1 Department of Neurology, Shenzhen People’s Hospital (The Second Clinical Medical College, Jinan University, The First Affiliated Hospital, Southern University of Science and Technology), 518020 Shenzhen, Guangdong, China
2 Department of Medical Record Management, Shenzhen People’s Hospital (The Second Clinical Medical College, Jinan University, The First Affiliated Hospital, Southern University of Science and Technology), 518020 Shenzhen, Guangdong, China
† These authors contributed equally.
Abstract
Objective: To describe the clinical features, laboratory data, treatment, and outcomes of anti-N-methyl-D-aspartate (NMDAR) encephalitis in Chinese patients. Methods: This retrospective study included hospitalized patients definitively diagnosed with anti-NMDAR encephalitis and positive for anti-NMDAR antibodies in the cerebrospinal fluid (CSF) in Shenzhen People’s hospital, between November 2015 and February 2020. The clinical manifestation, laboratory data, treatments and outcomes were collected retrospectively. Patients were followed up for more than 1 year. Results: The study included 31 patients (15 men, 48.4%) with a median age of 31 years (interquartile range 21–48). The most common clinical presentations were psychosis (n = 23, 74.2%), seizures (n = 20, 64.5%), and memory impairment (n = 20, 64.5%). Total magnetic resonance imaging abnormalities were found in 11 patients (35.5%), with the medial temporal and frontal lobes as the most commonly involved. Abnormal electroencephalogram was observed in 16 patients (51.6%). Five out of 31 patients (19.5%) were diagnosed as neoplasm, including five females with ovarian teratoma and one male with a central nervous system tumor. Multiple immune antibodies, including anti-SSA antibody in four patients (15.4%), anti-Ro52 antibody in four (15.4%), antinuclear antibody (ANT) in four (15.4%), anti-thyroglobulin antibodies (TGAb) in five (17.2%), and thyroid peroxidase antibodies (TPOAb) in three (10.3%) were present. All patients received first-line immunization therapy (intravenous immunoglobulin, glucocorticoids, or plasmapheresis alone or combined), and only two patients (7.3%) received second-line immunization therapy (rituximab). Mechanical ventilation was more necessary in women (37.5%) than in men (6.7%) (p = 0.04), and 29 (93.5%) had favorable clinical outcomes. At more than 12 months of follow-up, the median modified Rankin Scale score decreased from 4 to 0. Conclusions: Patients with anti-NMDAR encephalitis in China had high rates of psychosis and seizures, with low rates of underlying neoplasms. A higher proportion of female patients required mechanical ventilation. Complications with other positive autoimmune antibodies were a common clinical symptoms of anti-NMDAR encephalitis. Majority of the patients obtained satisfactory outcomes in combination with early first-line and long-term immunization therapy.
Keywords
- Anti-NMDA receptor
- Encephalitis
- Treatment
- Autoimmune diseases
- Retrospective
Anti-N-methyl-d-aspartate receptor (NMDAR) encephalitis is an autoimmune disorder that develops as a rapidly progressive encephalopathy. The incidence of NMDAR encephalitis is increasing globally with the popularization of antibody testing for autoimmune encephalitis [1]. This encephalitis may be associated with neoplasms or secondary to viral infection [2]. Anti-NMDAR encephalitis is a severe and treatable disease. Being able to assess prognosis early, timely, and accurately can help to individualize treatment plans. To provide the basis for early identification and diagnosis, we retrospectively analyzed the clinical symptoms and laboratory data of anti-NMDAR encephalitis in Shenzhen People’s Hospital, a single center in Shenzhen, China.
We retrospectively enrolled patients with anti-NMDAR encephalitis at Shenzhen People’s Hospital between November 2015 and February 2020. All patients with a definitive diagnosis of anti-NMDAR encephalitis and positive for NMDAR antibodies (cell-based analysis [Euroimmun, Lubeck, Germany]) in the CSF were included [3]. Patients lacking clinical data and diagnosed with infectious encephalitis or unknown cause encephalitis were excluded.
