Background: Osteosarcoma is a common bone tumor with extremely high malignancy, occurring mostly in children and adolescents. At present, the survival rate of osteosarcomas has made progress in some aspects; however, this can only be regarded as a partial success because substantial progress has not been made in the last few decades. Object: The kinesin superfamily is a group of proteins that play regulatory roles in various metabolic processes and are closely related to tumor metastasis. Increasing evidence shows that kinesins play key roles in the occurrence and development of human cancer. Purpose: This review summarizes the roles of the kinesin superfamily proteins in osteosarcoma and related functions.
Osteosarcoma is one of the most common primary malignant bone tumors, with a 5-year survival of 70% to 80% [1]. It usually occurs in adolescents aged between 10 and 25, and its prevalence is slightly higher in men than in women [2]. Osteosarcoma is a rare disease but is still considered the third most common cancer owing to its high metastasis and mortality rates [3, 4]. The focal feature of osteosarcoma is that approximately 80%–90% of tumors grow in the long bones of the limbs, 60% of which are located around the knees, although it may also occur in other bones such as the femur, tibia, fibula, humerus, ulna, and pelvis [5]. The malignancy of osteosarcoma is characterized by a high rate of metastases. Approximately 85%–90% of osteosarcomas are transferred to the lungs, 8%–10% are transferred to the bones, and a few are transferred to the lymph nodes [6, 7, 8]. At present, patients with osteosarcoma are treated by removing the primary lesions, removing small lung metastases, and inhibiting lung metastasis, combined with multi-drug chemotherapy using cisplatin, carboplatin, etoposide, and isocycline [9, 10]. In addition, phosphoramide, doxorubicin, and high-dose methotrexate can reduce the malignant transformation of primary tumors, but approximately 50% of osteosarcoma patients still relapse [11]. The prognosis of patients with primary osteosarcoma and lung metastases is still very poor, and the 5-year overall survival rate is only 25%. Therefore, it is important to continue studies on the mechanism of osteosarcoma and discover novel treatment methods and target molecules.
The kinesin superfamily is a class of conserved microtubule-dependent molecular motor proteins with adenosine triphosphatase (ATPase) activity and motor properties [12]. Studies have shown that abnormal expression of the kinesin superfamily proteins (KIFs) is related to the development and progression of various human cancers [13, 14]. For example, Wang et al. [15] showed that KIF2A is highly expressed in osteosarcoma, and inhibiting the expression of KIF2A can effectively prevent the spread and migration of tumors and the invasion of osteosarcoma cells in vitro, as well as block tumor growth and metastasis in mice [16]. Gu et al. [17] showed that KIF18B is highly expressed in osteosarcoma tissues and cells and that inhibiting the expression of KIF18B significantly inhibits the proliferation, migration, and invasion of osteosarcoma cells, as well as the tumor formation ability. Other studies have shown that KIFs can be used as biomarkers for the diagnosis and treatment of osteosarcoma. This review emphasizes the importance of KIFs in the pathobiology of osteosarcoma and discusses their potential clinical uses.
KIFs were first discovered in 1985. To date, 45 mouse- and human-derived KIFs have been identified, and they have been classified into 14 families (kinesin-1 to kinesin-14) according to their molecular characteristics [18, 19]. The original kinesin, also known as KIF5/kinesin-1, is a tetramer protein consisting of two motor active heavy chains (110–120 kDa) and two light chains (60–70 kDa). All KIFs heavy chains have a highly conserved motor domain, including ATP binding sequence and microtubule binding sequence, which can bind to ATP, hydrolyse ATP, and transfer chemical energy, enabling KIFs to carry out mechanical movement along microtubules, while by combining with specific cargo, including vesicles, organelles, macromolecules, chromosomes and spindle fibres through light chains [20, 21]. Thus, the KIFs protein is involved in a variety of biological functions within the cell.
