1 Department of Pharmacy, Daping Hospital, Army Medical University, 400042 Chongqing, China
Academic Editor: Pietro Gentile
Abstract
Platelets are small, anucleate cellular fragments, which are produced by megakaryocytes, and play a key role in hemostasis and thrombus formation. The differentiation of megakaryocytes from hematopoietic stem cells in bone marrow and the development of megakaryocytes into platelets is a complex process. Various regulatory factorsin megakaryopoiesis including cytokines, growth factors, transcription factors, and gene expression, are all involved in the process of thrombocytopoiesis and play distinct roles in different stages of megakaryocytes development. In this review, we summarize the current state of knowledge ofmultiple regulatory factors including the TPO/Mpl signaling pathway, transcription factors, RasGTPases family, estrogen, and microRNAs. Altogether, we aimed to discuss more molecular mechanisms of megakaryocytes differentiation and maturation, and possess a better understanding of platelet formation.
Graphical Abstract
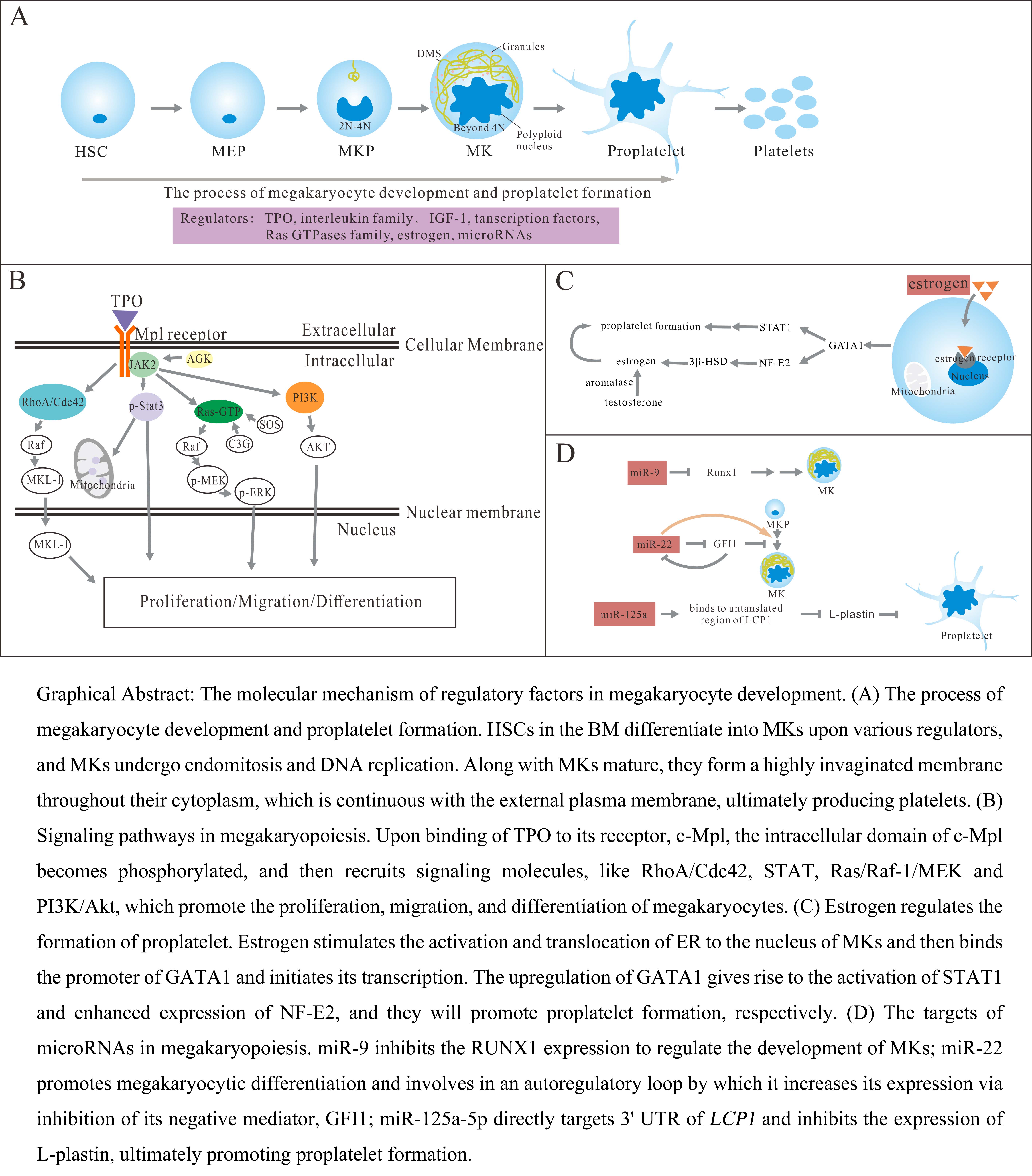
Keywords
- megakaryocyte
- thrombopoietin
- transcription factors
- Ras GTPases family
- estrogen
- microRNAs
Platelets are one of the crucial components in mammalian peripheral blood with small size, anucleate cellular fragments, and play a key role in bleeding and coagulation. The reference range of platelet count in normal humans is 150–400
TPO/myeloproliferative leukemia virus (Mpl) receptor signaling pathway is the key pathway mediating megakaryocyte development and platelet production. TPO can bind to the Mpl receptor and then signal in megakaryocytes and HSCs, resulting in dimerization of the Mpl receptors and phosphorylation of Janus kinase2 (JAK2) downstream. Moreover, phosphorylation JAK2 will in turn phosphorylate Tyr626 and Tyr631 in Mpl, and then recruit signaling molecules containing the Src homology domain, like Stat family molecules [10, 11]. Besides, TPO also regulates megakaryocyte development and platelet production in other ways. The megakaryogenesis process involves the interplay of multiple transcription factors, which ensure the expression of a specific set of mRNA and protein during the development. Transcription factors and Ras GTPases family members have been found as critical roles in megakaryopoiesis and thrombocytopoiesis, and they may have important implications for in vitro platelet production. The deficiency of them affects multiple stages of hematopoiesis [12, 13], here we discuss the new effects of them in megakaryocyte-specification bias. Traditionally, it is believed that the bone marrow niche does not exhibit sex-dependent morphological differences, however, many studies have shown that estrogen has an important effect on the fate decision of hematopoietic cells [14, 15]. Moreover, estrogen is a potential contributor in megakaryopoiesis [16]. In recent years, microRNAs with a role in regulating gene expression attract the interest of researchers. The development of MKs needs protein translation to generate proplatelet, and MK-specific knockdown of the miR-processing enzyme Dicer leads to platelet counts decreasing [17]. More functional characterizations of microRNAs and their targets selection in different stages of megakaryocytopoiesis or thrombocytopoiesis can provide new insights for benefiting management approaches. In this review, we discussed the molecular mechanism of multiple cytokines and gene expression in different stages of thrombocytopoiesis, and they can regulate several hematopoietic lineage cell fate decisions and ensure that megakaryocytes mature correctly into final differentiated blood cells.
Thrombopoietin, a glycoprotein primarily produced by liver cells, is a crucial factor relating to regulating circulating platelet production [18, 19]. The level of TPO in the bone marrow and peripheral blood is negatively correlated with platelet count, but platelet count is not the only determinant of TPO, some studies have shown that inflammatory stimulation can also affect the expression of TPO, such as IL-6, which can promote the transcription of TPO in the liver [20]. The TPO is a vital cytokine in megakaryopoiesis, and multiple underlying mechanisms have been found to promote the differentiation of hematopoietic stem cells into the Mk-lineage. TPO/Mpl pathway activates the Janus kinase family at first, and then regulates a series of downstream signal pathways through JAKs, including signal transducer activator of transcription (STAT), Ras/Raf-1/mitogen activated protein kinase (MAPK) and phosphoinositol 3 kinase (PI3K)/Akt pathway, afterward, promotes the survival, proliferation, and polyploidization of megakaryocytes [21].
The TPO/Mpl/JAK2/Stat3 signaling pathway is the most studied pathway which regulates megakaryocyte development and platelet production. Among them, mitochondrial-associated gene sets related to mitochondrial biosynthesis and function and stimulated by TPO signaling can be rapidly enhanced, like Ppargc1a, Nrf1, Sdfb, and Sdhc genes, and functionally promote a Mk-lineage bias [22]. HSCs live in a hypoxic niche and depend on anaerobic glycolysis for maintenance [23, 24]. Previous works have revealed that mitochondrial metabolism is inhibited in quiescent HSCs, for increased mitochondrial activity accompanied by loss of pluripotency in HSCs [24, 25]. TPO-stimulation on HSCs showed an enhanced mitochondrial gene signature and induced the increase of mitochondrial activity to exit dormancy of HSCs, exhibiting a myeloid-Lineage bias to Mk-lineage. Mk cells rich in mitochondria display the overexpression of CD9, which is a marker of Mk-lineage. One of the downstream signals that regulate HSC mitochondrial function in response to TPO is signal transducer and activator of transcription 3, which is phosphorylated at serine 727 and translocated to mitochondria, and then stimulates mitochondrial function by activating complexes I and II of the electron transport chain [26, 27]. Furthermore, Acylglycerol kinase (AGK), initially identified as a mitochondrial inner membrane protein, participates in megakaryocyte differentiation and platelet formation. Cytosolic AGK can bind to JAK2 in megakaryocytes/platelets and significantly enhance JAK2/Stat3 signaling activation in response to TPO in vitro and in vivo, ultimately promote thrombocytopoiesis [28].
