†These authors contributed equally.
Academic Editor: Paramjit S. Tappia
Background: Cardiovascular disease (CVD) has become one of the leading
causes of death and disability worldwide, and its incidence continues to increase
because of an aging population. Studies have shown that the function of
cardiomyocytes decreases during aging, leading to changes in the functional and
structural integrity of the heart, ultimately resulting in CVD. The decrease in
the number of functional cardiomyocytes has a negative impact on cardiac
function; thus, myocardial aging is one of the main factors that causes
heart-related diseases (such as CVD). Therefore, alleviating cardiac aging is one
of the main ways of treating aging-related cardiac diseases. In this study, we
evaluated the potential effect of taraxasterol on myocardial aging.
Methods: The effect of taraxasterol on the aging of cardiomyocytes was
analyzed in vivo and in vitro using a D-galactose treatment
mouse model of cardiomyocyte senescence. Furthermore, the effect of taraxasterol
on aging-induced desensitization of insulin signaling was also evaluated.
Results: The experimental results indicated that taraxasterol could
reduce cardiomyocyte senescence, which was evaluated using Sa-
Aging is an inevitable life process that is accompanied by physiological and
pathological changes in many tissues and organs [1]. However, with an increasing
aging population, society will face the pressure of having to deal with issues
specific to this aging population in the future [2]. Aging is accompanied by
changes in body shape and physiological functions of tissues and organs [3].
Microscopically, it is manifested as increased cell damage and slowed metabolism;
macroscopically, it is manifested as the appearance of organs and tissues aging
and the weakening of organ functions [4]. Many studies have shown that
age-related cardiovascular disease (CVD) has become the most important risk
factor affecting the health of the elderly [5]. Cardiovascular damage is a common
pathological state in heart disease [6], and it is the basis for the development
of heart disease. Heart function gradually declines with aging. Therefore,
anti-cardiovascular aging treatment can not only improve the quality of life of
the elderly, but also help reduce the morbidity and mortality associated with
CVD. Dandelion is a perennial herb. There has been a great deal of research on
the chemical constituents of dandelions, and researchers have extracted and
separated many chemical constituents, including flavonoids, carotene, pigments,
and volatile oils [7]. One of the many bioactive molecules of dandelions is
taraxasterol, which has the molecular formula C
However, up to now, the effect of taraxasterol on cardiovascular aging has not been explored. For this, a mouse model of cardiomyocyte senescence induced by D-galactose was constructed and served as an aging model to investigate the effect of taraxasterol on D-gal-induced aging of cardiomyocytes. The experimental findings illustrated that taraxasterol could significantly alleviate cardiomyocyte senescence in the in vitro cell model. Furthermore, we found that taraxasterol had the potential to alleviate cardiomyocyte senescence via the regulation of the SIRT1/p53/p21 signaling pathway. We also found that taraxasterol treatment alleviated cardiovascular aging and fibrosis in vivo. Taken together, we showed that taraxasterol could reduce cardiac aging and fibrosis, indicating that taraxasterol may be an effective drug or health food additive for treating/attenuating cardiac aging and fibrosis.
The BCA protein concentration assay kit was from
Pierce (Rockford, IL, USA). The cell counting kit-8 (CCK-8) and the
H9c2 cells were purchased from the American Type Culture Collection (ATCC) (Cat no. Crl-1446). The cells were cultured in DMEM (containing penicillin and streptomycin) with 10% fetal bovine serum (FBS). When H9c2 cells grew to a certain density, they were passaged at a ratio of 1:3. The primary human coronary artery endothelial cell line (HCAEC, PCS-100-020) was purchased from ATCC and cultured in DMEM.
Paraffin sections of heart tissue were baked in a 55 °C incubator for 30 min. The sections were dewaxed using xylene. Distilled water was used to rinse the sections, and they were stained with hematoxylin (5 min). After rinsing the sections with distilled water, they were stained with scarlet solution for 5 min and then rinsed with distilled water. The sections were incubated in phosphoaluminate phosphotungstic acid aqueous solution for 15 min. After washing, the sections were stained with aniline blue for 5 min. After the sections were washed with 1% glacial acetic acid solution and distilled water, they were dehydrated and soaked in 70% ethanol for 1 s, 95% ethanol for 1 s, and 100% ethanol for 30 s. The above process was repeated three times. The sections were placed in xylene for 3 min (and repeated 3 times). Neutral resin was used to seal the sections. The sections were observed and imaged under a microscope.