This study was conducted retrospectively, and the data were collected by two experienced physicians. Information collected included demographic data (age, sex), and clinical characteristics: primary symptoms, clinical presentation, level of consciousness at admission, presence of tumor, requirement of mechanical ventilation, onset to treatment time, immunological therapy, and residual deficits. The absolute indication for ICU admission was a requirement for mechanical ventilation due to the hospital’s limited resources and patients’ financial problems. Mechanical ventilation was required for respiratory failure due to encephalitis, excluding teratoma surgery. Auxiliary examinations were recorded, including serum albumin levels, immune indicators (anti-nuclear antibodies, anti-neutrophil cytoplasmic antibodies, and thyroid-related antibodies), brain magnetic resonance imaging (MRI), including the sequences of T1, T2, Flair and DWI and electroencephalogram results. CSF analysis included: white blood cell count, CSF protein, percentage of mononuclear cells, pleocytosis, glucose, chloride and related autoimmune and paraneoplastic antibodies testing. Anti-NMDAR antibody titers were tested both in the serum and CSF, using the cell based assay (CBA) method. Prognostic indicators, including discharge modified Rankin Scale (mRS) score, treatment, hospitalization stay, and costs, were also recorded. All patients had received at least one systemic tumor screening at onset. The study was approved by the Research Ethics Committee of Shenzhen People’s Hospital (ID: LL-KY-2021646). Written informed consent was obtained from each subject.
We followed-up all the patients regularly for more than 12 months. The mRS score was used for evaluating the effectiveness of treatment and long-term outcomes by two experienced physicians. Based on the mRS scores, we divided the patients into two groups: good outcome groups (mRS score 0–2) and poor outcome groups (mRS score 3–6). Patients exhibiting exacerbation of symptoms or showing new symptoms after 2 months of stability were defined as being in relapse.
Statistical analysis was conducted using SPSS version 16.0 (IBM, Armonk, NY,
USA). GraphPad Prism 5.0 (GraphPad Software, Inc., San Diego,
CA, USA) was performed to generate data. Quantitative data with a normal
distribution are denoted as mean
In total, 31 patients positive for anti-NMDAR antibodies in the CSF between November 2015 and February 2020 were enrolled, and all patients met the anti-NMDAR encephalitis diagnostic criteria [3]. The onset median age was 31 (range 14–68) years (Fig. 1). 16 (51.6%) patients were females, and 15 (48.4%) were males. Psychosis (23, 74.2%), seizures (20, 64.5%) and memory impairment (20, 64.5%) were the three most common clinical presentations in this study. Mechanical ventilation was more necessary in females (6/16, 37.5%) than in males (1/15, 6.7%) (p = 0.04). Five out of 31 patients (19.5%) were diagnosed as neoplasm, including five females with ovarian teratoma and one male with central nervous system tumor. Three out of four female patients with ovarian teratomas underwent tumor removal and one refused, whereas the patient with central nervous system tumor was treated medically and later died of cerebral hernia. The median hospitalization stay was 22 days, and the average medical cost was 50,500 yuan. The detail clinical symptoms were seen in Table 1.