MAPK is a key signaling pathway that regulates a variety of cellular processes, including proliferation, differentiation and apoptosis [29]. MAPK signal pathway includes three major kinases, MAPK kinase kinase (MAP3K), MAPK kinase (MAP2K) and MAPK, which can activate and phosphorylate downstream proteins. MAPKs cascade contains four groups of proteins: extracellular signal related kinase (ERK) 1 and 2, ERK5, p38MAPK and c-Jun amino terminal kinase (JNK) [30], and they are activated by dual phosphorylation of threonine and tyrosine residues. Research shows that JNK and p38 MAPK pathway are mainly related to cell stress and apoptosis, while MAPKs/ERKs signal pathway is closely related to cell proliferation and differentiation, and plays a pivotal role in cell signal transduction network [31, 32]. ERK1/2 can regulate cell signal transduction under normal and pathological conditions, and its expression is very important for cell development [33, 34]. In the ERK pathway, Ras, Raf, MAPK/ERK kinase (MEK) and ERK act as upstream activating protein, MAP3K, MAPKK and MAPK respectively, ultimately forming the Ras-Raf-MEK-ERK pathway. And this signal pathway contains various biological effects of cytokines, especially the activation of ERK1/2 involving in TPO/Mpl pathway [35]. After TPO stimulation, inhibition of MEK-ERK1/2 signal pathway induced an increase in the expression of immature marker CD34 and a decrease in late MK commitment marker CD42b, without affecting the expression of early megakaryocyte marker CD41. It is demonstrated that the MEK-ERK1/2 signal pathway plays a key role in the late-stage differentiation of MKs, and also plays an important effect on proplatelet formation [36].
The binding of TPO to c-Mpl receptor also leads to the activation of PI3K/Akt signal pathway, which reduces the cell apoptosis and increases proliferation [37]. Andre et al. [38] found that Tensin2, as a focal adhesion molecule, was a critical downstream protein in TPO/c-Mpl signal pathway. After the binding of TPO to c-Mpl, the intracellular domain of c-Mpl was phosphorylated, one site being at Y631, and then Tensin2 was recruited to this site and was phosphorylated. After that it may act to recruit the p85 subunit of PI3K, which then went on to activate Akt and affected downstream functions including cell growth. In addition, in the development of MKs, PI3K/AKT signal pathway is also activated by the stimulation of NOTCH signal, NOTCH pathway activation inhibits PTEN expression which is a negative regulator of the PI3K/AKT pathway and acts by inhibiting the activation of AKT, and promoting the genesis of megakaryocytes [39]. Moreover, NOTCH signal also induces the development of MKs by the other pathways [37]. Of note, keeping the balance between the activation of AKT and ERK1/2 is important for regulating proplatelet formation. Over-activation of AKT not accompanied by ERK1/2 over-activation result in hyperproliferation of immature megakaryocytes which possess a defective capacity to form proplatelets. In contrast, pathologic over-activation of ERK1/2 only, not accompanied by AKT over-activation, inhibits proplatelet formation. Eltrombopag increased megakaryocyte maturation and proplatelet formation by stimulating the phosphorylation of AKT and ERK1/2 [40].
Thus far, TPO is considered to be the most important cytokine in megakaryopoiesis [41]. However, TPO-deficient mice also appear healthy and show no symptoms of spontaneous hemorrhage [42]. Therefore, alternative/compensatory regulators exist in this process, and these regulators are likely to play a supplementary role for maintaining hemostasis, like IL-1
Transcription factors are crucial regulators of gene expression. In hematopoiesis, TFs form a complex network regulating the fate of HSCs. For instance, runt-related transcription factor 1 (RUNX1), GATA-binding protein 1 (GATA1), friend leukemia virus integration 1 (FLI1), Megakaryoblastic leukemia 1 (MKL-1), T-cell acute lymphocytic leukemia 1 (TAL-1), Nuclear factor erythroid 2 (NF-E2) and so on, all play a critical role in megakaryopoiesis and thrombocytopoiesis. These TFs act in a combinatorial manner to bind sequence-specific DNA within promoter regions to control lineage-specific gene expression, either as activators or repressors.
