San Diego, CA, USA), and the sequencing length was PE150. The raw sequencing reads were deposited into the NCBI Sequence Read Archive (SRA) database (Accession Number: PRJNA867020). Bioinformatic analysis of the transcriptome data was carried out using the online Majorbio Cloud Platform (https://www.majorbio.com) [23].
Total microbial genomic DNA was extracted from mouse colon content samples using the E.Z.N.A.® soil DNA Kit (Omega Bio-Tek, Norcross, GA, USA) according to the manufacturer’s instructions. The quality and concentration of DNA were determined by 1.0% agarose gel electrophoresis and a NanoDrop® ND-2000 spectrophotometer (Thermo Fisher Scientific Inc., Wilmington, DE, USA) and kept at –80 ℃ before further use. The hypervariable region V3-V4 of the bacterial 16S rRNA gene was amplified with the primer pairs 338F (5′-ACTCCTACGGGAGGCAGCAG-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′) [24] by an ABI GeneAmp® 9700 PCR thermocycler (ABI, Foster City, CA, USA). Purified amplicons were pooled in equimolar amounts and paired-end sequenced on an Illumina NovaSeq 6000 platform (Illumina, San Diego, CA, USA) according to the standard protocols by Majorbio Bio-Pharm Technology Co. Ltd. (Shanghai, China). The raw sequencing reads were deposited into the NCBI Sequence Read Archive (SRA) database (Accession Number: PRJNA863054).
Bioinformatic analysis of the gut microbiota was carried out using the online Majorbio Cloud Platform (https://www.majorbio.com) [23]. Based on the ASV information, rarefaction curves and alpha diversity indexes, including observed ASVs, Chao1 richness, Shannon index, and Good’s coverage, were calculated with Mothur v1.30.1 (https://www.mothur.org/wiki/Download_mothur). The similarity among the microbial communities in different samples was determined by principal coordinate analysis (PCoA) based on the unweighted UniFrac dissimilarity using R package vegan v2.5-3 (http://www.cran.r-project.org/package=vegan).
Statistical analyses were performed using GraphPad Prism 6
(GraphPad Software, San Diego, CA, USA). For multiple datasets, data were subjected to
one-way ANOVA followed by Dunnett’s multiple comparisons test when comparing
every mean to a control means. All p values
The total polyphenol content (TPC) and total flavonoid content (TFC) were determined using the photocolorimetric method with gallic acid and rutin as standards (Fig. 1). As shown in Fig. 1B, the TPC of the EA crude extracts was twice as high as the TFC. After secondary extraction with different polar solvents, the TPC and TFC were highest in TCM, followed by EAC. They were lowest in NBA, and the TFC was much lower than the TPC.
 Fig. 1.
Fig. 1.Total flavonoid and phenolic contents of various polarity extracts from D. nobile. (A) Gallic acid and rutin standard curves. A rutin standard curve was used to determine the total flavonoid content (TFC) at 510 nm. At 760 nm, a gallic acid standard curve was used to determine the total polyphenol content (TPC). (B) The content of TFC and TPC in the extracts of each group. NC, negative control; AC, aging control; PC, positive control; EA, ethanol extract; TCM, chloroform extract; EAC, ethyl acetate extract; NBA, n-butanol extract; OD, optical density.
We used a treadmill to test the endurance of each group of mice. The running time of mice in the AC group was significantly shorter than that of mice in the NC group. The running time of mice in the PC, EA and EAC groups was significantly increased compared to that of mice in the AC group and was similar to that of the NC group (Fig. 2A). Mice in the AC group had significantly higher body weights than mice in the NC group. The body weight of mice in the PC, EA and EAC groups was significantly lower than that of mice in the AC group and similar to that of the NC group. The body weight of mice in the TCM and NBA groups was significantly lower than that of mice in the AC group and much lower than that of mice in the NC group (Fig. 2B). Compared with mice in the AC group, the spleen coefficient of mice in the NBA group was basically the same as that in the AC group, while the spleen coefficient of mice in the NC, PC, EA and TCM groups was slightly higher than that in the AC group and that of mice in the EAC group was significantly higher than that in the AC group (Fig. 2C). Compared with the mice in the AC group, the thymus coefficient of the mice in the TCM and NBA groups was basically the same as that in the AC group, the thymus coefficient of the mice in the PC group was slightly higher than that in the AC group, and the thymus coefficient of the mice in the NC, EA and EAC groups was significantly higher than that in the AC group (Fig. 2D).