An SA-
D-gal was used to induce H9c2 cardiomyocytes to establish a cell aging model.
H9c2 cells were seeded into 6-well plates. After the cells grew to 90%
confluence, they were subcultured into a 12-well plate. When the cells grew to
30%–40% confluence, they were starved in DMEM containing 0.5% FBS for 12 h
and then replaced with DMEM without FBS. The various concentrations of D-gal were
added to induce cell aging, after which the cells were washed three times. Fresh
DMEM (plus 5% FBS) was added, and cells were cultured for three more days.
SA-
Mice were divided into two groups. The control group received drinking water only for 6 weeks, while the experimental group received taraxasterol (5.0 mg/kg body weight) given in the drinking water for 6 weeks [10].
The heart tissue or cell sample was fixed with 4% paraformaldehyde. The heart
tissue was embedded in paraffin, and then 5
The heart tissues from mice were taken out and then treated in 4% formaldehyde solution for 48 h. The fixed tissue was rinsed with running water to remove any residual fixative. The tissues were dehydrated in successively increasing percentages of ethanol: 70%, 80%, 90%, 95%, and 100%. The tissue was embedded in wax and then sectioned. The slides were incubated in hematoxylin dye for 3 min. After distilled water was used to wash tissue sections approximately 2–3 times, the slides were put into eosin staining solution for 3 min. The slides were put into 95% alcohol Ⅰ for 30 s, 95% alcohol Ⅱ for 30 s, absolute ethanol Ⅰ for 3 min, and absolute ethanol Ⅱ for 3 min. After the slides were sealed with neutral gum, the histopathological changes in the tissues were observed using a microscope.
For tissue samples, the appropriate amount of myocardial tissue was added to a
microtube, and then 1 mL Trizol was added to each microtube. The microtubes were
put in a grinder (30 Hz, 60 s) and then placed on ice. For cell samples, the
cells were washed three times with PBS. After adding 1 mL Trizol to each tube,
200
The animals used in the current experimental group (C57 mice) were purchased
from Beijing Huafukang Company (Beijing, China); 3-month-old mice (young group) and 18-month-old
mice (aged group) were chosen. The mice were placed in a clean animal room and
the temperature was maintained at 22
After the tissue/cell protein was extracted, the protein concentration was determined using a BCA kit. The protein sample was subjected to SDS-PAGE and then transferred to a PVDF membrane. The membranes were put into TBST solution containing 5% skim milk powder and incubated at room temperature for 3 h. After washing with TBST solution 2–3 times, the corresponding primary antibody was added and incubated at 4 °C overnight (the primary antibody could be recycled and reused). After washing the PVDF membrane with TBST solution for 6 min, the corresponding horseradish peroxidase coupled-IgG antibody was added and incubated at room temperature for 2–3 h. The secondary antibody solution was discarded, and the PVDF membranes were washed with TBST solution for 10 min/time (6 times). ECL chemiluminescence reagent was used as the detection agent, and the exposure time was selected according to the staining intensity of the PVDF membrane.
Cells (3
Cells were collected using centrifugation (1000 rpm). The levels of MDA and SOD were determined using an MDA/SOD kit according to the instructions provided with the kit.
Mitochondrial membrane potential was analyzed using a mitochondrial membrane potential kit according to the instructions provided with the kit.
The results of all experimental data are expressed as the mean
To induce senescence in H9c2 cells, H9c2 cells were pre-treated with various
concentrations of D-gal for 2 h (10 g/L, 20 g/L, 40 g/L, 60 g/L, and 80 g/L).
After fresh medium was added to the H9c2 cells and they were cultured for an
additional 48 h, senescence and apoptosis were evaluated. First, a CCK8 assay was
performed to evaluate cell viability. The experimental results showed that the
cell viability gradually decreased with increasing D-gal concentrations (Fig. 1A). Sa-
 Fig. 1.
Fig. 1.Establishment of the senescent cell model. (A) D-gal treatment
leads to the decline of cell viability of H9c2 cells. The cells were treated with
different concentrations of D-gal. Cell viability was detected using the CCK8
assay kit. (B) Sa-
In the pre-experiment, we used the CCK8 cell viability assay to determine the
concentration of taraxasterol (0–100
To study the effect of taraxasterol on cell aging, H9c2 cells were incubated in
D-gal at a concentration of 60 g/L to establish the cell aging model.