RUNX1, which is one isoform of the core binding factor Runt-related transcription factor, has been found to regulate megakaryocyte polyploidization, maturation, and thrombopoiesis through multiple biological mechanisms [47, 48, 49]. The gene mutation in Runx1 and activation/repression of Runx1 in humans can lead to various diseases, such as acute myeloid leukemia [50] or familial platelet disease [51]. Moreover, RUNX1 stimulates several signal pathways in the process of thrombocytopoiesis, including platelet factor 4, Notch families, nuclear receptor subfamily 4 group A member 3 [52], protein kinase c theta [53], myosin light chain 9 [54] and so on. Once these platelet genes are activated or inhibited, Runx1 transcription is strictly regulated, with changes in gene dosage and expression level affecting both the spatiotemporal onset of hematopoiesis and hematopoietic homeostasis [55, 56]. In mammals, Runx1 loci possess 2 promoters that control their transcription: a distal P1 promoter and a proximal P2 promoter [57]. The major protein generated by the P1 and P2 promoters are the RUNX1C and RUNX1B isoforms [58]. Performing a detailed experiment on the activity of RUNX1B and RUNX1C, researchers have confirmed that P1 has a crucial role in the total process of hematopoiesis, and is active in all Runx1-expressing subsets [59]. Julia et al. [60] established a novel mouse model which replaced the expression of RUNX1C with RUNX1B in physiological process, and then tipped the balance in favor of erythrocyte specification in bipotential megakaryocytic/erythroid progenitors and stayed away from megakaryopoiesis [61]. They further demonstrated that Runx1 P1 was the dominant promoter in the course of megakaryopoiesis, but differentiation and polyploidization defect of megakaryocytes lineage was not observed in the absence of RUNX1C, the mechanism underlying, therefore, appeared to directly mediate apoptosis signaling pathway and arrest the process of cell death in the megakaryocyte, finally resulting in a competitive preponderance of megakaryocyte lineage output. The expression of several important genes in megakaryopoiesis is regulated by RUNX1, and RUNX1 downstream targets can be pharmacologically manipulated to improve MK production. The Notch4 gene encoding a Notch receptor is a novel downstream target of RUNX1, and its expression is downregulated in a RUNX1-dependent manner [62]. Li et al. [62] strongly suggested that repression of Notch4 signaling by RUNX1 promoted MK formation. The underlying mechanism of the Notch4 signal is negatively controlled by RUNX1 via a novel regulatory DNA element within the locus, and it is involved in the process of hematopoietic progenitor cells and the inactivation of Notch4 promotes the development of megakaryopoiesis.
FLI1, a critical regulator for late megakaryopoiesis, and its overexpression inhibits the development of erythroid cells, resulting in megakaryocyte lineage bias in cell lines. In hematopoiesis, FLI1 knockout mice exhibit embryonic lethality because of loss of vascular integrity and defective megakaryopoiesis [63]. Meanwhile, the loss of FLI1 is the reason for thrombocytopenia in human Paris-Trousseau syndrome (PTSx) [64, 65]. Karen et al. [66] found that downregulated expression of FLI1 affects megakaryocyte potential, especially large CFU-MK potential, and heterozygous FLI1 loss observed in PTSx displayed a big defect of megakaryocyte and platelet. Furthermore, E26 transformation-specific proto-oncogene 1 (ETS1) is overexpressed in FLI1-deficient megakaryocytes, suggesting FLI1 negatively regulates ETS1 in megakaryopoiesis [66].
TAL-1, also known as stem cell leukemia, is a transcription factor with helix–loop–helix structure that regulates the activity of other transcription factors, like GATA-1/2 [67]. TAL-1 is essential for hematopoietic development due to embryonic lethality [68]. However, TAL-1 is non-indispensable for maintaining normal hematopoietic stem cells, the effects of TAL-1 loss in megakaryopoiesis were slight, and 5% residual expression of TAL-1 in megakaryocytes was sufficient to maintain relatively normal platelet number and function. One of the reason is its closely related family member, lymphoblastic leukemia 1 (Lyl1), which compensating for loss of Scl in megakaryopoiesis, meanwhile, TAL-1and Lyl1 all possess important roles in platelet production by modulating expression of partner proteins including GATA-1 and FLI-1 [69].
MKL1 is a member of the myocardin family of transcriptional coactivators of serum response factor (SRF), other members include MKL2 and myocardin [70]. This family has several highly domains, among them, a B-box and a glutamine-rich domain bind SRF, and a repeat sequence called RPEL, that is arginine, proline, glutamic acid, and leucine, bind to actin in the N-terminus. Studies have shown that MKL1 is a vital regulator wherein megakaryocyte development. MKL1-deficiency mice have displayed low ploidy of megakaryocytes and reduced the number of platelet counts in the peripheral blood [71]. MKL1 is mainly localized in the cytoplasm, where MKL1 binds to monomeric G-actin by its N-terminal RPEL domains, and cycles between the cytoplasm and the nucleus through a complex process [72]. When actin polymerizes to form F-actin in response to RhoA activation, MKL1 is dissociated from G-actin and gathered in the nucleus, and then binds SRF to activate transcription of SRF target genes [73, 74], such as cytoskeletal and adhesion molecule genes, to promote megakaryocyte development [75]. Simultaneously, the expression of MKL1 contributes to increased expression of GATA1, GATA2, GP5 and so on, thus enhancing megakaryocytic differentiation. Notably, TPO is a critical stimulator to promote MKL1 nuclear accumulation in megakaryocytes in vivo, but not the only one. After RhoA is activated by TPA stimulation, MKL1 accumulates in the nucleus, occupies chromatin target sites and becomes stabilized, and initiates the transcription of MKL1 target genes, but the unbound MKL1 goes back to the cytoplasm after the RhoA signal weakens [76]. Accordingly, MKL1 is another molecular mechanism mainly controlled by TPO to regulate megakaryocyte differentiation.
