1 Department of Cardiology, The First Central Clinical College of Tianjin Medical University, 300070 Tianjin, China
2 Department of Cardiology, Tianjin First Central Hospital, 300070 Tianjin, China
3 Department of Cardiology, Longgang People’s Hospital of Shenzhen, 518172 Shenzhen, Guangdong, China
4 Department of Cardiology, Dongguan Kanghua Hospital, 523080 Dongguan, Guangdong, China
5 Department of Nuclear Medicine, Shanghai Tenth People’s Hospital, Tongji University, 200072 Shanghai, China
Abstract
Background: Aldosterone is an important hormone in the renin-angiotensin-aldosterone system (RAAS), and playing a pivotal role in the development of hypertension, heart failure, and other cardiovascular diseases. Material and method: In this study, the role of the aldosterone in vascular calcification was underwent in rat model compared with other drugs. Vascular calcification, calcium concentration, activity of alkaline phosphatase (ALP), aldosterone, Urotensin II, mineralocorticoid receptor (MR) and Osteopontin (OPN) were detected or confirmed by the von Kossa staining, colorimetric assays, immunohistochemistry and radioimmunoassay, separately. Result: Results revealed that the aldosterone was significantly increased compared calcification + aldosterone group with calcification group, whereas it was notably decreased in calcification + Spironolactone group in the aortic wall. Compared with control group and aldosterone group, calcium content in vascular tissues was increased in calcification group and calcification + aldosterone group. As the immunoreactivity of the MR, OPN, Urotensin II, IL-6, monocyte chemoattractant protein-1, and deposition of collagen in calcification group and aldosterone group, they all were increased slightly, but were significantly increased in calcification + aldosterone group. Conclusion: It is implied that aldosterone may be involved in the development of vascular calcification, however, the mechanism needs to be further studied.
Keywords
- Aldosterone
- Vascular calcification
- Inflammatory factor
- Collagen
- Osteopontin
- Urotensin II
Vascular calcification is a complication in hypertension, diabetes mellitus, chronic renal insufficiency, and considered as a risk factor for cardiovascular event [1, 2, 3]. Two major types of vascular calcification are distinguished by their location and association with atherosclerotic plaque formation. First, atherosclerotic calcification, is located in the intimal layer and is associated with atherosclerosis. Atherosclerotic calcification involves cellular necrosis, inflammation, and lipid deposition. As lesions progress, osteogenesis, including osteoblast induction and lamellar bone formation, becomes increasingly evident. Second type is Monckeberg sclerosis, in which amorphous mineral forms circumferentially along or within one or more elastic lamellae of the medial layer. This second type also known as medial artery calcification, it is more prevalent in patients with diabetes and chronic kidney disease (CKD). Calciphylaxis is defined as a chronic progressive syndrome of arteriolar media calcification, thrombotic ischemia, and necrotic ulceration. The cumulative incidence of calciphylaxis among hemodialysis patients in the Europe and America has been reported to be within 1%, and the corresponding prevalence was estimated to be 4.1–4.4% [2]. The aforementioned pathological mechanisms often overlap with each other. Atherosclerotic calcification often accompanies with medial artery calcification in clinical trials. Vascular calcification is an active consequence of aging, which may increase vascular stiff, lessen vascular compliance, and cause myocardial ischemia and plaque rupture [1, 2, 3].
Amount of evidence pointed out a tightly regulated process was associated with
the competition of various factors, having the capability to promote
calcification and inhibitors of mineralization. However, the precise molecular
mechanisms on facilitating ectopic mineral deposition remain unclear [1, 2, 3, 4, 5]. It
has been reported that cardiovascular calcification recapitulates the processes
of orthotopic, skeletal, bone formation, accompanying by calcium deposition,
upregulation of the activity of alkaline phosphatase (ALP), and change of cell
phenotype [2]. aldosterone partly regulates Na
Alone or with combination, vitamin D3, warfarin, nicotine, and calcium chloride can induce artery calcification in rats and mice, which is an often used method for preparing animal models of vascular calcification. The operation is relatively simple, fast, with high survival rate and low cost [29]. The model preparation was briefly presented below.
Sprague-Dawley rats was induced to arterial calcification by Vitamin D3 plus
nicotine (VDN). Also, 40 samples of 7-week-old male SD rat (because the male rats
were rarely disturbed by the estrogenic hormone, then impossibly impacted the
aldosterone) were randomly divided into 5 groups and with 8 samples in each
group: control group, aldosterone group [aldosterone (20
Blood pressure measurement: Tail pressure method (Tail-cuff method) was used to acquire the blood pressure value noninvasively. Blood pressure values were obtained by placing the sensor in the tail of the mouse and monitoring the blood pressure signal through inflation and ventilation for the tail artery pressure and pressure release.
After model rats were anesthetized with 2% pentobarbital sodium (40 mg/kg), plasma, serum, and aorta (the whole length of thoracic and abdominal aorta) were collected for detection, detailed information are described as below.
After fixation, the abdominal cavity and thoracic cavity of rats were cut open
with scissors, and their heart was exposed. Venous blood of rats was extracted
with a 10 mL syringe, about 4 mL was put into a 5 mL plastic tube containing
heparin, ethylenediaminetetraacetic acid (EDTA), and aprotin, which was
thoroughly mixed, and centrifuged at 4
Carefully peeled the aorta to the full length of the abdominal aorta, placed it
in ice saline, and washed it, meanwhile, carefully peeled the surrounding
connective tissue with small tweezers, used the initial section of the thoracic
aorta (histological examination), placed it in 4% paraformaldehyde solution,
stored it for about 12–24 h, then rinsed it with running water for about 12 h,
and thereafter dehydrated and embed it. Took about 100 mg aorta tissue, divided
each 50 mg inserted in 1.5 mL EP (via measuring aldosterone and vasopressin
receptor 2 (V2R)), marked with oily pen mark, and refrigerated at –80
Measurement of the extent of calcification: The von Kossa staining for calcification showed that a positive staining, as black areas in the main, large, and nodular structures, was found in the vascular media.
Measurement of calcium content: The calcium content in vascular tissues was measured using a simple and rapid colorimetric method based on methylthymol blue, performed by Nanjing Jiancheng Bioengineering Institute (Nanjing, China).
Measurement of the activity of ALP: The activity of ALP was evaluated by using Alkaline Phosphatase Assay Kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). Briefly, the nitrophenol phosphate (pNPP) is a commonly used alkaline phosphatase color rendering substrate that can be catalyzed by ALP to generate nitrophenol (para-nitrophenol, p-nitrophenol) as a yellow product and absorbance can be detected at 400–415 nm. The darker the product yellow represents higher ALP activity, and vice versa, lower enzyme activity. Then, ALP levels can be quantified quantitatively by colorimetric analysis.