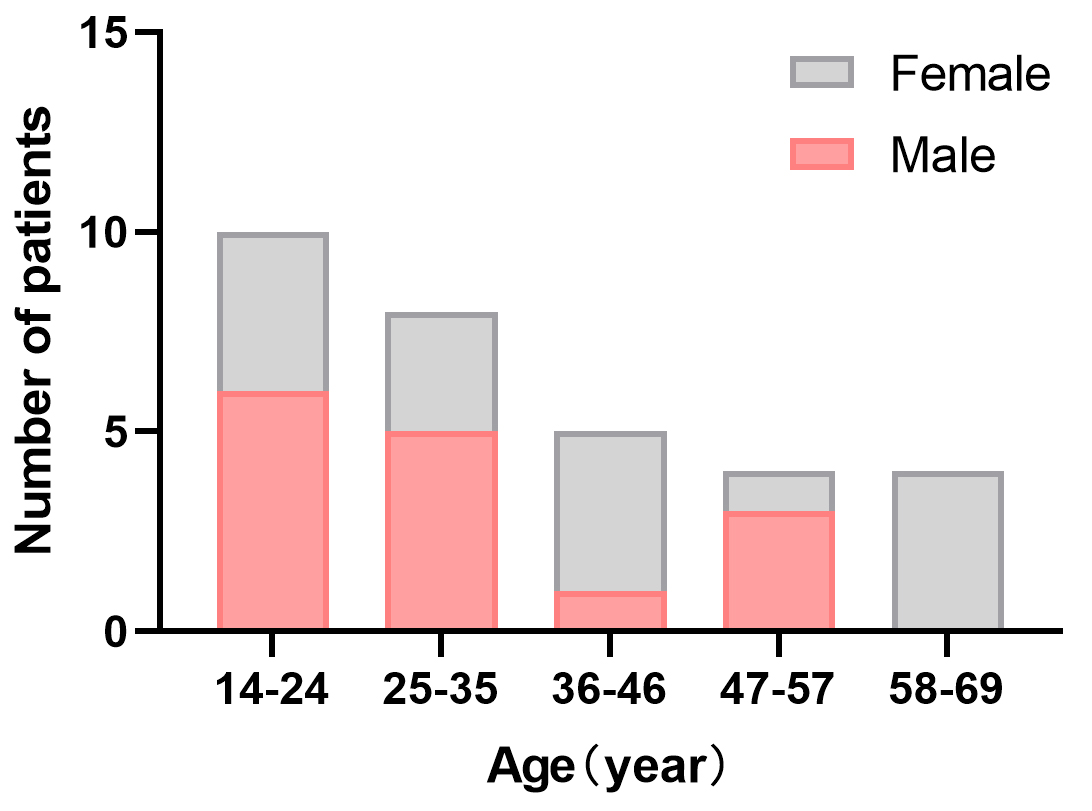 Fig. 1.
Fig. 1.Age/sex distribution of patients. The onset median age was 31 (range 14–68) years. Sixteen (51.6%) patients were females.
| Clinical symptoms | All | Male | Female | p value |
| (n = 31) | (n = 15) | (n = 16) | ||
| Median age, range (year) | 31 (14–68) | 28 (18–52) | 37 (14–68) | 0.12 |
| Psychosis | 23 (74.2) | 11 (73.3) | 12 (75) | 0.92 |
| Seizures | 20 (64.5) | 10 (66.7) | 10 (62.5) | 0.81 |
| Fever | 17 (54.8) | 9 (60) | 8 (50) | 0.58 |
| Decreased level of consciousness | 13 (41.9) | 8 (53.3) | 5 (31.3) | 0.21 |
| Memory deficit | 20 (64.5) | 8 (53.3) | 12 (75) | 0.21 |
| Speech disturbance | 7 (22.6) | 3 (20) | 4 (25) | 0.74 |
| Movement disorder | 8 (25.8) | 5 (33.3) | 3 (18.8) | 0.35 |
| Sleep disorder | 8 (25.8) | 3 (20) | 5 (31.3) | 0.47 |
| Headache | 11 (35.5) | 4 (26.7) | 7 (43.8) | 0.32 |
| Admission to the ICU | 7 (22.6) | 1 (6.7) | 6 (37.5) | 0.04 |
| Mechanical ventilation | 7 (22.6) | 1 (6.7) | 6 (37.5) | 0.04 |
| Central hypoventilation | 6 (19.4) | 1 (6.7) | 5 (31.3) | 0.08 |
| Arrhythmia | 8 (25.8) | 5 (33.3) | 3 (18.8) | 0.35 |
| Limb weakness | 2 (6.5) | 1 (6.7) | 1 (6.7) | 0.96 |
| Hospitalization expenses (ten thousand yuan) | 5.05 (3.13, 11.10) | 4.56 (1.70, 6.40) | 6.78 (3.65, 13.58) | 0.06 |
| Hospital stay (days) | 22 (14, 32) | 17 (13, 25) | 27 (17, 32) | 0.18 |
| Baseline mRS score |
26 (83.9) | 12 (80.0) | 14 (87.5) | 0.65 |
| Baseline mRS score |
23 (74.2) | 10 (66.7) | 13 (81.3) | 0.43 |
| Relapsed patient (n, %) | 4 (12.9) | 3 (20) | 1 (6.3) | 0.25 |
| Abbreviation: mRS, Modified Rankin Scale. | ||||
Brain MRI was performed for all patients at diagnosis. The brain MRIs with fluid-attenuated inversion recovery (FLAIR) sequence for 11 patients (35.5%) were abnormal, six of whom (19.4%) had lesions in the medial temporal lobe. Other such involved areas were the cerebellum, brainstem, frontal, parietal, and occipital cortices, and diencephalon, as in previous reports [4]. Two out of 31 patients (6.5%) were found with demyelinating lesions, including being positive for myelin oligodendrocyte glycoprotein (MOG) antibody, were diagnosed with multiple sclerosis. Abnormal electroencephalogram (EEG) presentation were found in 16 (51.6%) patients: 14 (45.2%) had slow activity and seven (22.6%) had epileptic discharges, showing no significant differences between males and females. Multiple immune antibodies, including SSA in four patients (15.4%), Ro52 in four (15.4%), ANT in four (15.4%), TGAb in five (17.2%), and TPOAb in three (10.3%), were present. Table 2 shows the test results of the patients.
