Preeclampsia is associated with an increased cardiovascular risk later in life. Anti-GPCR autoantibodies have been shown to contribute to the development of cardiovascular disease. We investigated whether anti-GPCR autoantibodies are elevated in women with a history of early-onset preeclampsia 8-11 years postpartum, and whether they correlate with clinical outcomes. We investigated data from the Preeclampsia Risk EValuation in FEMales cohort, a retrospective matched case-control study. Anti AT1R-, beta1AR-, ETAR-, PAR1- and CXCR3- autoantibodies were determined in 485 samples by using commercially available ELISA. Women with the lowest combined levels of autoantibodies and a history of early preeclampsia had significantly higher SBP, DBP and MAP (all p<0.001) compared to the controls. The individual titer levels of autoantibodies were not different between controls and former early PE groups 8-11 years postpartum. In conclusion, regulatory autoantibodies alone are not sufficient to explain hypertension or other cardiovascular pathologic conditions, but together with other risk factors such as a previous hypertensive pregnancy, lower levels of autoantibodies are associated with increased blood pressure.
Pregnancy may be considered as a window to future women’s health with adverse pregnancy outcome being a novel, female-specific, incompletely understood risk factor. Preeclampsia (PE) is a systemic disease, affecting 2-8% of pregnancies and a leading cause of maternal and neonatal morbidity and mortality worldwide (1- 3). Moreover, mounting evidence from clinical and epidemiological studies indicates it as an independent cardiovascular risk factor in later life (4, 5). Early PE is associated with an even higher susceptibility to cardiovascular disease (CVD) (6). As such, PE represents a considerable public health burden. Accordingly, improved diagnosis of this pregnancy complication and management of its cardiovascular sequelae are urgently warranted. In its new guideline for the prevention of CVD in women (7), the American Heart Association (AHA) already includes the history of PE and other pregnancy complications in the algorithm suited for identifying individuals at higher risk for cardiovascular disease, the Framingham cardiovascular risk score. AHA recommends postpartal referral by the obstetrician to a primary care physician or cardiologist for the guarded monitoring and control of risk factors (7).
In the latest 2016 European Society of Cardiology (ESC) guidelines CVD prevention, PE has also been acknowledged as a female-specific risk factor, but specific recommendations are lacking (8).
Agonistic autoantibodies against G-protein-coupled receptors (GPCR) have been shown to contribute to cardiovascular diseases such as dilated and peripartum cardiomyopathy, myocarditis, malignant, pulmonary and essential hypertension, and PE (9, 10). It has been suggested that autoantibodies against the angiotensin II receptor type 1 (AT1R-AA), beta-1 adrenergic receptor (beta1AR-AA), endothelin-1 receptor type A (ETAR-AA), and others may permanently stimulate G-protein-coupled signal cascades in an agonist-like manner and result in Ca2+ overload with cardiomyocyte apoptosis (11). On the other hand, after binding to their receptors, autoantibodies might also exert inhibitory effects by blocking the corresponding receptors and preventing their activation through the agonists (12).
We, and others, have shown that AT1R-AA are present in women with PE (13- 16), even >1 year after pregnancy (17). In animal models of PE, AT1R-AA increased reactive oxygen species, inflammatory factors, endothelin-1 and soluble fms-like tyrosine kinase-1 (sFlt-1) (18,19). Inhibition of AT1R-AA during pregnancy in reduced uterine perfusion pressure (RUPP) rat model prevented hypertension and several other maternal pathogenic factors associated with PE (20).
We thus hypothesized that agonistic (regulatory) autoantibodies against GPCR represent pathogenic drivers, a part of regulatory system that might shift the metabolic and immuno-balance towards pathophysiology. The rationale of our study was to evaluate the potential of the regulatory autoantibodies to identify women at particular risk for development of CVD and to assess whether the titer levels of these autoantibodies are increased in women with a history of early PE 8-11 years after the index pregnancy.