 Fig. 2.
Fig. 2.Endurance, weight gain and organ coefficient of aging mice
following D. nobile extract treatment. (A) Running
time during the endurance test. All groups ran on a treadmill at 10 m per minute
for the first 5 min and 20 m per minute thereafter until exhaustion. (B) Effects
of different compounds on body weight gains of D-galactose-induced ageing mice
after oral administration for 8 weeks. Bodyweight gain (%) = [(bodyweight of 8
weeks – bodyweight of 1 week)/bodyweight of 1 week]
As shown in Fig. 3, after 8 weeks of treatment, the MDA content in the skin of the AC group was significantly higher than that of the NC group, while the MDA content in the liver of the EA and EAC groups was significantly lower than that of the AC group (Fig. 3A,C). The EA extracts significantly upregulated SOD, CAT and GSH-Px activities in the skin compared to the AC group. In addition, EAC extracts significantly upregulated skin CAT and GSH-Px activities (Fig. 3A). In blood, EA extracts significantly upregulated SOD and CAT activities, while EAC extracts significantly upregulated SOD, CAT and GSH-Px activities (Fig. 3B). In the liver, SOD, CAT and GSH-Px activities were significantly upregulated in the EA group, while CAT levels were upregulated in the EAC group, and GSH-Px activities were upregulated in the NBA group (Fig. 3C). In brain tissue, SOD and GSH-Px activities were significantly upregulated in the EA group, while GSH-Px activities were upregulated in the EAC group (Fig. 3D). In summary, all D. nobile extracts were effective in increasing the activity of antioxidant factors in skin, serum, liver and brain, with the effects on skin and liver being more pronounced. In addition, the antioxidant activity was stronger in the EA and EAC groups.
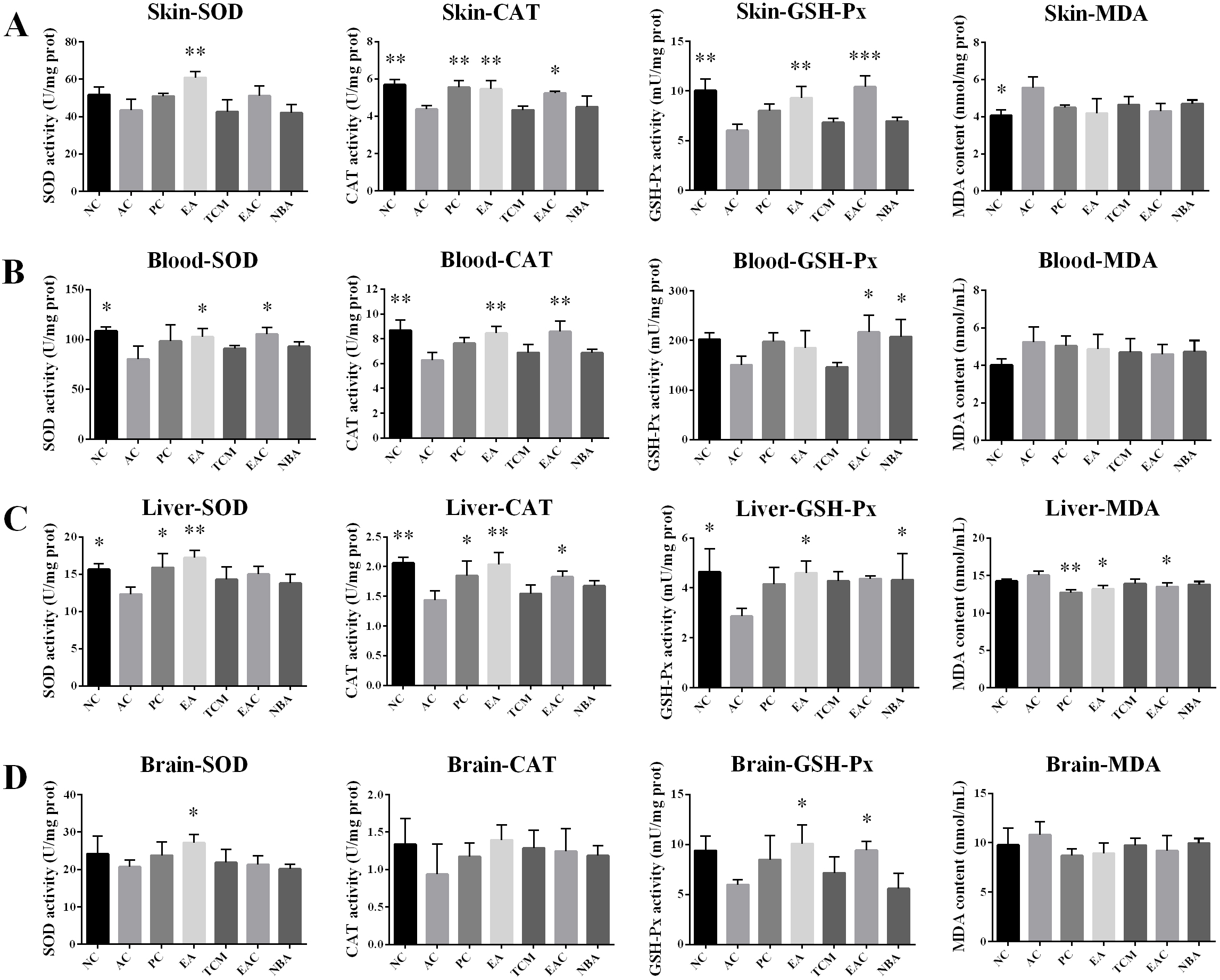 Fig. 3.
Fig. 3.Analysis of antioxidant indicators including SOD, CAT, and
GSH-Px activity and MDA content in the skin (A), blood (B), liver (C), and brain
(D). Values represent the mean