Sa-
 Fig. 2.
Fig. 2.Effects of Taraxasterol on cell senescence. (A)
Taraxasterol reduced the number of senescent cells induced by D-gal. (B) The
expression of p16, p21, and p53 was downregulated under taraxasterol treatment.
(C) The proportion of S phase cells was increased under taraxasterol treatment.
(D) The mitochondrial membrane potential also increased significantly in the
taraxasterol treatment group. Different low case letters above columns indicate
statistical differences at p
We examined the effect of taraxasterol on the cardiomyocyte SASP. In the D-gal
group, the mRNA expression levels of IL-1
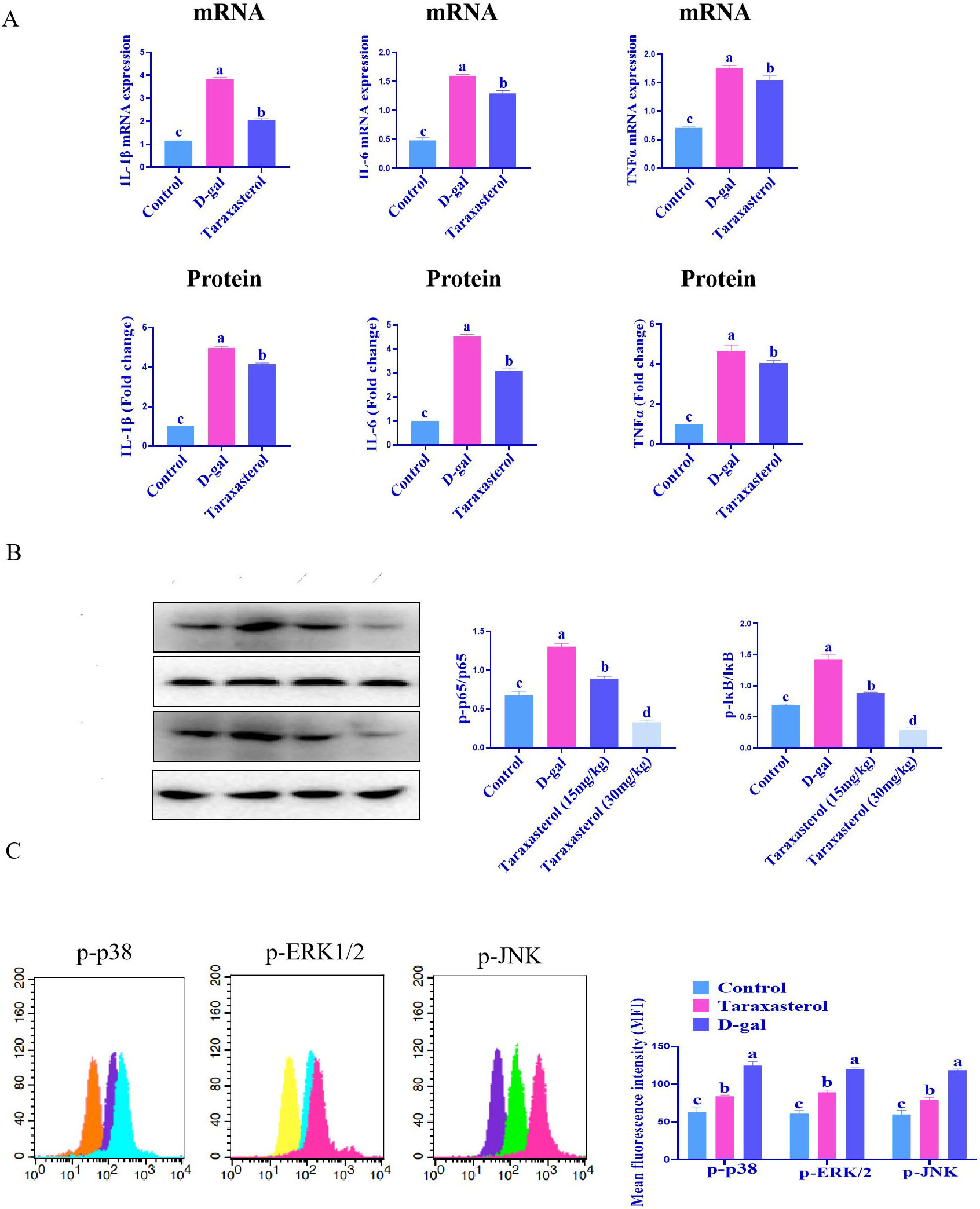 Fig. 3.