We investigated data from the PREVFEM (Preeclampsia Risk EValuation in FEMales) retrospective matched casecontrol study, which was performed in Zwolle, The Netherlands, from 2008 until 2010. The detailed description of study design, participants and aims is presented elsewhere (21). In brief, all women after early PE as well as an equal number of age-matched females without PE from the regular obstetric database in the same time period (1991–2007) were invited to participate in the PREVFEM study. Early PE was defined according to the International Society for the Study of Hypertension in Pregnancy (ISSHP) as an elevated diastolic blood pressure greater than or equal to 90 mmHg with proteinuria (greater than or equal to 0.3 g/24 h) between 20 and 32 weeks of gestation during index pregnancy. Metabolic syndrome was defined according to the ATP III criteria (22). We excluded women with other complications (gestational diabetes mellitus, gestational hypertension, abortion, fetal death and early PE superimposed with GDM) due to the heterogeneity within the groups and low numbers. The final cohort comprised 485 women in total.
The study was approved by the institutional review board of the Isala Klinieken in Zwolle and was carried out according to the 2nd Helsinki Declaration. All participating women gave written informed consent.
Autoantibodies against AT1-, beta1- adrenergic receptors, endothelin-1 receptor A, protease-activated receptor 1 (PAR1) and C-X-C3 chemokine binding receptors (CXCR3) were determined by commercially available ELISA (Celltrend, Luckenwalde, Germany). Intra- and inter-assay coefficients of variations were 3.9% and 5.1% (AT1R-AA); 6.3% and 8.3% (ETAR-AA); 4.7% and 6.1% (CXCR3-AA); and 1.9% and 6.62% (PAR1-AA), respectively. The lowest detection limit was 0.6 units/ml for AT1R-AA, 0.6 units/ml for ETAR-AA, 0.1 units/ml for PAR1-AA, 2.5 units/ml for beta1AR-AA and 0.5 units/ml for CXCR3-AA.
The distribution of all continuous variables was checked by the Shapiro-Wilk test. Normally distributed data were expressed as means with standard deviation (SD), and differences between the groups were compared by the independentsamples t-test. Non-normally distributed data were expressed as medians with interquartile range (IQR), and differences in the distributions were compared by the Mann-Whitney-U test. Binary (categorical) data were shown as absolute values and percentages. Likelihood-ratio chi-squared test and Fisher’s test were used to test for differences in distribution of the categorical characteristics as appropriate. Associations between the single regulatory autoantibodies and clinical maternal outcomes were assessed by multiple regression analyses. Models were adjusted for age, age at index partus, gestational age in weeks at index partus, years postpartum, body mass index (BMI), waist-to-hip ratio and smoking status. Multicollinearity analysis was performed, and the degree of multicollinearity between the variables was assessed by the variance inflation factor (VIF). If the VIF was > 5, the variable was dropped from the model to minimize the adverse effects of multicollinearity and to avoid misleading interpretations of the results. A two-sided p-value < 0.05 was considered significant, p-values 0.05 - 0.10 were considered as trends. We combined the levels of autoantibodies by summing up AT1R-AA, beta1AR-AA, ETAR-AA, PAR1-AA, CXCR3-AA. The rationale is based on the currently changing concept of autoantibodies. Autoantibodies are functional regulators of receptor function and present also in healthy donors. In a recent study (23) we were the first to show that a network of immunoglobulin G autoantibodies targeting GPCR, growth factors and their related tyrosine kinase receptors of healthy donors behaves differently compared to patients with disease, such as systemic sclerosis, Alzheimer's disease, and ovarian cancer. This novel concept suggests that regulatory autoantibodies primarily play a role in normal human physiology, while dysregulation of their functions causes autoimmune disease (24). This concept reinforces the interplay between various G-coupled receptors, creating a regulatory network. In an earlier study we measured several G-coupled receptors among other autoantibodies and could show that not the individual autoantibody, but more the cluster was associated to agitation or mood symptoms (25).
All statistical analyses were carried out in IBM SPSS Version 25 and R Version 3.5.0.. Graphpad Prism Version 6.0 and R Version 3.5.0. were used to create all graphs.