According to the histological analysis of H&E staining, mice in the NC group had a thick skin dermis with a clear and orderly structure, while mice in the AC group had a much thinner skin dermis with many adipocytes. Mice in the PC, EA and EAC groups had skin similar to that of the NC group, while mice in the TCM and NBA groups had skin similar to, but better than, that of the AC group (Fig. 4). Hepatocytes in the AC group were increased in size and had some edema, with a large amount of hepatocyte necrosis and steatosis. On the other hand, liver tissue from mice in the NC, PC, EA and EAC groups showed normal hepatocytes with no necrosis around the central vein. Pathological changes were also present in the TCM and NBA groups, but the symptoms were not as severe as those in the AC group (Fig. 4). In the NC group, the hippocampal CA1 region had tightly arranged pyramidal cells with normal cell morphology. The AC group had sparsely scattered pyramidal cells with more necrotic neuronal cells. The PC, EA and EAC groups had more tightly arranged pyramidal cells than the AC group, with less degeneration and necrosis of neurons, especially in the EA group (Fig. 4). Spleen pathology in the AC group showed blurred boundaries between the white and red marrow, severe loss of white marrow and a marked decrease in the white marrow/red marrow ratio. In addition, the spleen in the AC group had interstitial fibrous tissue hyperplasia and vascular growth. The severity of spleen lesions was greatly reduced in the EA and EAC groups compared to the AC group (Fig. 4). Follicle cells in the ovaries of the NC group were well developed with abundant corpus luteum tissue. Pathological lesions such as ovarian atrophy, follicular cell disintegration, corpus luteum and fibrous tissue hyperplasia were significantly higher in the AC group. Follicles in the EA and EAC groups grew well, and the number of follicular cells was appreciable compared to that in the AC group (Fig. 4). Thus, D. nobile extracts showed significant improvement in the pathological changes of the tissues.