KIFs participate in a variety of biological functions in cells, including mitosis, meiosis, membrane transport, mRNA and protein transport, signal transduction, and microtubule transport [12, 22, 23]. A growing body of evidence indicates the importance of KIFs in regulating many physiological events, including brain function, developmental patterns, and even tumorigenesis [19]. Abnormal expression of KIFs plays a key role in the occurrence and development of various human cancers, by affecting the even distribution of chromosomes, leading to changes in genetic material during mitosis and the formation of abnormal spindles, thereby resulting in cytokinesis defects and mitotic arrest. Defective genetic material induces multiple functional defects in daughter cells, which may promote tumorigenesis and tumor cell invasion and metastasis [14, 24]. Therefore, a better understanding of the function of KIFs may help to develop drugs for molecular targeted therapies against various human cancers.
Abnormal expression and functions of KIFs are closely related to the development of a variety of human cancer types (Table 1, Ref. [25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64]). KIF5B, a member of the kinesin-1 family, is mainly involved in lysosomal membrane and mitochondrial transport. KIF5B is highly expressed and may be used as a diagnostic marker in neurofibromas, breast cancer [25], lung cancer [26], bladder cancer [65], skin cancer [66], and gastric cancer [67].
| Kinesins | Member (s) | Expression in cancer | Tumor type |
| Kinesin-1 | KIF5B | High | Neurofibromatosis [27] |
| Breast cancer [25] | |||
| Lung cancer [26] | |||
| Kinesin-2 | KIF3A, KIF3B | High | Lung cancer [28] |
| Brain tumor [29] | |||
| Breast cancer [30] | |||
| Colorectal cancer [31] | |||
| Bladder cancer [32] | |||
| Kinesin-3 | KIF1A, KIF1B KIF14 | High | Brain tumor [33] |
| Colon cancer [34] | |||
| Oral cancer [35] | |||
| Kinesin-4 | KIF4A, KIF7 | High | Colorectal cancer [36] |
| Lung cancer [37] | |||
| Gastric cancer [38] | |||
| Oral cancer [39] | |||
| Breast cancer [40] | |||
| Kinesin-5 | KIF11 | High | Breast cancer [41] |
| Colorectal cancer [42] | |||
| Gastric cancer [43] | |||
| Ovarian cancer [44] | |||
| Oral cancer [45] | |||
| Kinesin-6 | KIF20B, KIF23 | High | Oral cancer [46] |
| Breast cancer [47] | |||
| Colorectal cancer [48] | |||
| Bladder cancer [49] | |||
| Pancreatic cancer [50] | |||
| Kinesin-7 | KIF10 | High | Liver cancer [51] |
| Breast cancer [47] | |||
| Kinesin-8 | KIF18A | High | Prostate cancer [52] |
| Gastric cancer [53] | |||
| Lung cancer [54] | |||
| Breast cancer [55] | |||
| Liver cancer [56] | |||
| Kinesin-10 | KIF22 | High | Breast cancer [57] |
| Kinesin-11 | KIF26B | High | Breast cancer [58] |
| Osteosarcoma [59] | |||
| Kinesin-12 | KIF15 | High | Breast cancer [60] |
| Osteosarcoma [61] | |||
| Kinesin-13 | KIF2C | High | Breast cancer [47] |
| Gastric cancer [62] | |||
| Colorectal cancer [63] | |||
| Kinesin-14 | KIFC1, KIFC3 | High | Breast cancer [64] |
KIF3A and KIF3B are members of the kinesin-2 family. Kinesin-2 is a heterotrimeric complex composed of a KIF3A/3B heterodimer and KAP3. KAP3 can bind KIF3A/KIF3B to other functional proteins, such as adenomatous polyposis coli and breast tumor kinase, which are involved in regulating the occurrence of tumors [14, 68]. Kinesin-3 family proteins act as organelle transporters. There are three members in this family, namely KIF1A, KIF1B, and KIF14, and these are mainly involved in the transport of mitochondria and synaptic vesicles [69, 70]. KIF1B plays a key role in nerve cell apoptosis; low expression of KIF1B can protect nerve cells and exert an anti-apoptotic effect, and therefore, low expression of KIF1B is positive for the 5-year overall survival rate and metastasis-free survival rate of breast cancer patients [71].