Immunohistochemistry: The mineralocorticoid receptor immunoreactivity, the immunoreactivity of OPN, the receptor immunoreactivity of the Urotensin II, interleukin-6 (IL-6), and monocyte chemoattractant protein-1 (MCP-1) in cardiovascular tissues were detected by using immunohistochemical assay.
Masson’s Trichrome staining: The deposition of collagen in cardiovascular
tissues was measured by Masson’s trichrome staining. Briefly, by use of the three
stains, Masson’s Trichrome staining technique is used for the detection of
collagen fibers in tissues such as the skin, heart, muscles. The samples are
formalin-fixed, paraffin-embedded sections, or frozen sections. Weigert’s
hematoxylin, an iron hematoxylin dye is used to stain the nuclei. This dye is
resistant to decolorization by acidic staining solutions. Biebrich-Scarlet Acid
Fuschin solution (No. S125, Bay Shore, NY, USA) stains all the acidic tissues
such as the cytoplasm, muscle, and collagen. Phosphomolybdic or phosphotungstic
acid is used as a decolorizing agent, making the Biebrich Scarlet-acid fuschin to
diffuse out of the collagen fibers, this leaves the muscle cells staining red.
Aniline blue stains the collagen along which 1% acetic acid is added to show a
difference in the tissue sections. The collagen fibers stain blue and the nuclei
stains black, with a red background. The detailed procedures are: Deparaffinize
and rehydrate using 100% alcohol, 95% alcohol, and 70% alcohol sequentially;
Wash in distilled water; For tissues fixed with Formalin, re-fix in Bouin
Solution for 1 hour at 56
Radioimmunoassay: The contents of Aldosterone in plasma and vascular tissue,
C-reactive protein, IL-6, tumor necrosis factor-
Enzyme immunoassay: The content of Osteopontin (OPN) in plasma was measured by enzyme immunoassay, conducted by Beijing Sino-UK Institute of Biological Technology (Beijing, China).
In this study, 8 fields were randomly selected from each group of slices, and image-pro Plus 6.0 software (version 6.0, Media Cybernetics, Inc., MD 20852, USA) was applied to calculate the percentage of positive staining area in the total area of slices. Multiple independent samples were used for nonparametric testing of the percentage of positive staining area between groups.
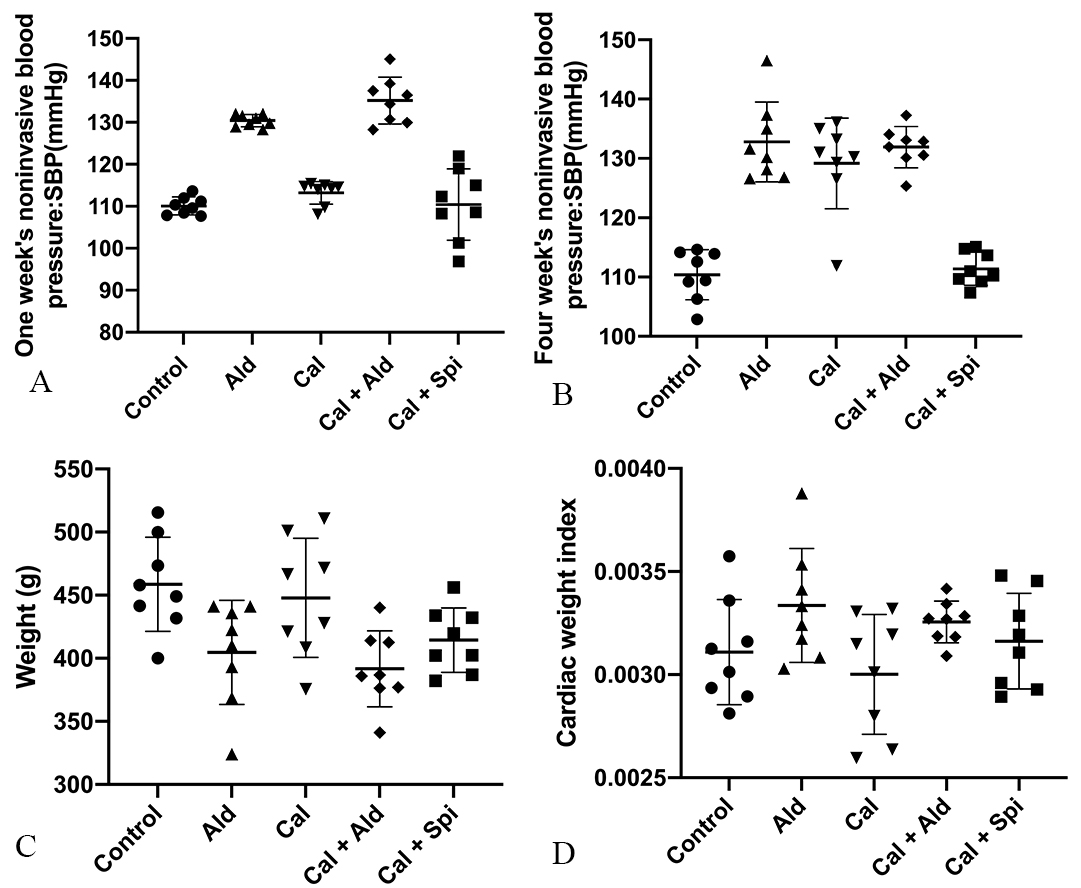
After 6 weeks of hypodermic injection of aldosterone, the noninvasive blood pressure and cardiac weight index of rats were increased, and we observed the weight loss, which proved aldosterone’s effect. Besides, the noninvasive blood pressure, weight loss, and cardiac weight index for calcification group were shown in Fig. 1.