In addition, others transcription factors, like GATA-1, ecotropic viral integration site-1(EVI-1), NF-E2 and so on, also play a critical role in megakaryopoiesis and thrombocytopoiesis. GATA-1/2, along with its cofactor FOG1, are key transcription factors that are essential for differentiation of megakaryocytes and erythrocytes [77]. EVI-1 is an oncogenic transcription factor in human myeloid leukemia, and plays a critical role in tissue development and in the proliferation and differentiation of HSCs through affecting the expression of GATA-2 [78]. NF-E2 is a key regulator in the last stage of MK maturation, and promote proplatelet formation [79]. Megakaryopoiesis is a complex process including lineage commitment, megakaryocyte maturation and platelet release, and it is regulated by various transcription factors who form a network to ensure platelets normal supply.
The Ras GTPases are the founding members of the monomeric small GTPases superfamily, which regulate many cellular processes, such as cell survival, cell cycle progression, actin cytoskeletal organization, cell polarity, adhesion and movement and so on [80]. The Ras superfamily contains more than one hundred members in human, like Ras family, Rho family. As GTPases, Ras superfamily proteins are interconvertible between an active GTP-bound and an inactive GDP-bound form. The cycle between the active and the inactive states of these small GTPase is controlled by guanine nucleotide exchange factors (GEFs), which promote the exchange of GDP and GTP, and is controlled by GTPase-activating proteins (GAPs), which discontinue the active state by stimulating the GTP hydrolysis [80]. In megakaryopoiesis, Ras GTPase family plays an important role for promoting megakaryocyte differentiation, polyploidization, maturation and proplatelets formation.
The Rho GTPases family which is most studied in the Ras protein superfamily plays a crucial role in platelet production and function and have more than 20 members which are divided into typical and atypical members [81]. Among them, typical Rho GTPases such as RhoA, Cdc42, and Rac1 are regulated by Rho-specific GEFs, guanine nucleotide dissociation inhibitors (GDIs), and GAPs. Simultaneously, these Rho GTPases are widely studied in the formation of MKs and platelets due to regulating actin cytoskeletal dynamics [82].
During mitosis, RhoA signaling is used to form the contractile ring at the cleavage furrow to complete cytokinesis [83]. However, in endomitosis, the contractile ring of Mk lacks non-muscle myosin IIA and contains slowly decreased levels of RhoA and actin in the process of polyploidy, until it is not detectable in higher ploidy cells [83, 84]. In previous studies, GEFs were found to participate in RhoA localization and activation during cytokinesis. GEF-H1 and Epithelial Cell Transforming Sequence 2 (ECT2), two mitotic GEFs, are downregulated during Mk polyploidization, and play a critical role in the RhoA signal pathway. After the rupture of the nuclear envelope during mitosis, the GEF ECT2 is released from the nucleus to the cytoplasm, and accumulated in the central spindle to establish the cleavage furrow and involve in the progression of the cell cycle [85]. Some evidence suggested that GEF ECT2 recruited RhoA into the cleavage furrow, but it might not be directly responsible for its activation [86]. GEF-H1, as a microtubule-associated protein, is dephosphorylated and released from microtubules to activate RhoA. Moreover, GEF-H1 and ECT2 play different roles in MK differentiation. MKL1 can downregulate GEF-H1 levels so that it can involve in the endomitosis of 2N cells to 4N. ECT2 downregulation is used for polyploidization beyond the 4N stage [86]. In the state of 2N cells to 4N transition, RhoA is recruited to the central spindle after ECT2 correctly localization, whereas the lack of GEF-H1 protein leads to inactivation of most RhoA. Beyond the 4N stage, although the expression level of GEF-H1 is restored, dramatically decrease expression of ECT2 protein also results in the failure of RhoA to be recruited to the central spindle.