KIF4A is a member of the kinesin-4 family and participates in the regulation of mitosis and cytokinesis of eukaryotic cells. Cells cannot complete mitosis and cytoplasmic separation in the absence of KIF14. This suggests that targeting KIF14 may be a new cancer treatment strategy. Additionally, KIF14 is a prognostic marker of breast cancer. At present, changes in the expression of KIF4A can be observed in different types of human cancers. For example, Narayan et al. [72] found that the expression of KIF4A mRNA in cervical cancer was much higher than that in normal tissues. Taniwaki et al. [37] demonstrated that inhibiting the expression of KIF4A in non-small cell lung cancer cells inhibited their growth and that patients with tumors with high KIF4A expression had a poorer prognosis than patients with tumors expressing KIF4A. KIF7 is another kinesin-4 family member. KIF7 is an effective inhibitor of the Hedgehog pathway [73] and is activated in a variety of tumors. Recent studies have shown that blocking the Hedgehog pathway may be a treatment-related cancer strategy [74, 75].
KIF11 is a member of the kinesin-5 family and is overexpressed in breast, colorectal, gastric, ovarian, and oral cancers. Overexpression of KIF11 is associated with cancer staging and recurrence [76, 77, 78]. For example, in laryngeal squamous cell carcinoma, high KIF11 expression is associated with lymph node metastasis, TNM staging, and poor prognosis [79]. In oral cancer, significantly high expression of KIF11 is associated with a shorter survival time [45].
KIF20B and KIF23 are kinesin-6 family members and have been identified as potential biomarkers that promote the progression of multiple cancers [46, 47, 48, 49, 50]. The kinesin-7 family member KIF10 can be used as a survival and prognostic biomarker as well as a potential therapeutic target for liver [51] and breast [47] cancers. The expression of KIF18A is abnormally increased in most cancer cells. Overexpression of KIF18B is associated with poor prognosis of liver cancer [80, 81]. Furthermore, KIF18B is an oncogene of cervical cancer [82]. Heriberto et al. [57] showed that KIF22 is overexpressed in hyperplastic breast cancer tissues and can also be used as one of the biomarkers of breast cancer. KIF26B plays an important role in kidney development and is involved in the occurrence and development of certain types of tumors, including breast cancer, esophageal adenocarcinoma, and colorectal adenomatous polyposis [58, 83, 84, 85]. KIF2C is significantly upregulated in gastric and colon cancer tissues, and the 5-year overall survival rate of gastric and colon cancer patients with high expression of KIF2C is much lower than that of patients with low expression of KIF2C [62, 63]. KIFC1 overexpression in breast cancer cells increases paclitaxel resistance, and the combination of KIFC1 inhibitors with paclitaxel is a novel treatment for breast cancer [64].
Since multiple members of the KIFs have tumorigenic properties, scientists were keeping research the specific inhibitor. Eg5, the protein is encoded by the gene KIF11 which involved in the progression and development in a variety of tumor types. Monastrol as a small molecules blocks tumor cell proliferation growth by targeting specifically Eg5 and not microtubules [86]. This finding was significant. Some traditional chemo drugs based on microtubule targeting agents, such as taxol, are associated with severe side effects [87, 88], since all cells, healthy and tumor cells, need microtubule functions. Therefore, a variety of Eg5 inhibitors have been developed, a certain of them have entered clinical trials, including Arry-520 (Sponsor: Array Biopharma) [89, 90, 91, 92]; LY2523355 (Sponsor: EliLilly) [93, 94]; 4SC-205 (Sponsor: 4SC); ALN-VSP02 (Sponsor: Alnylam) [95]; ispinesib (Sponsor: GSK) [96, 97, 98, 99, 100]; AZD4877 (Sponsor: AstraZeneca) [101, 102]; SB-743921 (Sponsor: Cytokinetics) [103]; ARQ 621 (Sponsor: ArQule); MK-0731 (Sponsor: Merck) [104]. Moreover, as the development of drug resistance, the strategies of reversing drug resistance were researching, and alterations in tubulin interactions are an important aspect of these strategies. Kinetin modulates docetaxel resistance by interacting with microtubules [13], and thus kinesin is assumed to be the main target of cancer chemotherapy. However, the mechanisms of PTX- and docetaxel resistance and their relationship with kinesin remain to be elucidated [64].