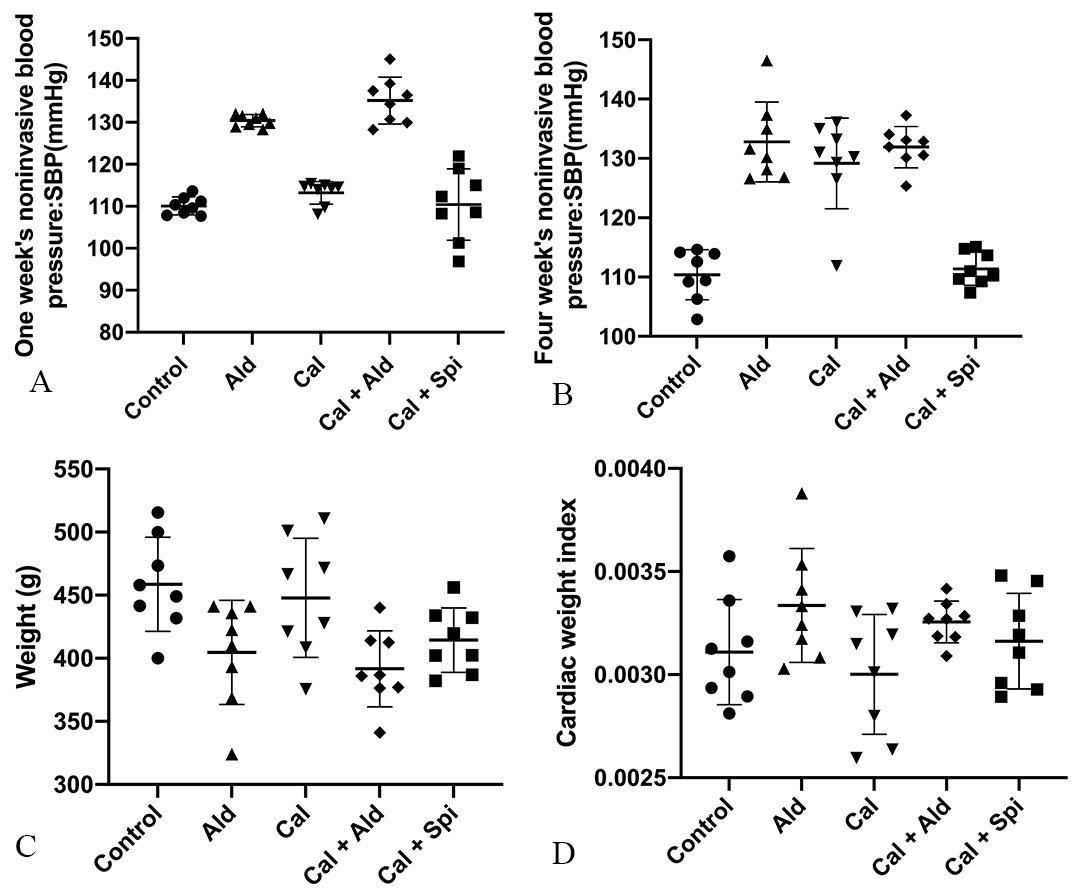 Fig. 1.
Fig. 1.Physiology index of experimental rats among the different groups. (A) Noninvasive blood pressure of rats after 1week. (B) Noninvasive blood pressure of rats after 4 weeks. (C) Weight of rats. (D) Rats’s cardiac weight index.
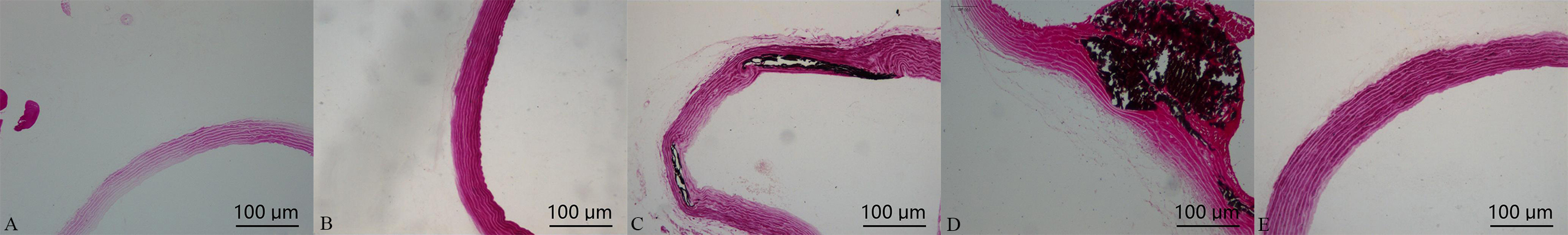
Von Kossa staining for calcification showed that a positive staining, noted black areas in the main, large, and nodular structures, was found in the vascular media. In comparison with calcification group, which was significantly increased in calcification + aldosterone group, whereas it was significantly decreased in calcification + spriolactone group, as illustrated in Fig. 2.
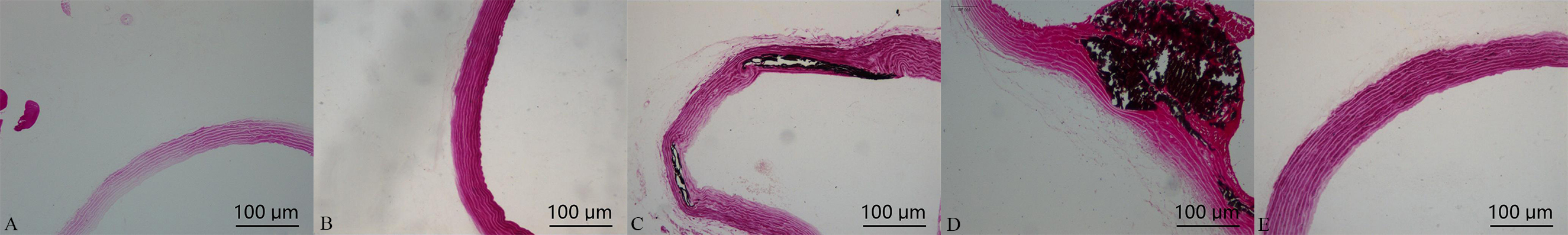 Fig. 2.