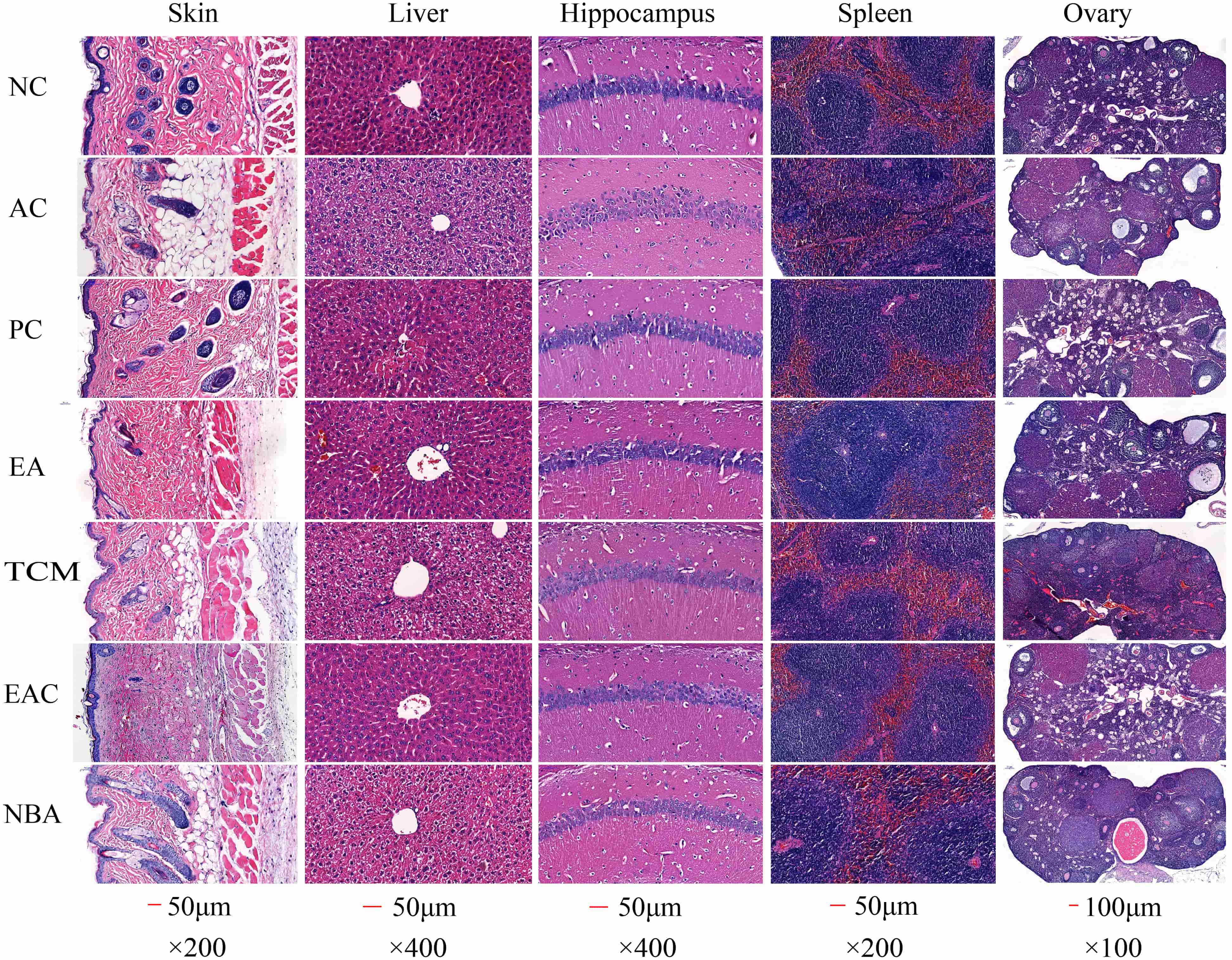 Fig. 4.
Fig. 4.Histopathological results of HE staining in mouse tissues. NC, negative control; AC, aging control; PC, positive control; EA, ethanol extract; TCM, chloroform extract; EAC, ethyl acetate extract; NBA, n-butanol extract.
A large number of studies have reported that aging can lead to the degeneration of brain function, which is the basis of neurodegeneration and dementia [25, 26]. In previous work, we found that EA and EAC reduced cellular senescence and oxidative stress in aging mice, so we wondered whether EA and EAC could regulate aging-related gene expression levels in the brain. We performed transcriptome sequencing analysis of brain tissue from D-galactose-induced aging mice in the EA and EAC groups. We found that 643 genes were upregulated and 707 genes were downregulated in aging mice of the AC group compared with normal mice of the NC group (Fig. 5A,B). EA and EAC reversed the same 235 upregulated and 72 downregulated genes associated with aging. In addition, EA alone reversed the upregulation of 134 genes and the downregulation of 68 genes. EAC alone reversed the upregulation of 25 genes and downregulation of 25 genes (Fig. 5A,B). KEGG analysis of these differentially regulated genes regulated by EA and EAC showed that most of the regulation at the gene level in aging mice was clustered in signaling molecule interaction pathways and signal transduction pathways (Fig. 5C,D). In addition, EAC regulates many genes in the endocrine system pathway (Fig. 5C). Cluster analysis of differentially expressed genes in these pathways revealed that important genes related to aging, such as Il1a, Il1b, Il1rn, Ccl3, Ccl4, Fos and Gck, were restored to low expression levels (Fig. 5E–G and Supplementary Fig. 1). In conclusion, EA and EAC have a significant downregulation effect on aging-related genes, indicating that EA and EAC have antiaging effects at the gene expression level.