Fig. 3.Effects of Taraxasterol on SASP. (A) Taraxasterol
could inhibit the D-gal-induced cardiac SASP. (B) Taraxasterol treatment
significantly reduced the phosphorylation level of NF-
In the current work, D-gal significantly increased the levels of ROS and MDA, but the level of SOD decreased significantly. However, in the taraxasterol treatment group, the levels of ROS and MDA decreased significantly, while SOD increased significantly (Fig. 4). These experimental results show that taraxasterol can alleviate oxidative stress.
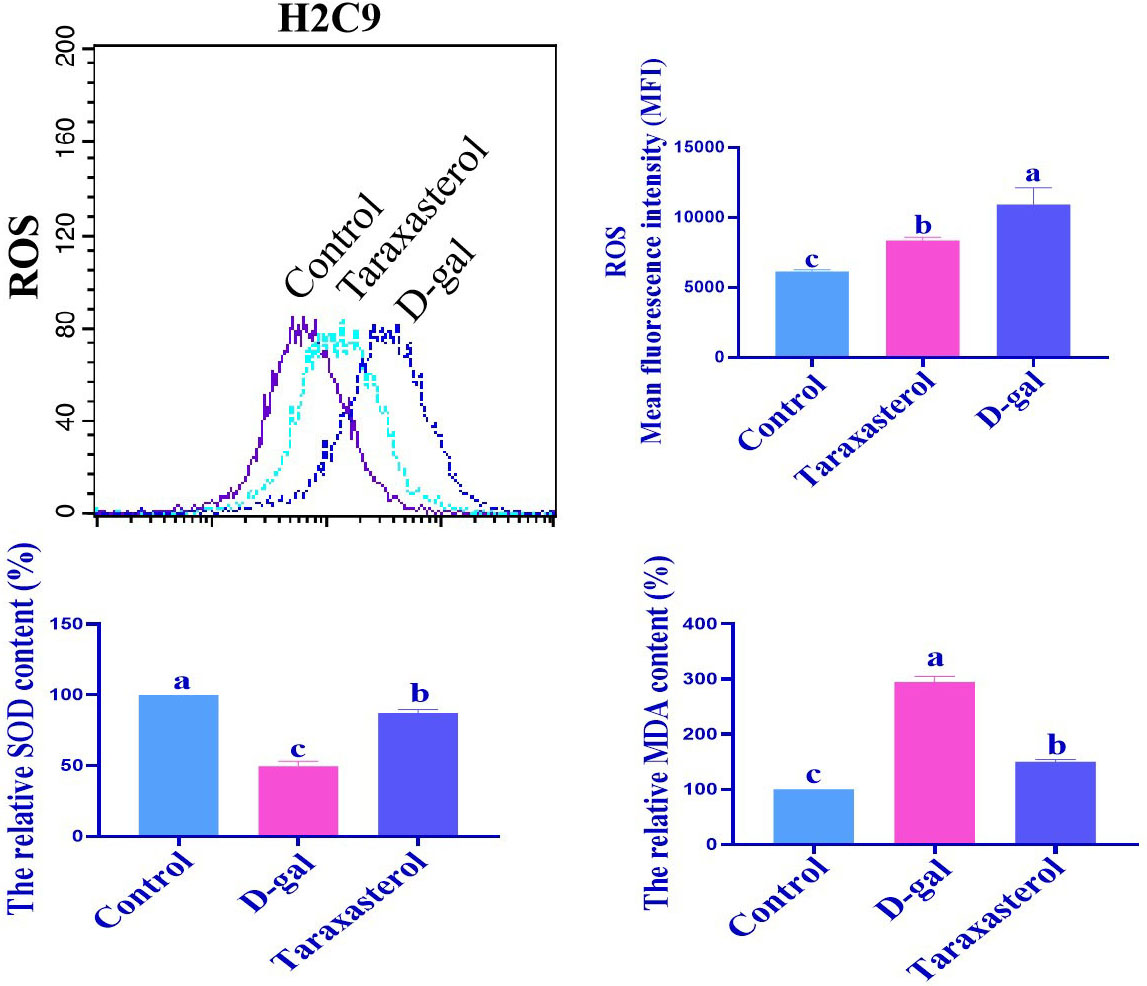 Fig. 4.