Fig. 2.Von Kossa staining in the aorta: positive staining was shown with black areas (10
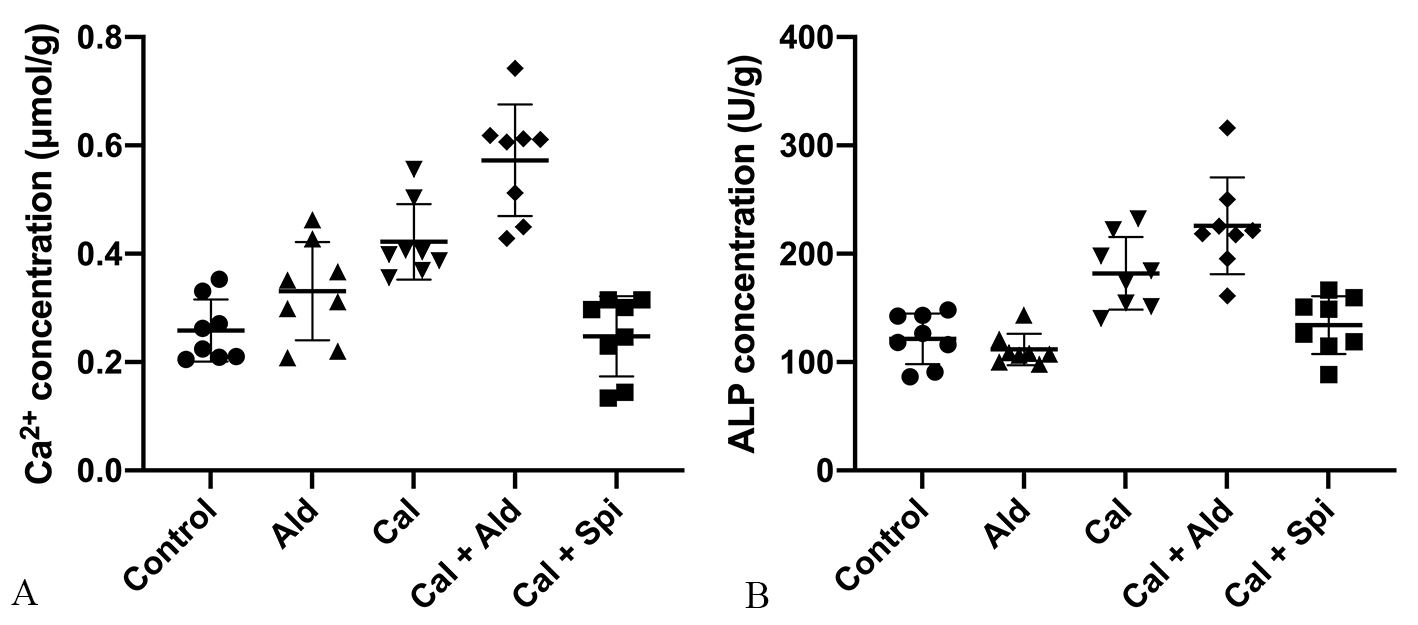
Compared with control group, the calcium content in vascular tissues was
increased by 63.45% (p
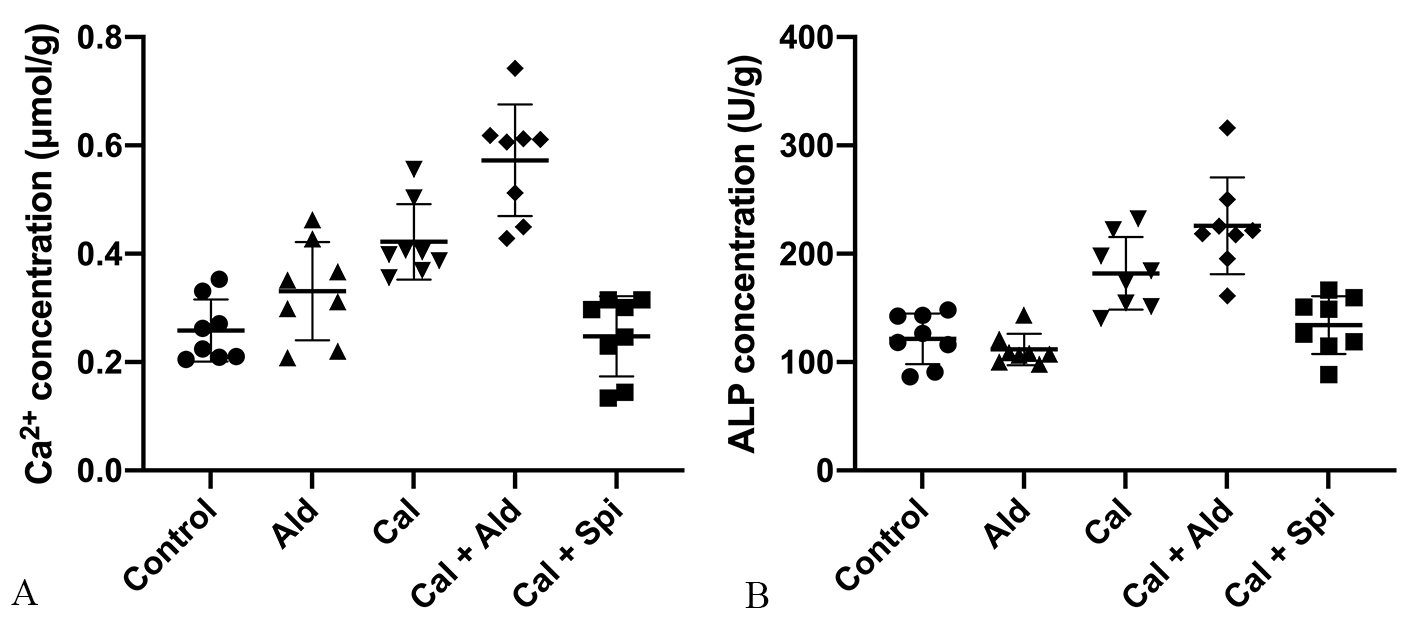 Fig. 3.
Fig. 3.The calcium content and the
activity of ALP in the aorta tissue. (A) Calcium content in the aorta (Mean
As for ALP activity in vascular tissues, compared with control group, which was
increased by 49.79% (p
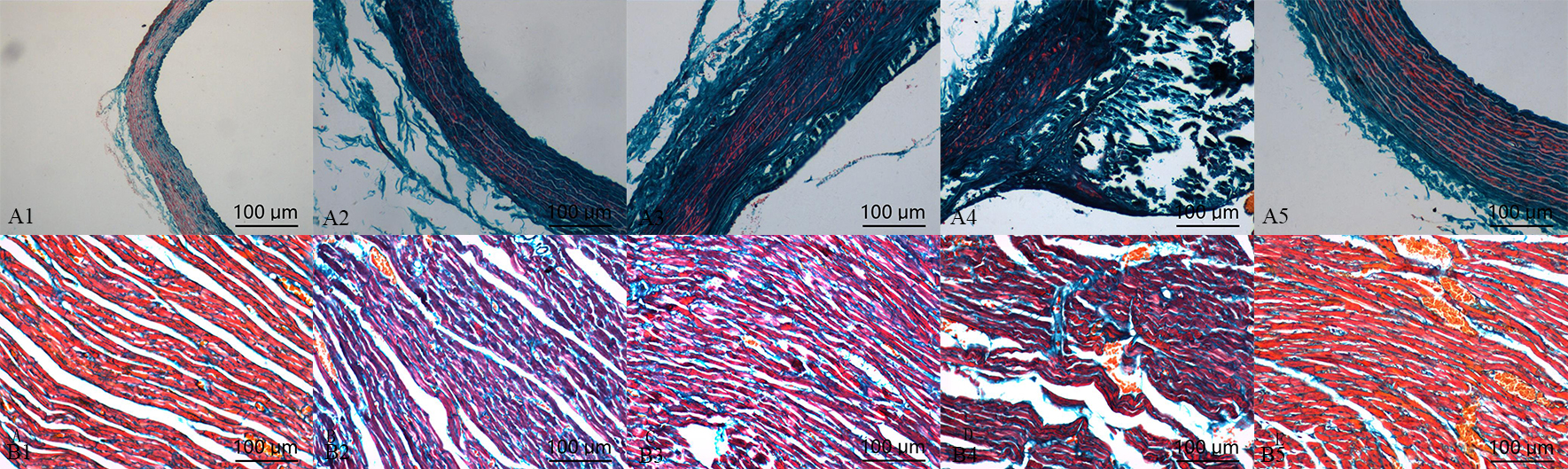
Compared with control group, the deposition of collagen in calcification group and aldosterone group was increased, but which was significantly increased in calcification + aldosterone group, and was slightly increased in calcification + spriolactone group (Fig. 4).