| All | Male | Female | p value | ||
| (n = 31) | (n = 15) | (n = 16) | |||
| Brain MRI | |||||
| Total with abnormal findings | 11 (35.5) | 4 (26.7) | 7 (43.8) | 0.32 | |
| Medial temporal lobe | 6 (19.4) | 1 (6.7) | 5 (31.3) | 0.08 | |
| Frontal lobe | 4 (12.9) | 1 (6.7) | 3 (18.8) | 0.32 | |
| Parietal lobe | 1 (3.2) | 0 (0) | 1 (6.3) | 0.52 | |
| Occipital lobe | 1 (3.2) | 0 (0) | 1 (6.3) | 0.52 | |
| Diencephalon | 2 (6.5) | 1 (6.7) | 1 (6.3) | 0.96 | |
| Cerebellum | 2 (6.5) | 0 (0) | 2 (12.5) | 0.16 | |
| Brainstem | 2 (6.5) | 1 (6.7) | 1 (6.3) | 0.96 | |
| Demyelinating lesion | |||||
| MS | 1 (3.2) | 0 (0) | 1 (6.3) | 0.52 | |
| MOG (+) | 1 (3.2) | 1 (6.7) | 0 (0) | 0.48 | |
| EEG | |||||
| Total with abnormal findings | 16 (51.6) | 7 (46.7) | 9 (56.3) | 0.59 | |
| Epileptic discharges | 7 (22.6) | 3 (20) | 4 (25) | 0.74 | |
| Slow activity | 14 (45.2) | 6 (40) | 8 (50) | 0.58 | |
| Other antibody tests | |||||
| SSA (+) |
4 (15.4) | 2 (18.2) | 2 (13.3) | 0.74 | |
| Antinuclear antibody (+) |
4 (15.4) | 2 (18.2) | 2 (13.3) | 0.74 | |
| Ro52(+) |
4 (15.4) | 2 (18.2) | 2 (13.3) | 0.74 | |
| TGAB (+) |
5 (17.2) | 2 (15.4) | 3 (18.8) | 0.81 | |
| TPOAB (+) |
3 (10.3) | 0 (0) | 3 (18.8) | 0.10 | |
| Abbreviation: | |||||
All patients underwent lumbar puncture, and CSF results before immunotherapy
were collected. The results showed a median opening pressure of 170 (IQR
135–195) mmH
| CSF analysis | Median (IQR) | Number (percentage) | |
| Opening pressure (mmH |
170 (135–195) | ||
| WBC (×10 |
20 (4–41) | ||
| Pleocytosis | 21 (67.7) | ||
| Percentage of mononuclear cells (%) | 95 (77.7–99.5) | ||
| Protein (g/L) | 0.30 (0.21–0.42) | ||
| Protein (g/L) |
8 (25.8) | ||
| Glucose (mmol/L) | 3.60 (2.3–5.6) | ||
| Chloride (mmol/L) | 123 (101–133) | ||
| Abbreviation: CSF, cerebrospinal fluid; WBC, white blood cell. | |||
The average time from symptom onset to confirmative diagnosis was 15 days (IQR 7–26 days). Once the diagnosis was established, immunological therapy was started immediately, and sometimes empirical treatment began even before the diagnosis. The median days of hospital stay were 22 (IQR 14–32) days, while seven patients requiring intensive care were hospitalized for up to 75 days. Overall, 31 patients (100%) obtained first-line immunization therapy, with most receiving combination therapy of steroids and immunoglobulin (IVIG). All patients (100%) received steroid therapy, including 21 patients (67.7%) who received pulsed intravenous methylprednisolone and 10 patients who received low-dose intravenous dexamethasone. Twenty-six (83.9%) patients received IVIG, two (6.5%) underwent PE, two (6.5%) received RTX, and one (3.2%) received AZA. In general, only a small number of patients were given second-line immunotherapy, and low-dose steroids lasted for half a year.