Clinical and demographic characteristics of study participants are shown in the Table 1. Women in all groups did not differ in age, BMI or smoking status, however, the prevalence of hypertension was significantly higher in women with a history of early PE (42.7% vs. 8.3% in the control group, p < 0.001). Correspondingly, systolic (SBP), diastolic blood pressure (DBP) and mean arterial pressure (MAP) were significantly higher in early PE group compared to the control group (SBP: 125.00 (23.00) mmHg vs. 116.00 (14.00) mmHg, respectively, p < 0.001; DBP: 85.00 (15.00) mmHg vs. 77.00 (13.00) mmHg, respectively, p < 0.001; MAP: 98.00 (18.33) mmHg vs. 90.167 (12.25) mmHg, respectively, p < 0.001, Figure 1. Former early PE patients were also more likely to develop metabolic syndrome compared to the control group (17.7% vs. 8.9%, p = 0.006, respectively).
 Figure 1.
Figure 1.
Systolic (SBP, A), diastolic blood pressure (DBP, B) and mean arterial pressure (MAP, C) levels in women 10 years post index pregnancy. Comparisons between groups were performed by Mann-Whitney U-test.
| Participant characteristics (n = 485) | Early PE (n = 293) | Control (n = 192) | p-value |
| Age (years) | 39.00 (6.00) | 39.00 (6.00) | 0.541 |
| Years post index partus | 8.84 (4.90) | 11.01 (4.64) | <0.001 |
| Age at index partus | 29.73 (3.76) | 28.55 (3.73) | 0.001 |
| Pregnancies | 2.00 (1.00) | 3.00 (1.00) | 0.004 |
| GA at index partus (wks) | 31.00 (6.00) | 40.00 (2.00) | <0.001 |
| Birth weight at index partus (g) | 1235.00 (1016.00) | 3500.00 (648.00) | <0.001 |
| Current smoking (n) | 47 (16.0) | 33 (17.2) | 0.739 |
| HR (beats/min) | 68.00 (17.00) | 69.00 (15.00) | 0.703 |
| Hypertension (n) | 125 (42.7) | 16 (8.3) | <0.001 |
| Antihypertensive use (n) | 60 (20.5) | 0 (0.0) | <0.001 |
| Diabetes mellitus (n) | 4 (1.4) | 1 (0.5) | 0.653 |
| Metabolic syndrome (n) | 52 (17.7) | 17 (8.9) | 0.006 |
| BMI (kg/m2) | 25.45 (7.60) | 24.80 (5.70) | 0.081 |
| Waist-hip ratio | 0.83 (0.08) | 0.83 (0.07) | 0.963 |
| LDL cholesterol (mmol/L) | 2.89 (1.13) | 2.80 (0.91) | 0.629 |
| HDL cholesterol (mmol/L) | 1.47 (0.46) | 1.52 (0.52) | 0.078 |
| Triglycerides (mmol/L) | 0.88 (0.56) | 0.84 (0.48) | 0.086 |
| Total cholesterol (mmol/L) | 4.82 (1.14) | 4.82 (1.01) | 0.894 |
| CRP (mg/l) | 1.72 (3.60) | 1.50 (3.15) | 0.684 |
| Leukocytes | 6.06 (2.42) | 5.88 (2.43) | 0.605 |
| Albumin | 45.60 (3.20) | 45.70 (3.30) | 0.989 |
| Glucose (mmol/L) | 4.78 (0.65) | 4.82 (0.59) | 0.969 |
| HbA1c (percent) | 5.20 (0.30) | 5.20 (0.30) | 0.949 |
| Insulin (mIU/L) | 10.00 (10.10) | 8.70 (9.30) | 0.176 |
| TSH | 1.70 (1.18) | 2.00 (1.45) | 0.054 |
| Data are shown as median ± IQR or mean ± SD as appropriate, binary variables as absolute values with percentages. GA = gestational age, BMI = body mass index, HR = heart rate, LDL cholesterol = low density lipoprotein cholesterol, HDL cholesterol = high density lipoprotein cholesterol, CRP = C-reactive protein, HbA1c = hemoglobin A1c, TSH = thyroid-stimulating hormone. | |||
The titer levels of AT1R-, beta1AR-, ETAR-, PAR1- and CXCR3- autoantibodies were not significantly different between controls and women with a history of pregnancy complications 8-11 years after index pregnancy, Figure 2. Single autoantibodies were highly interrelated. Intercorrelations between regulatory autoantibodies and clinical and demographic characteristics for different pregnancy outcomes are shown in the Figure 3. Even though years post index partum were slightly different in both groups, Table 1, there was no dependency between autoantibodies levels and years postpartum (data not shown).