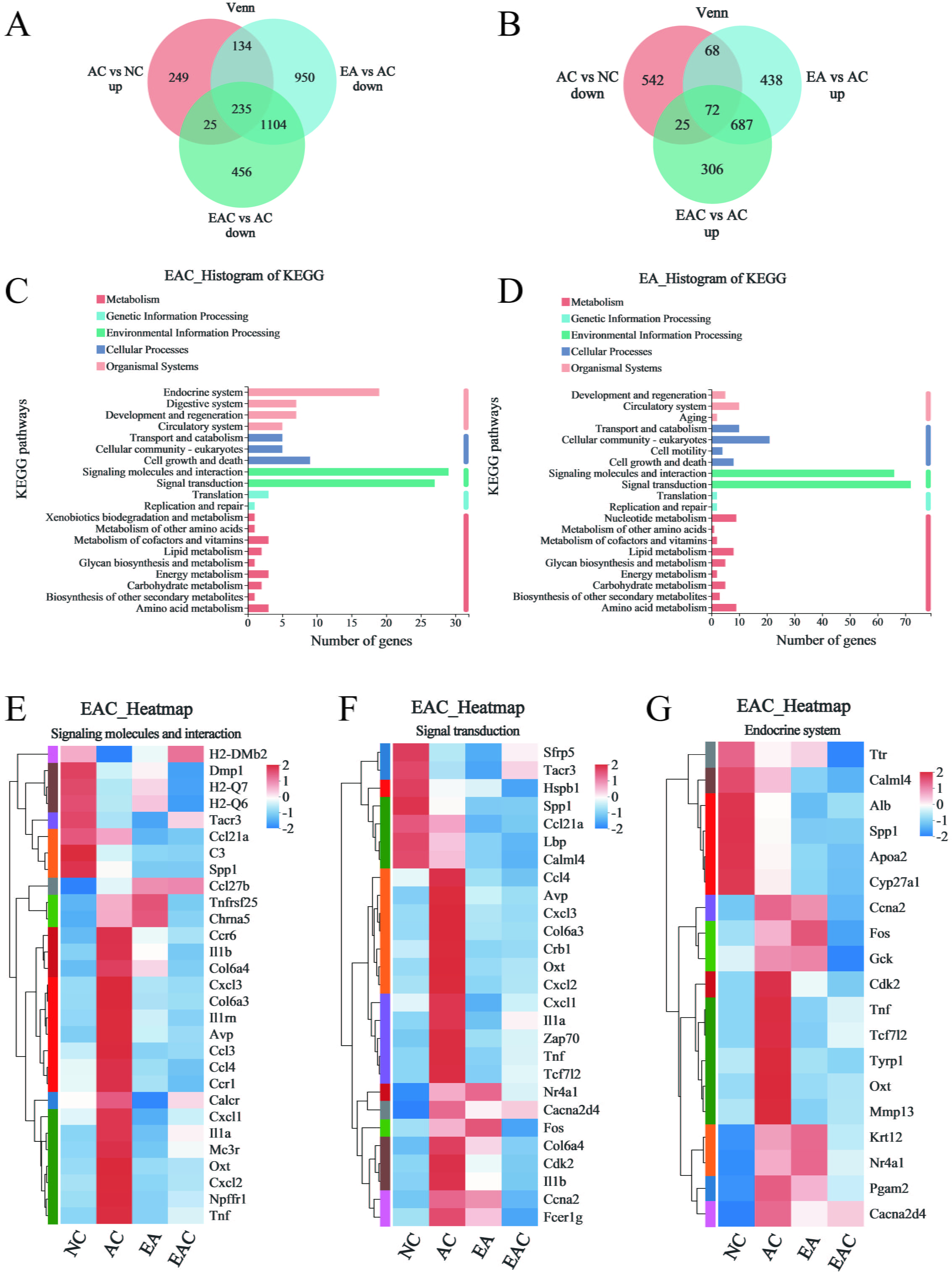 Fig. 5.