As noted previously, there are 14 KIFs [12, 18] that are mainly involved in formation of the intracellular spindle in cells, chromosomal reorganization and arrangement, and cytokinesis [105]. The abnormal expression and functions of KIFs are closely related to the development of a variety of human cancers. Increasing evidence shows that KIFs may be used as molecular therapeutic targets for human cancers. Furthermore, the detection of KIFs expression in human cancers may provide biomarkers suitable for early detection and prognosis of human cancers [24].
At present, there are relatively few studies on KIFs in osteosarcoma. Available literature suggests that KIFs may be used as diagnostic biomarkers and targets for the treatment of osteosarcoma; however, it is necessary to further study the roles of KIFs in osteosarcoma to discover novel markers for the clinical diagnosis and identify novel targets for the prognosis and treatment of osteosarcoma. Based on the important roles of KIFs in various cancers, we have summarized the functional roles of KIFs in osteosarcoma in Table 2 (Ref. [16, 17, 59, 61, 106]) and the following sections.
| Kinesins | Member (s) | Preclinical or clinical model of OS | Expression in osteosarcoma | Functions |
| Kinesin-2 | KIF3B | 143B, MG-63, U2OS, SOSP9607, SJSA-1, and HOS | High | Proliferation, migration, invasion [106] |
| Kinesin-11 | KIF26B | SJSA-1, G-292, Nude Mouse Tumor Model | High | Proliferation and tumorigenesis [59] |
| Kinesin-12 | KIF15 | MNNG/HOS, U2OS, human osteosarcoma tissue | High | Proliferation, migration, invasion [61] |
| Kinesin-13 | KIF2A | MG-63 and U2OS human osteosarcoma tissue | High | Proliferation, migration, invasion, tumorigenesis [16] |
| Kinesin-8 | KIF18B | HOS, U2OS, and Saos-2, human osteosarcoma tissue, Nude Mouse Tumor Model | High | Proliferation, migration, invasion, tumorigenesis cell cycle [17] |
Osteosarcoma is a highly invasive and metastatic tumor. Current research on the functional roles of KIFs in osteosarcoma shows that some KIFs are closely related to the migration and invasion ability of osteosarcoma cells. For example, Gu et al. [106] showed that KIG3B levels are high in osteosarcoma cell lines and can promote the proliferation, migration, and invasion of osteosarcoma cells. Pu et al. [59] and others found that inhibiting the expression of KIF26B increased the sensitivity of osteosarcoma cell lines to a variety of drugs, including doxorubicin, etoposide, methotrexate, cisplatin, and carboplatin. Wu et al. [61] reported that the expression of KIF15 in osteosarcoma tissues was much higher than that in adjacent tissues. Knockdown of KTF15 in osteosarcoma cells significantly inhibited the proliferation of osteosarcoma cells and DNA synthesis in the S phase and promoted cell apoptosis. Similarly, inhibition of KTF15 expression reduced the migration and invasion of osteosarcoma cells [61].
Wang et al. [16] found that the expression of KIF2A in human osteosarcoma tissues was much higher than that in adjacent tissues, and KIF2A promoted the proliferation, migration, and invasion of osteosarcoma cells. Inhibiting the expression of KIF2A in osteosarcoma cell lines reduced their tumorigenesis and their ability to metastasize to the lung [16]. A study by Gao et al. [17] demonstrated that KIF18B is a potential oncogene and is highly expressed in osteosarcoma tissues and cells; furthermore, they demonstrated that KIF18B can promote osteosarcoma in vivo and increase osteosarcoma cell proliferation and migration in vitro.
High metastasis is a malignant manifestation of osteosarcoma. Metastatic osteosarcoma has a poor prognosis and a high recurrence rate. Therefore, the study of genes closely related to metastasis of osteosarcoma from the molecular biology perspective may facilitate the identification of targeted drugs for osteosarcoma.
Because osteosarcoma is highly metastatic, the prognosis of patients with metastatic osteosarcoma is very poor, with a 5-year overall survival rate of only 25%. Therefore, timely and accurate diagnosis is of great significance for effective treatment of the disease and improvement of the overall survival rate of patients. At present, the diagnosis of osteosarcoma is very limited. More than 90% of the early diagnosis of osteosarcoma in children and young/adult patients occurs based on bone pain and a non-pathological fractures. Some patients experience local swelling and a limited range of motion of the affected limb. Overall, the clinical symptoms are usually mild, lasting for several months, and are often not valued by doctors and patients [107, 108, 109]. Therefore, accurate or auxiliary diagnosis is particularly important for the early recognition of osteosarcoma.