Overall, 29 patients (93.5%) improved, and two patients (6.5%) died after
treatment in the acute period. Two of the patients underwent antibody monitoring,
and the improvement of clinical symptoms was consistent with the decrease of NMDA
antibody concentration both in CSF and blood. We have followed up the 29 survival
patients for at least 1 year (range 12–60 months). Four (12.9%) patients
experienced relapse, of whom three (75%) were female and one (25%) had abnormal
EEG. Their median age was 20 years (IQR 15–21). All relapsed patients were
re-treated with first-line immunization therapy and achieved favorable outcomes.
At more than 12 months of follow-up, 28 (90.3%) patients had obtained
satisfactory outcomes, including mRS scores of 0 in 26 patients, mRS scores of
1–2 in two patients, compared with five (16.1%) patients with an mRS score
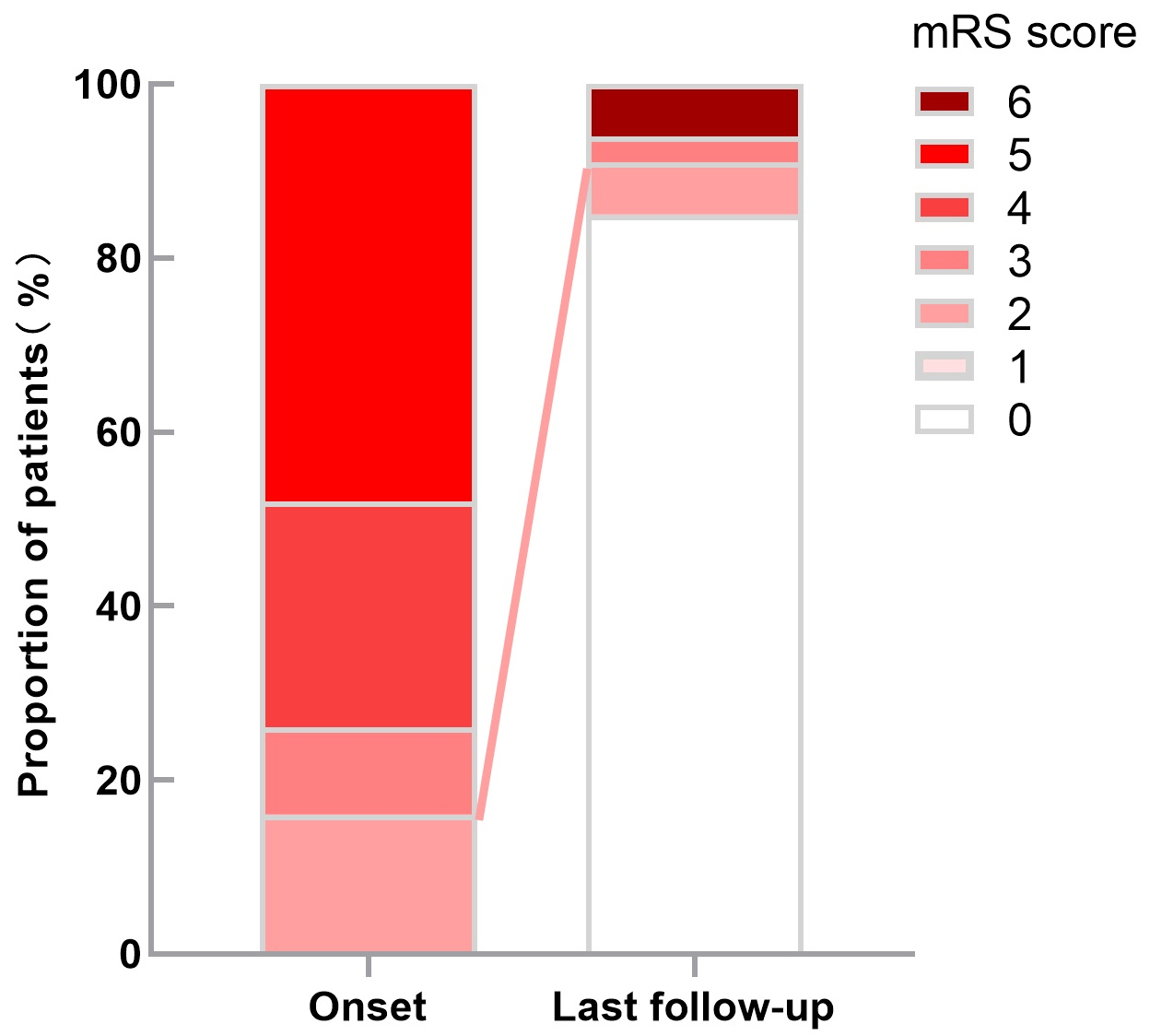 Fig. 2.