Cdc42, which belongs to the Rho GTPases family, is another key regulator in controlling MK differentiation and maturation at BM in vivo. The formation of giant cells from MKP needs to undergo several steps, including polyploid with DNA replication (endomitosis) and cytoplasmic maturation. The cytoplasmic maturation involves the generation of an internal demarcation membrane system, which serves as a membrane reservoir for the production of platelets in the terminal stage [3]. In previous studies, mice with deletion of Cdc42 in MKs led to macrothrombocytopenia, which was linked to decreased PPF of Cdc42-deficient MKs in vitro [81, 82]. Simultaneously, researchers revealed that Cdc42 positively regulated DMS formation/polarization and PPF in CD34
In addition, the other members of Ras subfamily, such as SOS/Ras/Raf, C3G/Rap1 and Rasa3/Rap1 pathway, also participate in the process of megakaryopoiesis. Son of sevenless (SOS) can bind to growth factor receptor-bound protein-2 (Grb2), and then the complex will activate the downstream signal pathway Ras/Raf/MAPK to promote megakaryopoiesis. C3G (CrkSH3-binding guanine nucleotide exchange factors), also known as RAPGEF1, is a guanine nucleotide exchange factor for Rap1 and R-Ras, and activated via Crk adaptor protein. Previous studies have shown that the activation of ERKs in TPO pathway has 2 components, a Ras-dependent transient ERK activation and a Rap1-dependent sustained ERK activation [91]. TPO/Mpl complex activates the JAK kinases that promote tyrosine phosphorylation. These phosphorylations act as binding sites for various signaling molecules, like Shc, which recruits Grb2/SOS to activate Ras and then causes a transient activation of ERKs. Sustained activation of ERKs that required in the differentiation of megakaryocytes needs the activation Rap1 and B-Raf [92]. The recruitment of a Crk/C3G complex to Mpl has been shown to trigger the activation of Rap1. Simultaneously, C3Gcan regulate the expression of p21, which plays a critical role in the proliferative arrest of cells during MK differentiation, and then contributes to cell cycle arrest. Moreover, GATA-1 could be a potential regulator of C3G during megakaryocytic/platelet differentiation, and overexpression of GATA-1 can increase C3G expression during TPO-induced MKs maturation [93]. Rasa3 is a member of the GAP1 family of Ras GAPs, which targets Ras and Rap1. And Ras GAPs are tumor suppressors as the loss of their GAP activity allows uncontrolled Ras, Rho and Arf activities and promotes disease [94]. Rasa3 controls Rap1 activation and integrin signaling during megakaryocyte differentiation to promote cell adherence, migration, actin cytoskeleton organization and differentiation into proplatelet. Inactivation of Rasa3 in mouse had a megakaryocytic dysplasia accompanied with a severe thrombocytopenia, and these megakaryocyte alterations were related to an increased active Rap1 level and a constitutive integrin activation [94, 95]. However, a new molecular mechanism has been found that dysregulated integrin signaling in the Rasa3-mutant mouse model may contribute to increased platelet clearance in various inherited and acquired thrombocytopenia [96].
More and more studies have shown that estrogen, as a primary female sex hormone, possesses a variety of functions outside the reproductive system in vivo. It was demonstrated that estrogen had an intrinsic ability to promote MK polyploidization, maturation, and platelet formation [97, 98]. A clinical study has shown that there are differences in platelet counts between postmenopausal women and young women. Lower platelet levels have been found in postmenopausal women, but the number of platelets would be dramatically increases after receiving estrogen replacement therapy [99]. Similarly, a survey of 32,000 inpatients and outpatients in Germany from birth to adulthood showed that the platelet level of adolescent women was higher than that of men when ovaries began to develop and secrete large amounts of estrogen to maintain sexual development [100]. Estrogen plays different biological functions mainly through nuclear estrogen receptor (ER) ER
In previous studies, various humoral factors that regulated proplatelet formation were sought. Steroid hormone receptors expressed in bone marrow megakaryocytes was described by Khetawat et al. [104]. among them, estradiol secreted by megakaryocytes was detected in the media, and its autocrine mechanism was initiated when the expression of 3
MicroRNAs, the endogenous non-coding RNA species with approximately 22-nucleotide in length and highly conserved through evolution, have been involved in various processes of the development and establishment of tissue identities, such as proliferation, differentiation, apoptosis and the others [109, 110]. Different miRNAs have distinct roles to determine cell fate of HSCs based on the complementarity of the miRNA seed sequences with the 3’-untranslated region in the respective target mRNA, then the synthesis of protein could be reduced through translational repression or mRNA degradation [111]. Notably, researchers have found that a large number of miRNAs play a critical role in controlling myeloid cell proliferation, differentiation, maturation, and activation [112]. Moreover, dysregulation of miRNA has an unfavorable impact on the cellular physiological process and even gives rise to diseases, like leukemia, osteosarcoma, myelodysplastic syndrome and so on [113, 114, 115].