Fig. 4.Taraxasterol can alleviate oxidative stress. The levels of ROS,
MDA, and SOD were determined using the corresponding assay kit as described in
the Materials and Methods section. Different low case letters above columns indicate statistical differences at p
To determine the potential anti-aging molecular mechanism of taraxasterol, we
assessed the expression of p53 as well as its upstream regulatory factor SIRT1
and downstream factor p21 in cardiomyocytes to confirm whether taraxasterol’s
anti-aging effect may be related to the SIRT1/p53/p21 pathway. The results showed
that compared with the normal group, in the aging cardiomyocyte model group, the
expression levels of SIRT1 and cyclin D1 proteins decreased significantly, while
the expression levels of p16, p21, and p53 proteins increased (Fig. 5).
Taraxasterol upregulated the expression levels of SIRT1 and cyclin D1 proteins,
and downregulated the expression levels of p16, p21, and p53 proteins (p
 Fig. 5.
Fig. 5.Taraxasterol might delay the aging of cardiomyocytes through the
SIRT1/p53/p21 signaling pathway. p
To clarify the effect of taraxasterol on insulin resistance in aging
cardiomyocytes, western blot assays were conducted. A previous study had
demonstrated that the insulin receptor (IR)-mediated signaling pathway (signaling
proteins p-IRS-1, p-AKT, and p-GSK-3
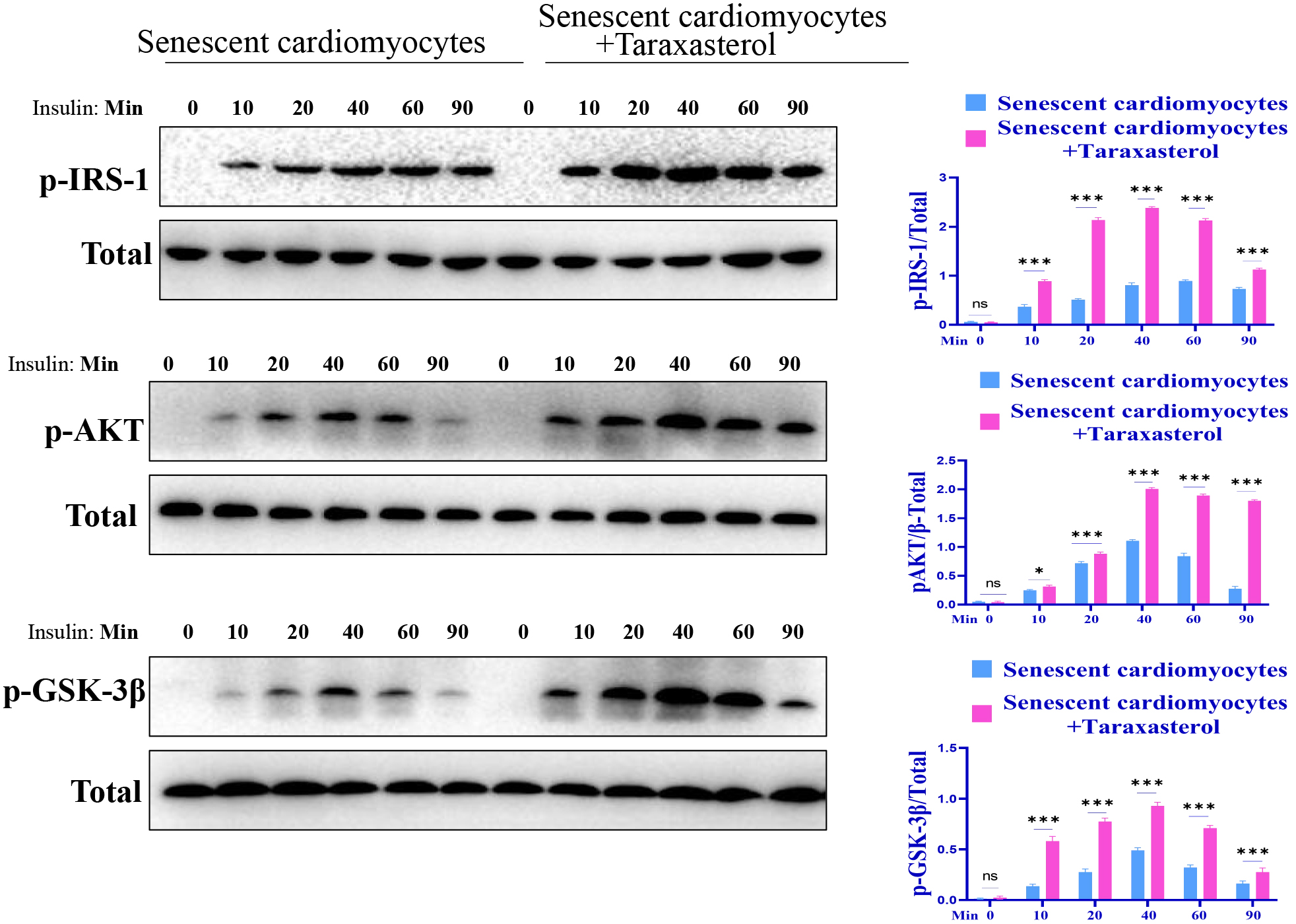 Fig. 6.
Fig. 6.Taraxasterol may improve insulin sensitivity. Different low case letters above
columns indicate statistical differences at p
We used the aged mice model in which mice were treated with taraxasterol
provided in their drinking water to study the effect of taraxasterol on aging
in vivo. We evaluated the aging of the heart in the aged mice and found
that the SA-
 Fig. 7.
Fig. 7.Evaluation of the anti-aging effect of
taraxasterol in vivo. (A) The taraxasterol treatment group showed a