Fig. 2.The scores of mRS distribution at admission and last follow-up.
At more than 12 months of follow-up, 28 (90.3%) patients had obtained
satisfactory outcomes, including mRS scores of 0 in 26 patients, mRS scores of
1–2 in two patients, compared with five (16.1%) patients with an mRS score
This study shows that anti-NMDAR encephalitis more frequently occurs at a young age. Previous studies have demonstrated that the age of onset of NMDAR encephalitis is younger than 35 years, with a median age of onset between 21 and 28 years [4, 5]. Shenzhen, one of the fastest growing cities in China, is dominated by young people. As the population increases, the incidence of anti-NMDAR encephalitis is increasing in Shenzhen. In this study, the proportion of anti-NMDAR encephalitis was equal between men and women, and the median age of onset was 31 years, which may be due to the fact it typically occurs in younger adults. Furthermore, we found that psychosis and cognition defects were the most common symptoms, which is consistent with the results of previous data [4, 6], remaining that mental and cognitive symptoms anti-NMDAR encephalitis should be cause for concern. In addition, behavioral disorders such as stress disorder, emotional instability, and agitation are more prominent in anti-NMDAR encephalitis patients [7, 8], compared with primary psychiatric patients. Psychobehavioral disorders and cognitive dysfunction can be long-term coexistence [9, 10, 11]. The severity of other psychiatric symptoms of anti-NMDAR encephalitis varies. Most symptoms such as personality changes, anxiety and irritability are mild, but can also be accompanied by persistent depression that may even lead to suicide [12, 13]. Therefore, it is necessary to conduct rigorous neuropsychological monitoring of these patients after immunotherapy and pay attention to distinguishing patients from relapsed patients.
Tumors are one of the important causes of anti-NMDAR encephalitis, and the prevalence of tumors varies in different studies. Thirty-eight percent of patients have been reported to have tumors, and 45% of Asian patients are more likely to have teratomas [4]. Guan et al. [14] reported that only 19.5% of patients have tumors and 29.4% of the females have ovarian teratomas. In our study, only 16.1% of patients have tumors and 25% of females had ovarian teratomas. Studies in Asia also reported low prevalence of tumors [15, 16]. The differences between the studies may be due to sample size, as well as or selection bias, which require further investigation. Besides tumors, it has been reported that herpes simplex virus (HSV) infection is another possible trigger. One case of anti-NMDAR encephalitis in our study was clearly diagnosed as HSV infection 6 months before onset by next-generation sequencing. However, the gold standard for a definitive diagnosis of infection is polymerase chain reaction testing. For this validation is relatively expensive and time-consuming, empirical treatment is started prior to confirmation of a definitive diagnosis of HSV infection in most patients. As a result, anti-NMDAR encephalitis after HSV could not be evaluated in our study due to the number of cases and the intensity of exclusion.
Notably, Guan et al. [14] reported that 5% of patients with anti-NMDAR encephalitis have overlapping demyelination, besides tumor and infection factors. Maarten et al. [17] reported the overlap of anti-NMDAR encephalitis and demyelinating diseases. Demyelinating diseases can occur before or after anti-NMDAR encephalitis, or at the same time. These finding suggest the existence of two simultaneously active immune mechanisms at the same time. We found two cases of overlapping syndrome in our study, one with MS [18], and the other with a positive MOG antibody. Demyelination-related diseases, including AQP-4 antibody and MOG antibody-related and MS, may be a potential cause of anti-NMDAR encephalitis. Among these patients, immunological studies usually show that these diseases are independent, but co-existing immune mechanisms, such as AQP4 and MOG antibodies, are related to NMDAR antibodies. In addition, the outcome data indicate that compared with anti-NMDAR encephalitis, demyelination episodes are more difficult to be treated, often leading to residual defects, highlighting the importance of timely diagnosis and treatment. ANA antibodies are associated with autoimmune diseases, which may be related to the disease activity of MS [19] and NMOSD [20]. Our research showed that some patients have elevated levels of SSA, Ro52, antinuclear antibodies, TPOAB, and TGAB, and the relationship between antibodies and disease activities still needs further investigation.