MicroRNA-9 (miR-9) is a short and single-stranded non-coding RNA that controls cell proliferation, migration and differentiation [116]. And one of its direct targets is Runx1, which is an essential transcription factor that controls the differentiation and function of myeloid-derived suppressor cells. MiR-9 was negatively correlated with Runx1 expression. Researchers found that 3’ untranslated regions (3’ UTR) of the Runx1 gene had two highly conserved seed sequences (position 185–190 and position 653–658 in mice) for miR-9 by several bioinformatic databases, and the binding mediated posttranscriptional gene repression by degrading target transcripts or inhibiting protein translation [117]. Moreover, CREB, as a transcription factor involved in various cellular processes, has been discovered that it has a potential binding capacity within the miR-9 promoter region, and then directly regulates the expression of miR-9 [117]. What is more, Sanjeev et al. [118] further studied the regulatory mechanisms between miR-9 and Runx1 in human megakaryocyte development. They observed developmental differences in the expression levels of the miR-9 in neonatal vs adult MKs. The high expression in neonatal produced abundant small low ploidy MKs but it is low expression in adult forms mature MKs. Then they firstly provided the evidence that the higher level of miR-9 might lead to the developmental difference and disease susceptible phenotype of neonatal MKs via regulating the RUNX1 expression.
Researchers found that MicroRNA-22 (miR-22), a non-coding RNA, was upregulated in megakaryocytes differentiated from mouse fetal liver in vitro [119] and in a dual functional human erythroleukemia cell line, K562 [120, 121]. In humans, miR-22 is encoded by genes localized on chromosome 17 [122]. miR-22 is defined as a protooncogene that causes the development of myelodysplastic syndrome with increased expression levels [123]. And recently, upregulation of miR-22 has been displayed to inhibit erythroid maturation and promote megakaryocytic differentiation in adult mice [124, 125]. Previous works revealed that miR-22-3p was the predominant strand based on sequencing analysis, and its expression was significantly increasing in the final phase of MKs differentiation based on sequencing analysis [59, 126]. For assessment of miR-22 targets that may regulate the polyploidization and maturation of megakaryocyte, it has been found that GFI1, a transcription factor [127], possess conserved miR-22-3p seed region in its 3’-UTRs in human and mice. And researchers elucidated that miR-22 in megakaryopoiesis played a positive regulation role through direct binding and repression of the GFI1 [124]. Interestingly, miR-22 is not predicted to bind to GFI1B, which is a paralog of GFI1 with a difference only in the intermediate domain. Furthermore, Jiang et al. [128] described a new relationship between GFI1 and miR-22, and they found a recognition site of GFI1 in the promoter of MIR22HG, which downregulated the expression of miR-22 via epigenetic silencing of GFI1-recruited TET1. Therefore, GFI1 was defined as upstream of miR-22. Altogether, miR-22 involve in an autoregulatory loop by which it increases its expression via inhibition of its negative mediator, GFI1.
Guo and his colleagues found a single microRNA, MicroRNA-125a (miR-125a), could positively regulate HSC cell-state specific [129]. Moreover, miR-125a, which is located on chromosome 17, is one of the most highly expressed genes in long-term hematopoietic stem cells/progenitor cells (HSPCs) [130]. And it is also the candidate functional downstream targets through which miR-125a may be able to modulate HSPC fate in mice model via a genetic screen [131]. Furthermore, during normal human thrombopoiesis, the downstream key regulator of miR-125a-5p, that is gene LCP1, has been found [132]. MiR-125a-5p is related to human platelet counts but does not influence leukocyte or hemoglobin levels, and the expression of MiR-125a-5p regulates the process of MK proplatelet formation to affect platelet releasing. LCP1 gene encodes the actin-bundling protein, L-plastin, which is a 65-kDa protein and involved in the actin filament organization process [9]. High expression of L-plastin in early-stage Mk Progenitors enhances actin stress fibers and cell migration, and low expression of L-plastin enhances MK DMS and promotes the formation of podosome and proplatelet formation [133]. Taken together, miR-125a-5p directly targets 3’-UTR of LCP1 and inhibits the expression of L-plastin, ultimately promotes proplatelet formation. Nevertheless, miR-125b was noticed with decreasing expression during megakaryopoiesis, and it suggested that miR-125b mightcontrol cell proliferation and survival of neonatal megakaryocytes [133].
Various microRNAs also display vital roles in megakaryopoiesis and thrombocytosis. For instance, overexpression of miR-181 affects megakaryocytic differentiation through repressing Lin28 expression after 12-O-tetradecanoylphorbol-13-acetate (TPA) stimulation in K562 cells [134]. Similarly, miR-27ahas an important role in megakaryocytic differentiation via binding with Runx1 [135]. More and more miRNAs have been discovered yet in MKs but new miRNAs are still continuously emerging. Here we discussed several microRNAs and elucidated their targets selection and regulatory roles in thrombocytopoiesis.