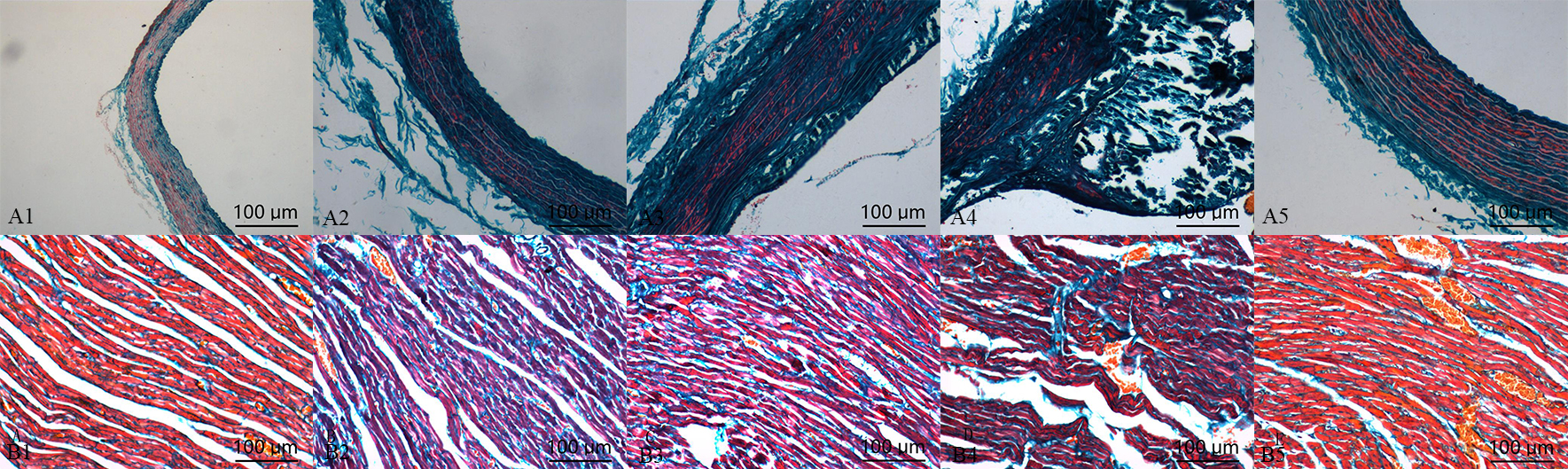 Fig. 4.
Fig. 4.Masson’s trichrome staining
in the aorta (A) and myocardium (B): positive staining was illustrated with blue
areas (10
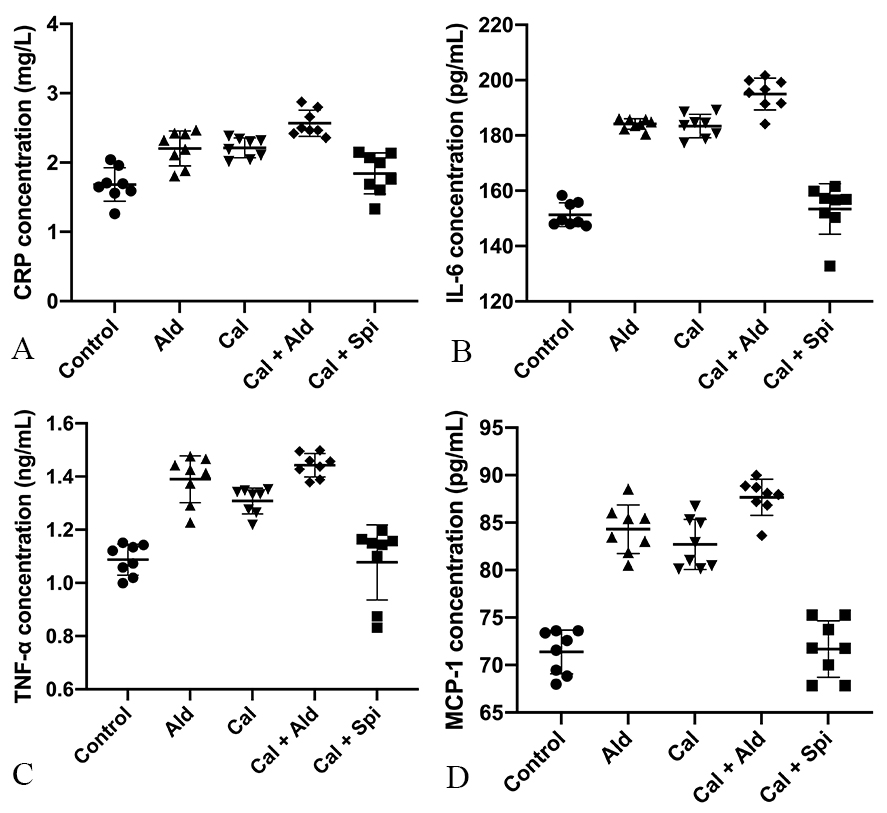
Compared with control group, the content of the C-reactive protein was increased
by 30.35% (p
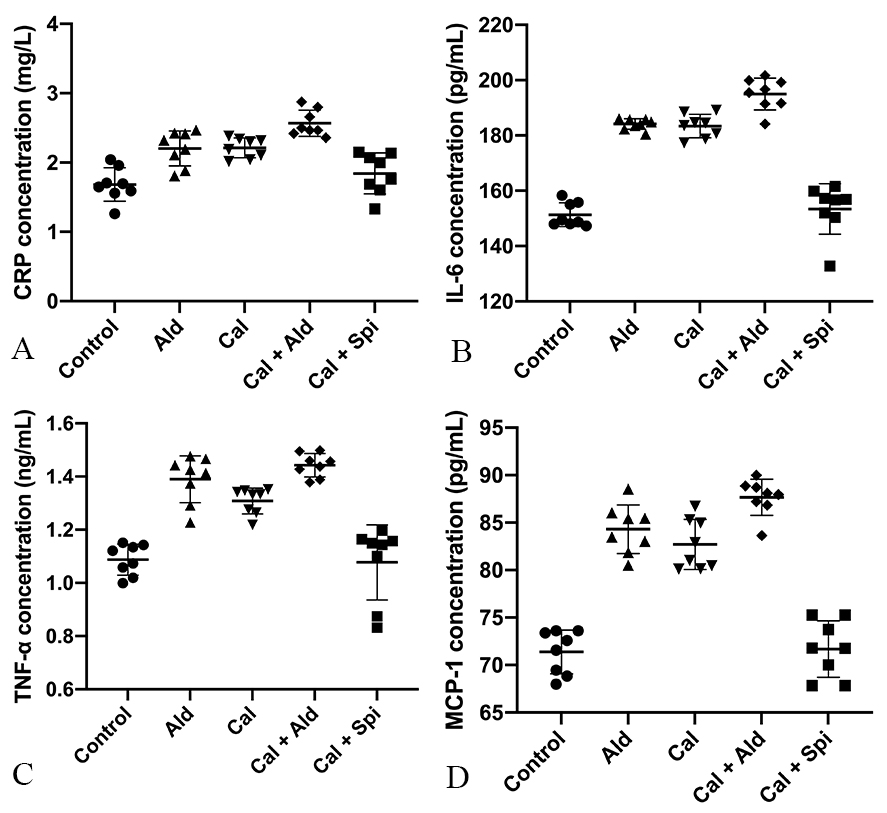 Fig. 5.