Studies have shown that KIF3B, KIF26B, KIF15, KIF2A, and KIF18B are all highly expressed in osteosarcoma tissues and cells and can act on oncogenes to promote the proliferation and migration of osteosarcoma cells. Groth-Pedersen et al. [110] showed that interference with the expression of KIF11, KIF20A, KIF21A, and KIF25 in human osteosarcoma U2OS cells significantly reduced their proliferation. Therefore, the auxiliary detection of KIFs expression in patients with suspected osteosarcoma may assist in the diagnosis of osteosarcoma, and KIFs may be used as a new biological marker for the diagnosis of osteosarcoma.
Currently, the most common osteosarcoma treatment involves surgical resection of the lesion, supplemented by chemotherapy and radiotherapy. However, these treatments have certain limitations, for example, poor curative effect on patients with metastatic osteosarcoma, high recurrence rate, and poor prognosis [111]. Therefore, exploring new treatments is extremely critical for patients with metastatic osteosarcoma. Liu et al. [112] found that intrathecal injection of a KIF17 antisense oligodeoxynucleotide into an osteosarcoma mouse model could relieve pain, inhibit tumor growth, and increase the survival rate after surgery. Saeki et al. [113] indicated that inhibiting the expression of KIF15 and KIF11 in osteosarcoma cell lines U2OS and HOS that are anti-S-trityl L-cysteine can significantly reduce the anti-S-trityl L-cysteine ability of these cells. Pu et al. [59] found that inhibiting the expression of KIF26B in the osteosarcoma cell line SJSA-1 reduced the resistance of these cells to multiple drugs such as doxorubicin, etoposide, cisplatin, and carboplatin. Gao et al. [17] found that inhibiting the expression of KIF18B in vivo decreased the tumor growth in U2OS osteosarcoma cells. Overall, these findings suggest that KIFs may be used as new targets for the treatment of osteosarcoma.
The identified studies indicate that KIFs participats in multiple modes of
regulation for the development of osteosarcoma. (a) High-expression of KIF15
promotes osteosarcoma proliferation. (b) High-expression of KIF2A promotes
osteosarcoma proliferation and metastasis. (c) CircRNA0032462 is high expression