significant reduction in the aging of the heart. (B) p16 and p21 stained cells
decreased significantly under taraxasterol treatment. (C) Taraxasterol treatment
inhibited the expression of proinflammatory molecules. (D) The effect of
taraxasterol on SOD, GSH, MDA, and ROS content. p
The effects of taraxasterol on SOD, MDA, and GSH in serum of aging mice were
also analyzed. The results illustrated that in aging mice, the contents of SOD
and GSH in serum were lower than those in the control group (p
In the process of aging, the heart experiences obvious pathological changes,
with one of the most important being myocardial fibrosis. Therefore, we studied
the effect of taraxasterol on myocardial fibrosis in aged mice. Masson staining
showed that the fibrotic staining area in the taraxasterol treatment group was
significantly reduced (Fig. 8A). In addition, in the taraxasterol group, the
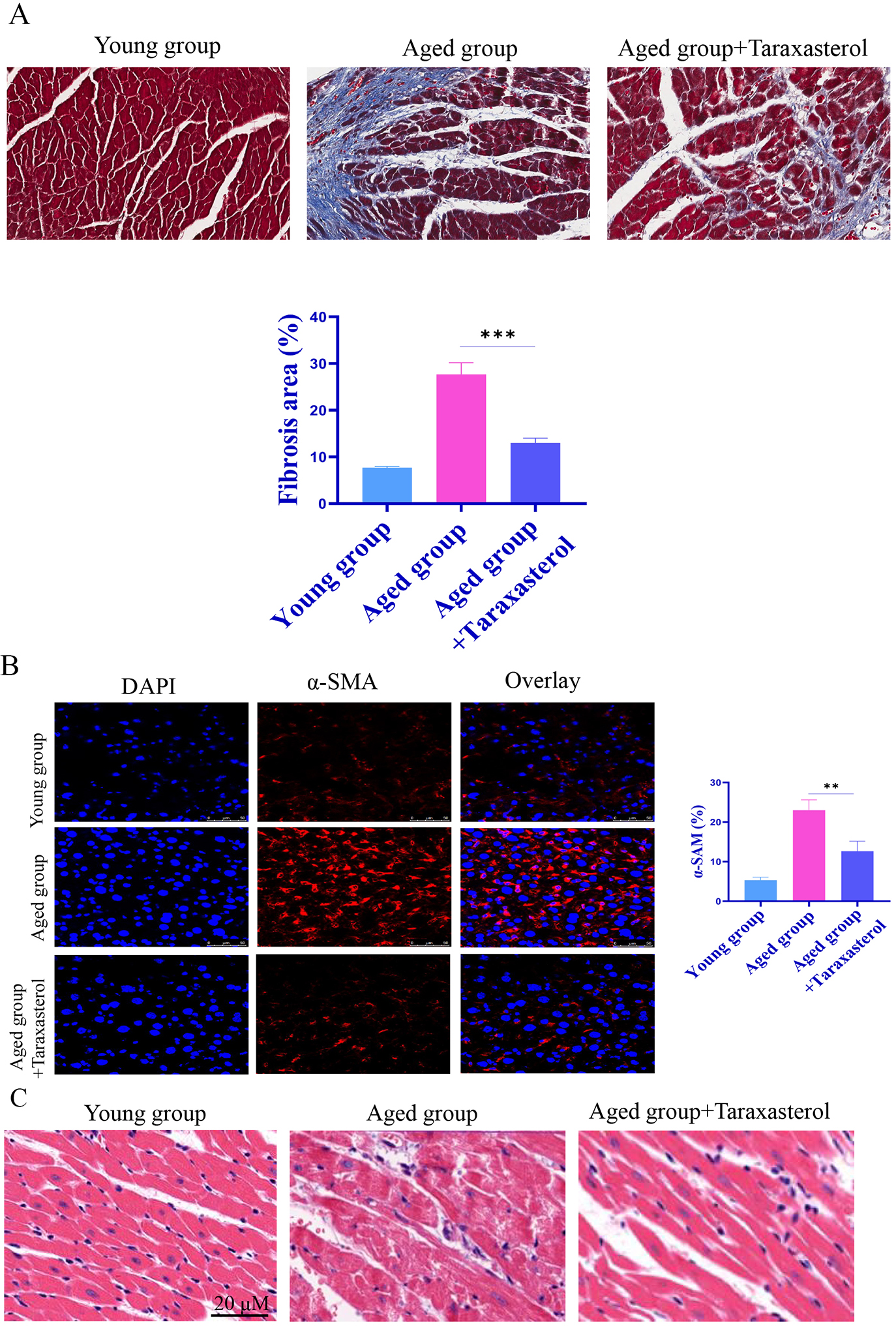 Fig. 8.
Fig. 8.Effects of Taraxasterol on myocardial fibrosis. (A)
Masson staining showed that the fibrotic staining area in the taraxasterol
treatment group was significantly reduced. (B) The
In the above-mentioned study, we analyzed the effect of taraxasterol on
cardiomyocyte aging. Here, the effect of taraxasterol on the aging of HCAEC was
studied. The HCAEC were stimulated with 60 g/L D-gal. Sa-
 Fig. 9.
Fig. 9.Effect of taraxasterol on cardiomyocyte aging. (A)
Sa-
The incidence of CVD continues to increase because of population aging, and CVD has become one of the leading causes of death and disability worldwide [12]. During myocardial aging, cardiomyocytes gradually lose their functional and structural integrity, resulting in a reduction in the number of cardiomyocytes capable of functioning normally, and ultimately leading to CVD. Cardiomyocyte aging is a key factor in the decline