Fig. 5.Transcriptome sequencing analysis of mouse brain tissue. (A,B)
Venn plots indicate the number of genes that are regulated by EA and EAC in
D-galactose-induced ageing mouse brains, and “up” and “down” represent the
changes in gene expression levels of the former compared with the latter,
p
The antiaging effect was better in the EA and EAC groups. We selected the EA, EAC, NC, and AC groups of mouse colon contents for 16S rRNA sequencing to obtain ASV data for each sample. The ASVs of each group were analysed by Pan and Core species analysis and rarefaction curve analysis, and it was found that all gut microbiota samples were representative and stable (Fig. 6A–C). Combining the rarefaction curves and Venn diagrams of each group revealed that D-galactose injection significantly reduced the gut microbiota diversity of mice. The gut microbiota diversity of mice in the EA and EAC groups increased significantly after the intervention, although the number of gut microbiota in the EAC group remained low (Fig. 6C,D). Genus-based PCoA showed that the gut microbiota of the NC, EA, and EAC groups was clustered, while that of the NA group was disorganized and discrete (Fig. 6E). We analysed gut microbiota taxonomy at the phylum level and found that the EA and EAC groups significantly reversed the decrease in abundance of the dominant mouse flora bacteroidota and the increase in abundance of actinobacteria caused by aging. The EA group also reversed the increase in Firmicutes abundance, but the two treatment groups did not show a reversal effect on the increase in Proteobacteria abundance (Fig. 6F). A genus-level-based heatmap revealed that the EAC and EA groups reversed 11 and 18 D-galactose-induced changes in the abundance of the Firmicutes subtype, 5 and 11 changes in the abundance of the Bacteroidetes subtype, and 3 and 4 changes in the abundance of the Actinobacteria subtype in the gut microbiota, respectively (Fig. 6G). These results indicated that both ethyl acetate and ethanol extracts of D. nobile could improve the gut microbiota dysbiosis of aging mice to varying degrees, with the ethanol extract being the most effective.
 Fig. 6.
Fig. 6.Active ingredients of D. nobile regulate gut microbiota dysbiosis in aging mice. The colonic contents of mice in the EA, EAC, NC, and AC groups were selected for 16S rRNA sequencing to obtain ASVs for each sample, and the ASV data from each sample were drawn flat and processed. (A,B) The ASV data of the gut microbiota were based on Pan and Core species analysis at the genus level. (C) The ASV data of the gut microbiota were based on rarefaction curve analysis at the ASV level. (D) Venn diagram analysis of ASV data of gut microbiota based on ASV levels. (E) Unweighted UniFrac PCoA based on the genus level of gut microbiota ASV data. (F) Community Barplot analysis based on gut microbiota ASV data at the phylum level. (G) Community heatmap analysis of gut microbiota ASV data based on the genus level. NC, negative control; AC, aging control; PC, positive control; EA, ethanol extract; TCM, chloroform extract; EAC, ethyl acetate extract; NBA, n-butanol extract.
Older people are more prone to fatigue, so we tested the endurance of each group of mice (Fig. 2A). The running time of mice in the EA and EAC groups was significantly higher, indicating that EA and EAC extracts had a significant effect on improving physical fitness. Obesity accelerated ageing, and the body weight of mice in the AC group was significantly higher than that in all other groups (Fig. 2B). The body weight of mice in the EA and EAC groups was similar to that of mice in the NC group, suggesting that EA and EAC have a good effect on maintaining a healthy body weight. The body weight of mice in the TCM and NBA groups was much lower than that of mice in the NC group, suggesting that the TCM and NBA extracts may be a burden on normal body metabolism. Fatigue is also associated with immune disorders [27], so we analysed the organ coefficient of the thymus and spleen of mice. The spleen coefficient of mice in the EAC group was significantly higher than that of the AC group (Fig. 2C), and the thymus coefficient of mice in the EA and EAC groups was significantly higher than that of the AC group (Fig. 2D). This finding indicates that EA and EAC extracts have significant effects on improving immunity.