In recent years, our knowledge of the highly complex molecular mechanism of the platelet production process has considerably increased. In this review, we focus on the influence of various regulatory factors in different stages of megakaryopoiesis and thrombocytopoiesis. Several critical regulators of the process, including TPO, transcription factors, Ras GTPases family, Steroid hormones, and microRNAs, have been investigated by researchers. However, new molecular mechanisms remain to be elucidated. New emerging data suggest that TPO/Mpl complex can regulate megakaryocyte development and platelet production through a series of downstream signal pathways, like JAK/STAT, Ras/Raf-1/MAPK and PI3k/Akt pathway, moreover interleukin family and IGF-1, as alternative/compensatory regulators, could play a supplementary role for TPO. It has been observed that transcription factors control lineage-specific gene expression, either as activators or repressors, to promote the development of megakaryopoiesis. A special insight is given to the Ras GTPases family that regulates the polyploidy and maturation of MKs and the formation of proplatelets. Estrogen is a steroid hormone that is a possible contributor to the regulation of proplatelet formation and platelet production by enhancing expression of GATA1 and NF-E2, and yet aromatase is the rate limiting-enzyme to control estrogen biosynthesis. The genetic expression of microRNAs displays a distinct role in thrombocytopoiesis through their target selection and regulatory role in MK physiology, like microRNA-9, microRNA-22, microRNA-125a and so on. The regulatory factors all bring complex effects during the different stages of megakaryopoiesis and thrombocytopoiesis. Currently, Eltrombopag as a thrombopoietin receptor agonist has been used the treatment of chronic immune thrombocytopenia [136], and another TPO-receptor agonist, romiplostim, can bind to the Mpl receptor on megakaryocyte precursors in bone marrow [137], and activate many of the same signal pathways as TPO, leading to an increase in platelet counts [138]. Therefore, a more detailed understanding of the various cytokines and gene expression in MKs can contribute to novel therapeutic targets exploration and insights for diseases comprehension in hemopoietic system, and promote the manipulation of platelet formation against hemopoietic diseases, such as thrombocytopenia.
AGK, Acylglycerol kinase; BM, bone marrow; C3G, CrkSH3-binding guanine nucleotide exchange factors; DMS, demarcation membrane system; ERK, extracellular signal related kinase; ETS1, E26 transformation-specific proto-oncogene 1; EVI-1, ecotropic viral integration site-1; ECT2, Epithelial Cell Transforming Sequence 2; ER, estrogen receptor; FLI1, friend leukemia virus integration 1; GATA1, GATA-binding protein 1; GEFs, guanine nucleotide exchange factors; GAPs, GTPases activating proteins; GDIs, guanine nucleotide dissociation inhibitors; Grb2, growth factor receptor-bound protein-2; HSCs, hematopoietic stem cells; HSPCs, hematopoietic stem cells/progenitor cells; IGF-1, Insulin-like growth factor-1; JAK, Janus kinase; JNK, Jun amino terminal kinase; Lyl1, lymphoblastic leukemia 1; MKs, megakaryocytes; MKP, megakaryocyte progenitor; Mpl, myeloproliferative leukemia virus; MAPK, mitogen activated protein kinase; MAP3K, MAPK kinase kinase; MAP2K, MAPK kinase; MEK, MAPK/ERK kinase; MKL1, Megakaryoblastic leukemia 1; miR-9, MicroRNA-9; miR-22, MicroRNA-22; miR-125a, MicroRNA-125a; NF-E2, Nuclear factor erythroid 2; PPF, proplatelet formation; PI3K, phosphoinositol 3 kinase; PTSx, Paris-Trousseau syndrome; RUNX1, Runt-related transcription factor 1; STAT, signal transducer and activator of transcription; SCF, stem cell factor; SRF, serum response factor; SOS, son of sevenless; SRC3, steroid receptor coactivator-3; TPO, thrombopoietin; TLR,toll like receptor; TAL-1, T-cell acute lymphocytic leukemia 1 gene; TPA, 12-O-tetradecanoylphorbol-13-acetate; 3’ UTR, 3’ untranslated region; WASp, Wiskott-Aldrich syndrome protein.
LLi and YLiu contributed to the idea for this review article. The literature search and analysis were performed by LLi, RN, ZL, YM, LLiu, DP, YC, YW, TJ and YLi. Further evidences collection, analysis and arrangement were accomplished by LLi and RN. The first draft of the manuscript was written by LLi. Then, the manuscript was critically revised by YLiu (Corresponding Author). All authors contributed in writing manuscript, reviewing and editing, and final approval.
Not applicable.
Not applicable.
The work was supported by The Chongqing Clinical Pharmacy Key Specialties Construction Project and The Chongqing Special Project for Technological Innovation and Application Development (NO. CSTC2021jscx-gksb-N0013) and Chongqing Technology Foresight and System Innovation Project (NO. cstc2020jsyj-zzysbAX0024).
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