of cardiac function and the development of CVD such as cardiac hypertrophy and heart failure [13]. With an increasing aging population, the study of cardiomyocyte aging and its corresponding protection strategies has important clinical significance for preventing the occurrence and development of CVD. To this end, in the current study, we evaluated taraxasterol myocardial aging in vivo and in vitro and found that taraxasterol exhibited an anti-aging effect on myocardial cells, which provided a theoretical basis for intervening with or even reversing cardiomyocyte aging.
Cellular senescence is the irreversible arrest of cell growth [14]. Cellular senescence can be divided into two categories: replicative senescence and premature senescence [15]. Cellular senescence is the basic unit of biological aging and the common basis for the pathogenesis of human geriatric diseases [16]. During physiological aging, the total number of cardiomyocytes decreases. It has been suggested that the aging of cardiomyocytes is involved in many cardiovascular events, and aging cardiomyocytes exhibit many functional and structural changes. In in vitro experiments, the effect of taraxasterol on the aging of cardiomyocytes was first evaluated. Taraxasterol is a pentacyclic three-shielded compound, an active ingredient extracted and isolated from dandelions. We evaluated a series of senescence-related markers and found that taraxasterol significantly alleviated cardiomyocyte senescence. In addition, previous studies have found that taraxasterol has a range of biological activities. It has been reported that taraxasterol exhibited a protective effect on acute lung injury in mice [17]. Furthermore, taraxasterol has anti-inflammatory and anti-arthritic effects [18]. In addition, taraxasterol exhibited protective effects on ethanol-induced liver injury [19].