SOD levels slow down the metabolism of lipid peroxide and accelerate cellular senescence and death [28]. D. nobile extracts, especially EA and EAC extracts, increased the activities of antioxidant enzymes in biological tissues, abnormal ROS metabolism is a common symptom of various diseases and leads to various biotoxic reactions. Antioxidant enzymes such as SOD, CAT and GSH-Px balance the production and scavenging of ROS. Lower SOD, CAT and GSH-Px in the skin, blood, liver and brain and reduced levels of MDA in tissues (Fig. 3) indicated their good antioxidant activity. Furthermore, EA and EAC extracts significantly improved D-galactose-induced skin damage (Fig. 4). Decreased antioxidant enzyme activity leads to reduced free radical scavenging and skin damage [29]. Our results thereby suggested that EA and EAC extracts delayed skin ageing by increasing antioxidant enzyme activity. Free radicals damage many cellular components, including DNA, proteins and lipids [30, 31, 32]. D-galactose contributes to increased oxidative stress and ROS formation, which can lead to hepatocyte damage or death [33]. Liver tissues from the EA and EAC groups showed normal pericentriolar hepatocytes without necrosis, unlike the model group with oedema, massive hepatocyte necrosis and steatosis. Fig. 4, demonstrating that they have good hepatoprotection. Cognitive impairment induced by ageing has become increasingly common, particularly damage to neurons in the hippocampus, a region closely associated with learning and memory [34]. Sustained oxidative stress in the brain can lead to neuronal cell death, which can result in cognitive decline. Oxidative stress is a major trigger of neuronal damage in the hippocampus [35, 36]. Oxidative stress is known to be induced in the brains of D-galactose-treated mice [37, 38], which in turn leads to learning and memory deficits. Cone cells in the PC, EA and EAC groups were more closely arranged and had less neuronal degeneration and necrosis (Fig. 4), indicating that the EA and EAC extract has a good protective effect on the neurons of the brain. Compared with the AC group, the splenic white and red bone marrow boundaries were clear in the EA and EAC groups (Fig. 4), which indicates that EA and EAC extracts have a good protective effect on the spleen. Premature ovarian failure is a common disease that occurs in women before the age of 40, causing emotional instability and even infertility [36, 39]. The follicles in the EA and EAC groups grew well, and the number of follicular cells was appreciable compared to that in the AC group (Fig. 4), proving that they delayed ovarian aging.
Every organ in the body is impacted by aging, which also causes dementia and neurodegeneration [26] and alters the biological clock of the brain [25]. Both the inflammatory chemokines Ccl3 and Ccl4, which rise with aging and indicate the prevalence of age-related disorders [40, 41], have been linked to Alzheimer’s disease. In this work, both EA and EAC effectively reduced the expression of both in the brain tissue of aging mice and were reflected in the signaling molecules and interaction pathways and signal transduction pathways (Fig. 5E,F and Supplementary Fig. 1). Il1a, Il1b, and Il1rn all have positive relationships with aging, while EA and EAC hurt their expression (Fig. 5E,F and Supplementary Fig. 1). Genes such as Fos and Gck in the endocrine system pathway were also downregulated by EAC (Fig. 5G). Overall, aging-related genes were significantly regulated in the brains of aging mice by both EA and EAC extracts. The regulation mode of the EAC may be more expansive than that of the EA. EAC participates in the endocrine system route in addition to the signaling molecules and interaction pathway and the signal transduction pathway. In contrast, EA extracts concentrated more on the first two pathways and influenced more genes along each of these two pathways.
In the intestine of aging mice, both the EA and EAC extracts improved the gut microbiota disorder caused by aging to different degrees, and the EA group showed more extensive and significant effects. Both of them lowered the Firmicutes/Bacteroidota ratio (Fig. 6F), which increased in D-galactose-induced aging mice [42]. Increasing the abundance of probiotic Lactobacillus could inhibit oxidative stress and senescence in aging mice [43, 44]. In this study, the abundance of Lactobacillus was significantly higher in the EA group (Fig. 6G), suggesting that the EA group achieved antioxidant and antiaging effects by increasing the abundance of Lactobacillus. Lactobacillus can lower the expression of the proinflammatory cytokine Il1b [45, 46], downregulate the expression of the chemokine Ccl4 [47] and negatively correlate with the expression of the chemokine Ccl3 [48]. According to the transcriptome results of brain tissue, both the EA and EAC groups showed decreased expression of Il1b, Ccl3 and Ccl4, suggesting that changes in gut microbiota may be synergistic with the expression of inflammatory factor genes. In addition, the abundance of Muribaculum was upregulated in the EA and EAC groups, while Muribaculum was negatively associated with aging and oxidative stress processes [44]. Furthermore, the EA group had a reduced abundance of pathogenic bacteria such as Staphylococcus, Corynebacterium, and Brevibacterium, and the EAC group had a reduced abundance of pathogenic bacteria Brevibacterium and Corynebacterium (Fig. 6G).