Fig. 5.Content of the C-reactive
protein, IL-6, TNF-
Compared with control group, the content of the IL-6 was increased by 20.55%
(p
Compared with control group, the content of the TNF-
Compared with control group, the content of the MCP-1 was increased by 18.37%
(p
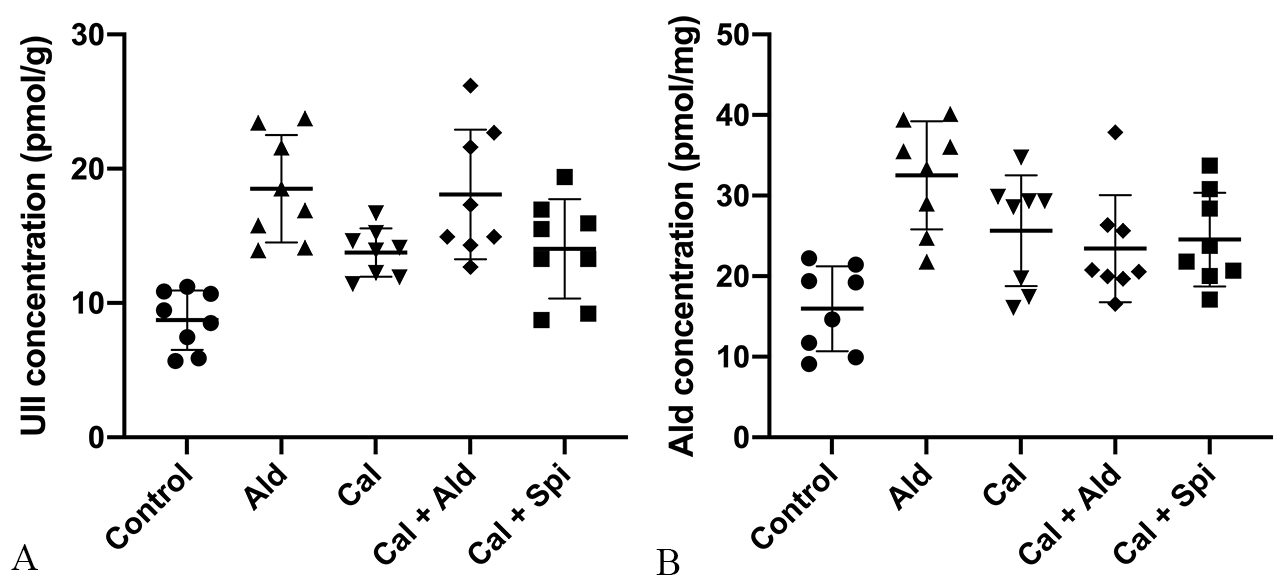
Compared with control group, the content of the aortic Urotensin II was
significantly increased by 121.15% (p
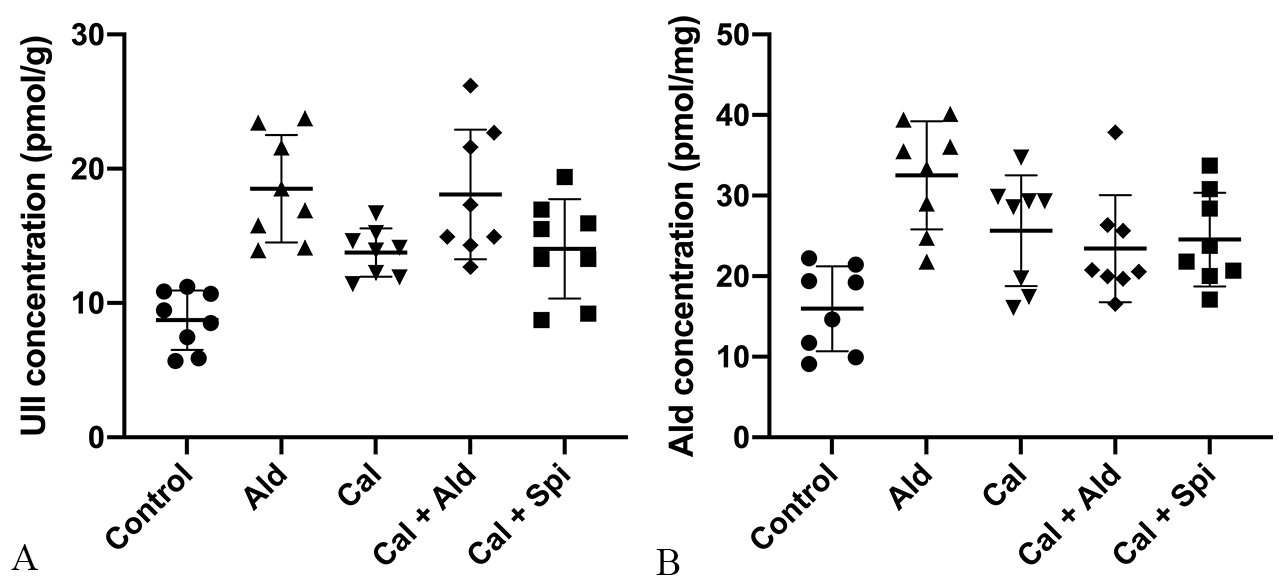 Fig. 6.