With aging, there is a progressive increase in the proinflammatory response in
the body. Franceschi et al. [20] were the first to name this phenomenon
inflammatory aging. Inflammatory aging is closely related to a variety of
geriatric diseases. The proinflammatory factors and anti-inflammatory factors in
the elderly change, and the final manifestation is the excessive proinflammatory
response and the imbalance of inflammatory homeostasis, which leads to
inflammatory aging. In the current study, we found that taraxasterol
significantly reduced proinflammatory factor expression (such as IL-6 and
IL-1
Oxidative stress is closely related to aging [21]. The theory of free radical aging was first proposed by Harmna, who believed that free radicals attack and destroy biological macromolecules, causing damage to tissue cells and finally leading to aging [22]. Oxidative stress refers to the imbalance between the antioxidant system and the oxidative system whereby the body’s antioxidant system is insufficient to resist and repair foreign oxidants (active oxygen free radicals and reactive nitrogen free radicals) when the body is subjected to various harmful stimuli. This results in tissue and cellular damage. In the current study, we found that taraxasterol has significant anti-oxidative stress effects in vitro and in vivo.
We further analyzed the molecular mechanism by which taraxasterol exhibited the anti-aging effect. The results showed that taraxasterol upregulated the expressions of SIRT1 and cyclin D1 and downregulated the levels of p53, p21, and p16. At the same time, taraxasterol upregulated the mRNA levels of SIRT1 and cyclin D1 and decreased the mRNA levels of p53, p21, and p16. These findings suggest that taraxasterol exhibited the anti-aging effect possibly via p21 and the p16-Cyclin D1 signaling pathway. However, the in-depth molecular mechanism still needs to be elucidated in the future.
Aging can cause myocardial fibrosis [23], and this leads to a significant increase in the incidence of and mortality from heart failure in the elderly. Aging is an inevitable process and one of the recognized causative factors of CVD. Myocardial fibrosis refers to the transformation of fibroblasts into myofibroblasts caused by various pathological factors. In CVD, the synthesis and degradation of collagen become unbalanced, the proportion of collagen is unbalanced, the arrangement of cells is disordered, and the deposition of extracellular matrix is common. Elucidating the role and mechanism of aging in myocardial fibrosis may help prevent and treat age-related CVD and improve the quality of life in elderly people. In the current study, we found that taraxasterol could inhibit myocardial fibrosis in vivo.
Studies have confirmed that aging can lead to a decline in insulin sensitivity in the heart (insulin resistance) [24]. In the current study, we found that taraxasterol could alleviate cardiac aging, so it could correspondingly increase insulin sensitivity (insulin sensitivity will significantly decrease because of cardiac aging). The results showed that taraxasterol partially improved insulin sensitivity.
The incidence of CVD such as myocardial ischemia, heart failure, and myocardial fibrosis also increases with aging [25, 26]. Therefore, scientists are looking for anti-cardiovascular aging solutions. In addition to cardiomyocytes, we also assessed the potential effect of taraxasterol on arterial aging and found that taraxasterol was able to significantly alleviate vascular endothelial cell aging.
Taken together, this work illustrates that taraxasterol could reduce cardiac aging and fibrosis, indicating that taraxasterol may be an effective drug or health food additive for treating cardiac aging and fibrosis. The current study provides a rationale for intervening in and treating cardiac aging.
CVD, cardiovascular disease; PBS, phosphate buffered saline; MMP, Mitochondrial membrane potential; IR, insulin receptor.
GL and DZ designed the research study; SW, NJ performed the research; YQ provided help and advice on the conclusions; GL and NJ analyzed the data; GL wrote the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript.
The establishment of the experimental mouse model was approved by the Animal Ethics Committee of Jiangsu College of Nursing (2021-0301).
Thanks to Dr. Wang for their guidance on this work.
This work was partially supported by the by the Huai’an Natural Science Research Plan (Guiding) Project, No.: HABZ201929.
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