and promotes osteosarcoma proliferation and metastasis by enhancing the
expression of KIF3B. (d) MiR-20a-5p can be used as a potential targeted drug to
inhibit drug resistance of osteosarcoma by inhibiting the expression of KIF26B.
(e) KIF18B upregulates
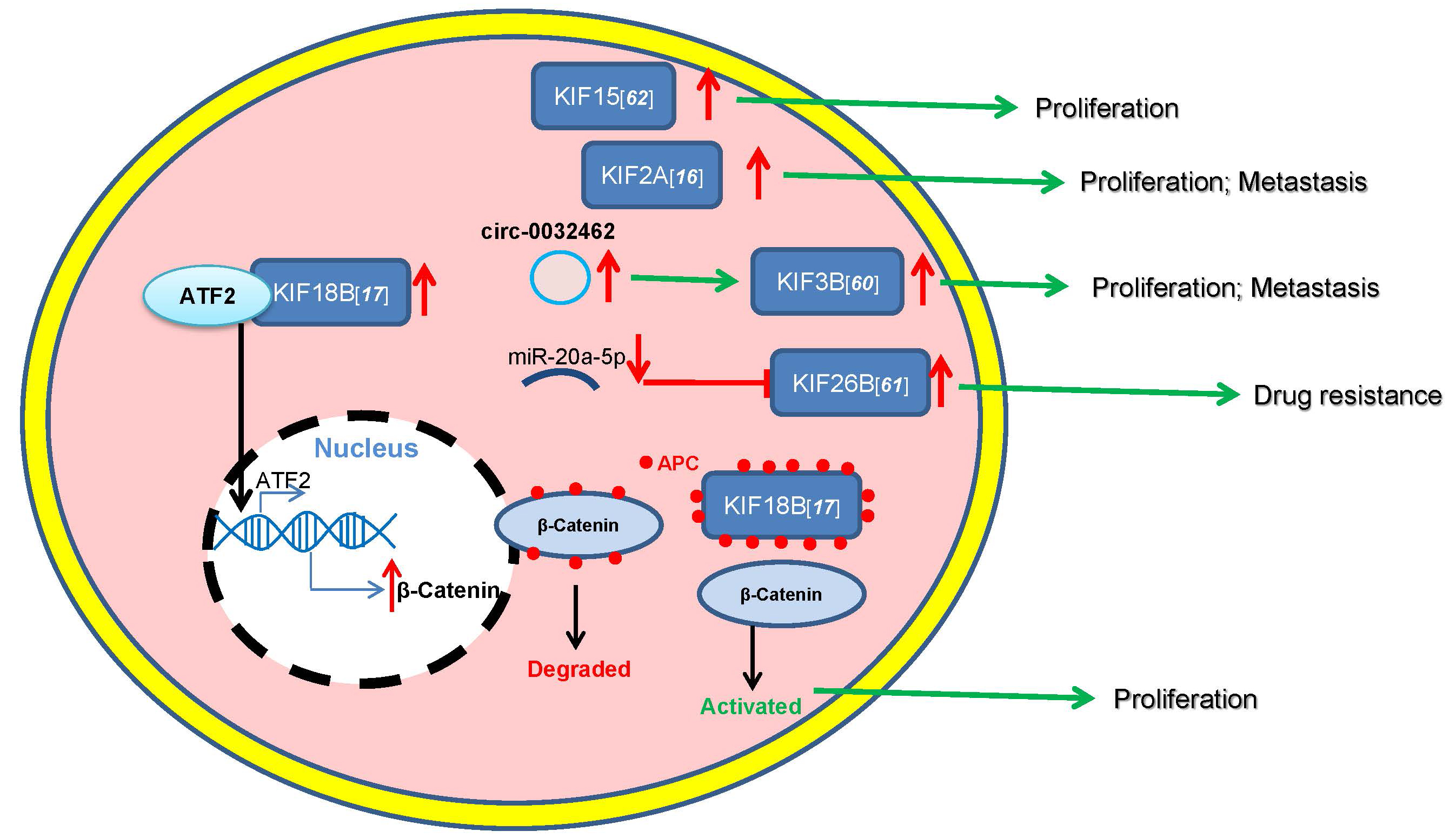 Fig. 1.
Fig. 1.Roles of KIFs in osteosarcoma.
Osteosarcoma is the most common primary bone malignant tumor in children and adolescents, with high metastatic rates [114]. Routine surgical treatment combined with systemic chemotherapy and directed radiotherapy can increase the 5-year survival rate of patients to 70%. However, the long-term survival rate of patients with metastatic or recurrent osteosarcoma is still less than 20% [115].
Abnormal KIFs expression and function are closely related to the development of a variety of human cancer types. KIFs play key roles in osteosarcoma. A variety of KIFs (KIF3B, KIF26B, KIF15, KIF2A, and KIF18B) are highly expressed in osteosarcoma tissues and cells and are closely related to the invasion and metastasis of osteosarcoma. KIF11, KIF20A, KIF21A, and KIF25 can regulate the proliferation of osteosarcoma cells, and inhibiting the expression of these KIFs can reduce the proliferative ability of osteosarcoma cells. In addition, interfering with the expression of KIF17 in the mouse sheath can relieve pain and inhibit tumor growth, as well as improve postoperative survival, whereas inhibiting the expression of KIF15, KIF11, and KIF26B can reduce the resistance of osteosarcoma cell lines to multiple drugs. Therefore, in-depth research on KIFs may provide novel markers and targets for clinical diagnosis as well as prognosis and treatment of osteosarcoma.
YL, TS, XX, GLC and PPH designed this review. YL, TS and XX drafted the manuscript. GLC and PPH revised and approved the final version of the manuscript. All authors critically revised the manuscript, and read and approved the final version of the manuscript.
Not applicable.
This work was supported by Pan-Pan Huang and Gao-Lu Cao. I thank Tao Song, Xue Xue for their critical reading of this review.
This research received no external funding.
The authors declare no conflict of interest.
ATPase, adenosine triphosphatase; KIFs, kinesin superfamily protein.