Fig. 6.Content of Urotensin II and
aldosterone in the aorta tissue. (A) Urotensin II (Mean
Compared with control group, the content of the aortic aldosterone was increased
by 103.76% (p
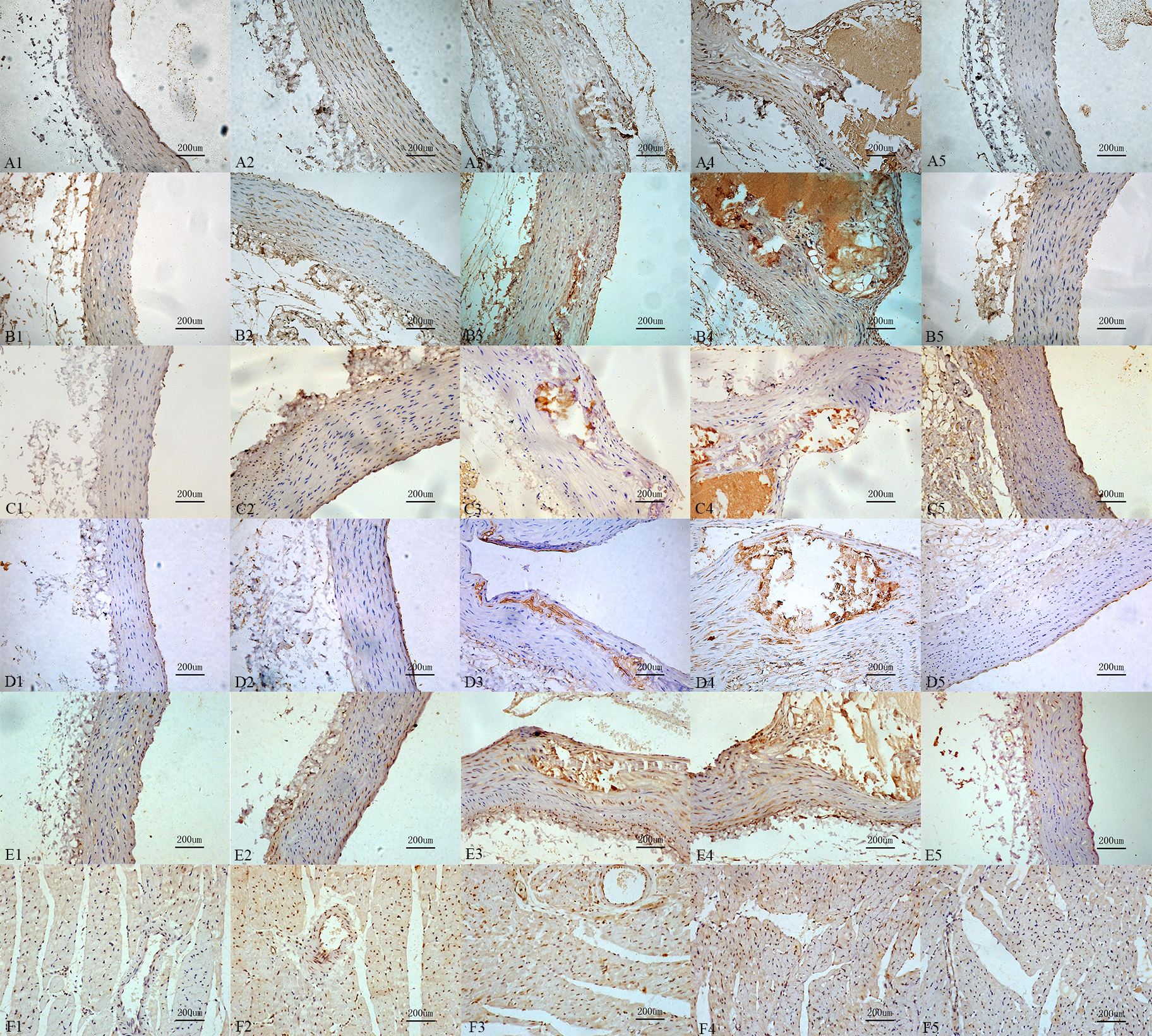
Compared with control group, the mineralocorticoid receptor immunoreactivity, the immunoreactivity of OPN, the receptor immunoreactivity of the Urotensin II, IL-6, MCP-1 in calcification group and aldosterone group were increased, those also were significantly increased in calcification + aldosterone, and slightly increased in calcification + spriolactone group (Fig. 7).
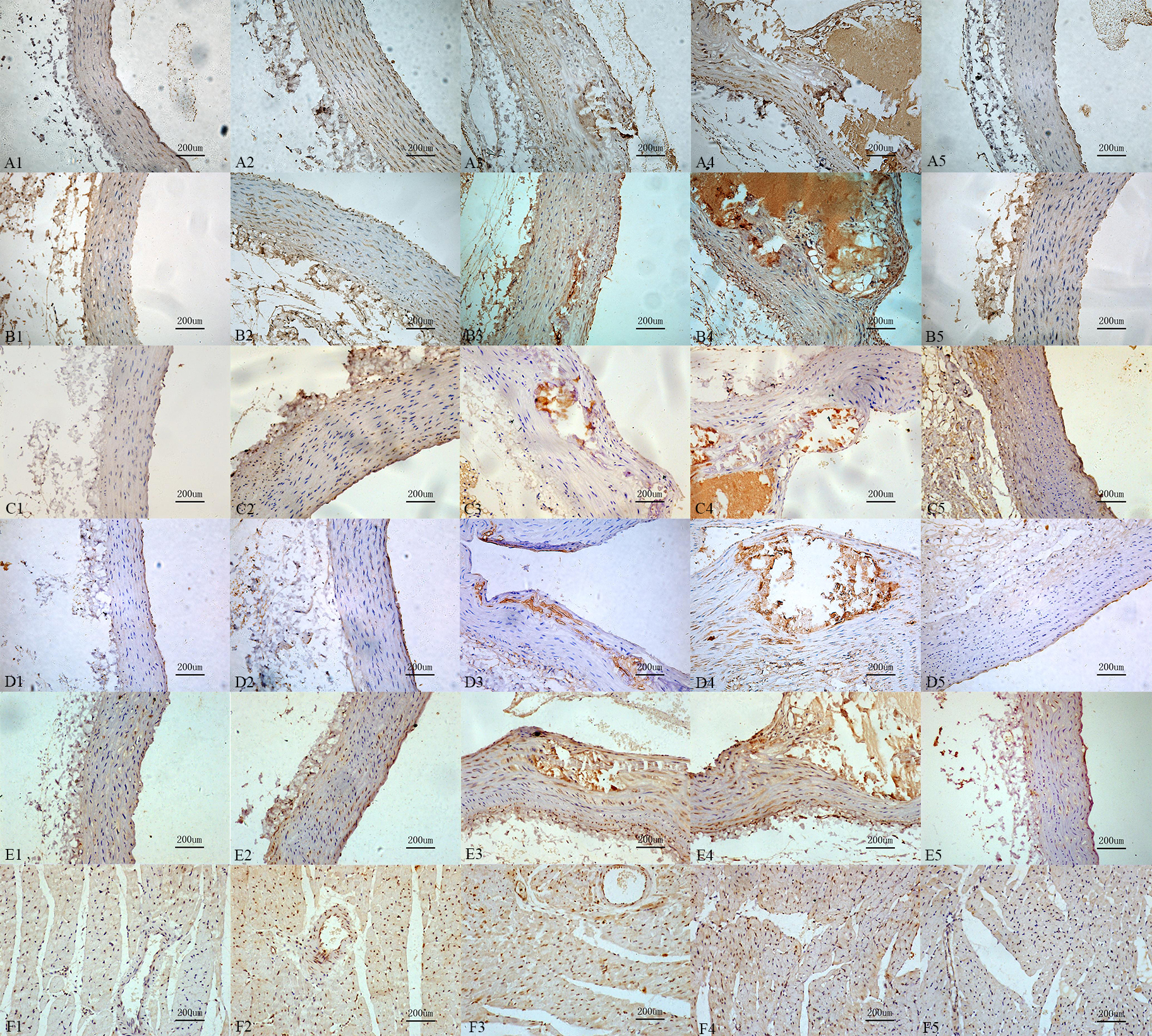 Fig. 7.