The findings of brain MRI further confirm that anti-NMDAR encephalitis is a “diffuse encephalopathy” [6]. Guan et al. [14] reported that 35.9% of patients have abnormal signals, mainly in the temporal, frontal, parietal, and occipital lobes. In our study, 35.5% of patients had abnormal signals mainly in the temporal and frontal regions. Other brain areas affected by anti-NMDAR encephalitis were the cerebellum, brainstem, frontal, parietal, and occipital cortices, and diencephalon. Abnormal EEG is one of the criteria for the diagnosis of anti-NMDAR encephalitis, and the change in EEG is closely related to brain injury severity. The most the common EEG manifestations of anti-NMDAR encephalitis are diffuse slow waves [3, 21]. It is a possible that NMDAR specifically binds to anti-NMDAR antibodies played a role in the mechanism, leading to cell depolarization shorten [22], and causing diffuse slow waves. Meanwhile, another important cause is that the loss of afferent impulses in the cortex lead by subcortical lesions [14]. In our study, abnormal EEG findings were found in 16 (51.6%) patients, including 14 (45.2%) patients, showing no significant differences compared with males and females. The proportion of slow waves is slightly lower than that reported in literature [23], which may be related to the severity of the disease.
Previous studies had shown that intravenous immunoglobulin was common chosen
following glucocorticoids. In addition, therapies including at least 2 forms of
immunotherapy had a higher efficacy rate than therapy using only one form [24].
In our study, repeated first-line immunotherapy occurred frequently in the
treatment of anti-NMDAR encephalitis. However, only a minority of patients
received second-line immunotherapy because of the cost, hospitalization
requirements, concerns about adverse effects, and the off-label drug use of RTX
in China, similar to previous reports [14]. In a cohort study, Titulaer
et al. [4] reported that 435 of 563 patients (75%) with mRS scores
The relapse rates were approximately 36.3% reported by Guan et al. [14] and 12.9% (4/31) in this study, which was significantly lower than that reported in the literature. This may be associated with the absence of a unified standard for the definition of relapse. Some studies are based on clinical findings. Nevertheless, a thorough examination is required to rule out other diseases and confirm the diagnosis. At present, there is no ideal clinical index to detect and evaluate the recurrence of anti-NMDAR encephalitis, for MRI is usually inconspicuous and serum antibody titers did not match the disease severity perfectly [25]. What’s more, some recurrent antibodies only could be tested in the CSF [25], which is difficult to achieve continuous CSF during follow-up.
The limitations of this study include its small sample size, and single center and retrospective nature. We have only a few patients undergoing dynamic monitoring of NMDA antibody concentration. More rigorous research is needed to study the relationship between the changes in antibody concentration, recurrence and therapeutic effects. Investigating and validating more clinical features are necessary in large sample sizes and prospective multicenter studies in the future.
In this study, we describe the clinical features, immunology treatment protocol, clinical outcomes, and long-term prognosis of patients with single-center anti-NMDAR encephalitis in Shenzhen, China. Patients with anti-NMDAR encephalitis in China had high rates of psychosis and seizures, with a low rate of underlying neoplasms. A higher proportion of female patients required mechanical ventilation. Complications with other positive autoimmune antibodies were a common clinical symptoms of anti-NMDAR encephalitis. Majority of the patients obtained satisfactory outcomes in combination with early first-line and long-term immunization therapy. Although recurrences are relatively common, most of treatment outcomes are satisfactory.
QHX: Conceptualization, Data Curation, Formal Analysis, Writing—Original Draft. YZ, QW, JH, FJM, SLZ, SYC, CYZ, MG, ZWL, XGL: Data Curation, Validation. XJF, YH: Conceptualization, Data Curation, Writing—Review & Editing.
Written informed consent was obtained from each subject. The study was approved by the Research Ethics Committee of Shenzhen People’s Hospital, code ID: LL-KY-2021646.
Not applicable.
This research received no external funding.
The authors declare no conflict of interest.