Studies have reported that resveratrol can improve oxidative stress, alleviate inflammatory responses, improve mitochondrial function, and regulate apoptosis, thereby preventing and treating aging and aging-related diseases [49]. In this study, the EA and EAC groups showed better antioxidant and anti-aging effects than the PC group administered resveratrol. The anti-ageing effects of the extracts were not significantly and positively correlated with the TPC and TFC contents. EAC contains relatively less TPC and TFC than TCM (Fig. 1B), but EAC has higher antioxidant and age-delaying activity, indicating that the best components are concentrated in EAC. In contrast, TCM has a reduced age-delaying effect, perhaps due to its toxic effects. EA as a total extract contains both highly potent and less potent substances, not only with better activity but also with lower toxicity, indicating a reconciliation effect between the multiple components. In the next study, we will further explore the active specific components in D. nobile. There are still many unanswered mysteries about the mechanisms of aging, and free radical theory and disordered glucose metabolism can only partially explain the process of aging [50]. Furthermore, a typical mouse model of D-galactose-induced senescence does not fully mimic the pathophysiological changes in organs in the natural aging state. Therefore, we need to further confirm the antiaging effect of D. nobile using natural aging models and investigate the underlying mechanisms to extend longevity.
D. nobile extracts, particularly the EA and EAC extracts, improved immunity with antifatigue properties and against degenerative changes in the spleen and thymus, protected skin, liver, hippocampus, and overy tissues from ROS damage and degenerative changes by increasing antioxidant enzymes such as SOD, CAT and GSH-Px and reducing MDA levels. Moreover, the EA and EAC extracts remodelled the intestinal microecosystem by improving the abundance of Lactobacillus and Muribaculum, which have a synergistic effect with antioxidants and anti-ageing. Both slowed brain aging by downregulating the expression of senescence-related genes Il1a, Il1b, Il1rn, Ccl3, Ccl4, Fos and Gck. Both the ethyl acetate extracts and the ethanol extract from D. nobile could be used as drugs or functional foods to delay aging.
The datasets used and/or analysed during the current study are available from the corresponding author upon reasonable request. Raw data for transcriptome sequencing can be accessed by logging on to the website https://www.ncbi.nlm.nih.gov/sra/PRJNA867020. 16S rRNA gene sequencing data can be obtained from the website https://www.ncbi.nlm.nih.gov/sra/PRJNA863054.
These should be presented as follows: RW and FH designed the research study. XG, JL, YLuo, YLei, and WL performed the research. KW, JZ, ML, NY, and HZ provided help and advice on paper writing. JL, YLuo, and YLei analysed the data. RW, XG and JL wrote the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript.
The Life Ethics Committee of Southwest Medical University (Ethical No. SWMU20210365) approved all animal handling and experimental methods, which were carried out following the Guidelines for the Care and Use of Laboratory Animals (National Institutes of Health, Bethesda, MD, USA).
We express heartfelt thanks to Li Cai from the Pathology Department at the Affiliated Hospital of Southwest Medical University for preparing hematoxylin-eosin (HE)-stained tissues.
This research was funded by the Key Research and Development Project from the Science & Technology Department of Sichuan Province (Grant No. 2019YFS0176), Science & Technology Program from the Administration for Market Regulation of Sichuan Province (Grant No. SCSJ2021009), Sichuan Provincial College Student Innovation and Entrepreneurship Training Program (Grant No. S202110632237), Strategy Project of Luzhou Science & Technology Bureau (Grant No. 2017CDLZ-S01), Open Fund of the Key Laboratory of Medical Electrophysiology of Ministry of Education and Sichuan Province, China (No. KeyME-2020-001), and University-level Science and Technology Program of Southwest Medical University (Grant No. 2019ZQN023).
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