Fig. 7.Immunoreactivity change for
the different recptors and proteins. Interleukin-6 (A1: 9.87%, A2: 37.16%, A3:
66.38%, A4: 75.75%, A5: 17.87%,
Vascular calcification is a basic pathology of atherosclerosis, hypertension, diabetes, chronic kidney disease, and aging. Accumulating evidence have mentioned a tightly regulated process, associating with a competition between various factors in promoting calcification and inhibitors of mineralization, but the cellular mechanisms remain unclear [1, 2, 3, 4, 5]. Aldosterone causes retention of sodium water and can promote collagen deposition and fibrosis, leading to fibrosis and structural remodeling of the heart. In this study, we used Vitamin D3 plus nicotine (VDN) to create vascular calcification model rat, and we found that aldosterone was involved in vascular calcification.
Firstly, we found that exogenetic aldosterone could increase vascular calcification in rats. The expression of aldosterone and MR were increased in calcific rats’ aorta. The MR was increased significantly, the activity of ALP was up-regulated, and mineral deposition was increased treated with aldosterone in calcific rats. The expression of collagen deposition, Urocortin II and its receptor, and inflammatory factor and its receptor were increased in calcific rats’ aorta. Besides, it was released that the aldosterone can promote the expression of all the factors to increase vascular calcification.
Vascular calcification is a complicated dynamic process, involving several regulation factors, such as up-regulated activity of ALP, expressive type transformation of vascular smooth muscle cell, and crystal deposition [1, 2, 3, 4, 5]. Vitamin D3 with its receptors could increase vascular calcification in vitro by promoting the expression of matrix vesicle, elastin, and collagen [3]. The up-regulation of the activity of ALP is an early biochemical marker for vascular calcification. The ALP modulates vascular calcification by decreasing the levels of inorganic pyrophosphate; pyrophosphate is a substrate for ALP and a potent inhibitor for vascular calcification [3]. We found that ALP and the content of aldosterone were increased in calcific rats’ aorta, and exogenetic aldosterone could increase the effects; however, that was lightened by spironolactone, demonstrating that endogenous and exogenetic aldosterone could increase vascular calcification in rats, and the effect can be lightened by spironolactone. We found that there were no significant differences in plasma aldosterone contents among the 5 groups, thus, the effect of the aldosterone might be involved in the development of vascular calcification, in a paracrine and/or autocrine manner. Besides, the calcified rats’ aorta is calcification or segmental, therefore, the expression of aldosterone in calcification + aldosterone group of aorta was not increased, compared with the aldosterone group [1, 2, 3, 4, 5].
We confirmed that collagen [30] and an inflammatory factor [31, 32] are involved in vascular calcification, and demonstrated that exogenetic aldosterone could increase their expressions to aggravate calcification. Inflammatory stimulation could increase formation of calcium nodes through AKT and Wnt signaling pathways [33, 34]. In addition, it was revealed that collagen is hypertrophy in calcific nodules, and also collagen may be associated with the baseball Brackets for vascular calcification, in favor of the expression of the factors, promoting calcification. In addition, it was revealed that collagen is hypertrophic in calcified nodules. The factors promoting vascular calcification coordinated and interacted with each other, and eventually the occurrence and development of vascular calcification were noted.
Urotensin II is a vasoconstrictive peptide that exerts its activity by binding to GPR14-the specific receptor of Urotensin II [35]. Previous research showed that it could increase the inflammatory factor to promote cardiac fibrosis, and demonstrated that Urotensin II is involved in vascular calcification by stimulating the inflammatory factor and collagen through the protein kinase C, mitogen-activated protein kinase, calcineurin, Rho kinase, and/or Ca2t signal transduction pathway [36]. Besides, aldosterone can significantly enhance its effectsl.
Ectopic calcification is exacerbated in OPN deficient mice, and this process is mitigated by adding exogenous OPN, that is phosphorylated and contains the arginine-glycine-aspartate (RGD) sequence [37]. Detailed analyses of OPN inhibition of hydroxyapatite and calcium oxalate (nephrolithiasis) indicated that phosphorylation of OPN defines its inhibitory potential. However, OPN is a factor, promoting fibrosis [38, 39]. Due to the collagen is hypertrophy in calcific nodules, so we conjectured that OPN may be an inhibitor for mineralization, which is phosphorylated and contains the RGD sequence.
Calcium and phosphorus metabolism disorders, activation of phosphate signaling
channels [40, 41, 42]; BMP (bone morphogenetic protein)/Smads, bone
protection element (OPG), nuclear factor kB receptor activated receptor protein
(RANK) regulation [43, 44, 45, 46, 47], Wnt/
In conclusion, aldosterone and mineralocorticoid receptor are significantly increased in calcified vessels, suggesting that aldosterone may be involved in the development of vascular calcification, in a paracrine and/or autocrine manner, and this effect is mediated by the mineralocorticoid receptor. The aldosterone regulates vascular calcification by promoting the expression of collagen, Urotensin II and its receptor in the aorta wall, upregulating the expression of the inflammatory factor and the corresponding receptor in the aorta wall. In addition, OPN is a contentious factor for inhibiting vascular calcification, as well as promoting vascular fibrosis in a paracrine and/or autocrine manner.
Aldosterone may be involved in the development of vascular calcification, and the regulation of it has a promising effect in inhibiting the vascular calcification. However, gene or protein expressions of IL6, MCP1, OPN during this pathology process should be further explored, and the signaling mechanisms needs to be further studied.
XZ (Xusheng Zhang), ZH, XF and XT carried out the studies, participated in collecting data, and drafted the manuscript. XZ (Xusheng Zhang) performed the statistical analysis and participated in its design. CL, XZ (Xusheng Zhang) and JY participated in acquisition, analysis, or interpretation of data and draft the manuscript. XZ (Xiaoou Zhou) carried out the experiment and drafted the manuscript during the study. All authors read and approved the final manuscript.
The study protocol was approved by the Ethics Committees of the Tianjin Medical University (TMU-2020-LLSC-0106).
Not applicable.
This work was supported by research grants from the National Natural Science Foundation of China (grant number 81970303).
The authors declare no conflict of interest.
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
VDN, Vitamin D3 plus nicotine; IL-6, interleukin-6; ALP, alkaline phosphatase;
CKD, chronic kidney disease; RAAS, renin-angiotensin-aldosterone system; MR,
mineralocorticoid receptor; OPN, Osteopontin; TNF-