 Figure 2.
Figure 2.
Autoantibody levels by diagnosis: AT1R-AA (A), ETAR-AA (B), Beta1AR-AA (C), PAR1-AA (D), CXCR3-AA (E), and combined levels of autoantibodies (F). Comparisons between groups were performed by Mann-Whitney U-test.
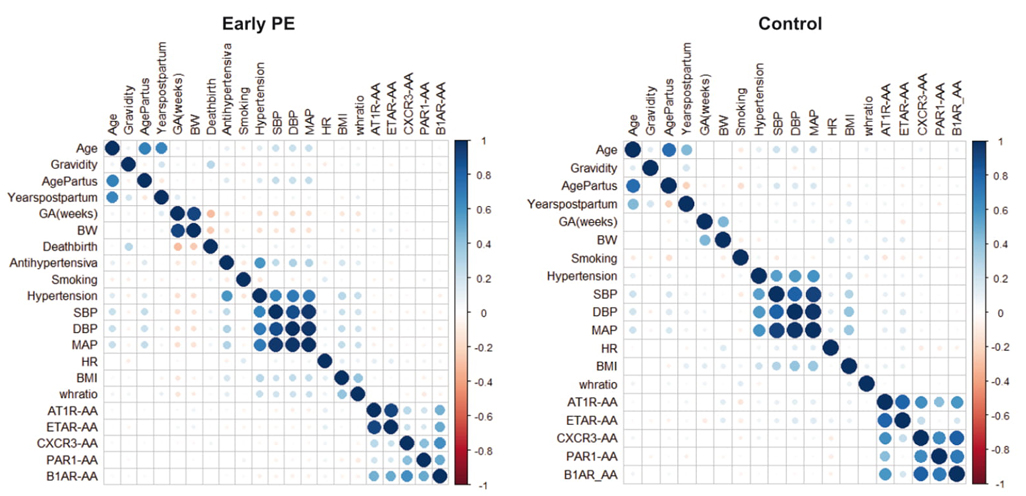 Figure 3.
Figure 3.
Intercorrelations between the regulatory autoantibody levels, clinical and demographical characteristics of the participants stratified by the pregnancy outcome.
When AT1R-, beta1AR-, ETAR-, PAR1- and CXCR3- autoantibodies were combined, women with the lowest combined levels of autoantibodies and a history of early PE showed significantly higher SBP (mean difference = 11.54 mmHg, 95% CI (4.39; 18.70), p < 0.001), DBP (mean difference = 7.75 mmHg, 95% CI (2.42; 13.08), p < 0.001), and MAP (mean difference = 9.02 mmHg, 95% CI (3.24; 14.79), p < 0.001) compared to the control group in the same lower quantile, Figure 4. No differences could be detected in the heart rate (HR). The BP was not different between the sub-groups in women with the highest combined autoantibodies levels, Figure 4. The combined autoantibodies levels were not significantly different between both sub-groups, Figure 5.
 Figure 4.
Figure 4.
SBP (A), DBP (B), MAP (C) and HR (D) in women with the highest vs. lowest total levels of autoantibodies, stratified by the diagnoses. Highest 0.25Q = upper 0.25 quantile, lowest 0.25Q = lower 0.25 quantile. Comparisons were performed by the 2-way ANOVA with Tukey’s correction for multiple comparisons.
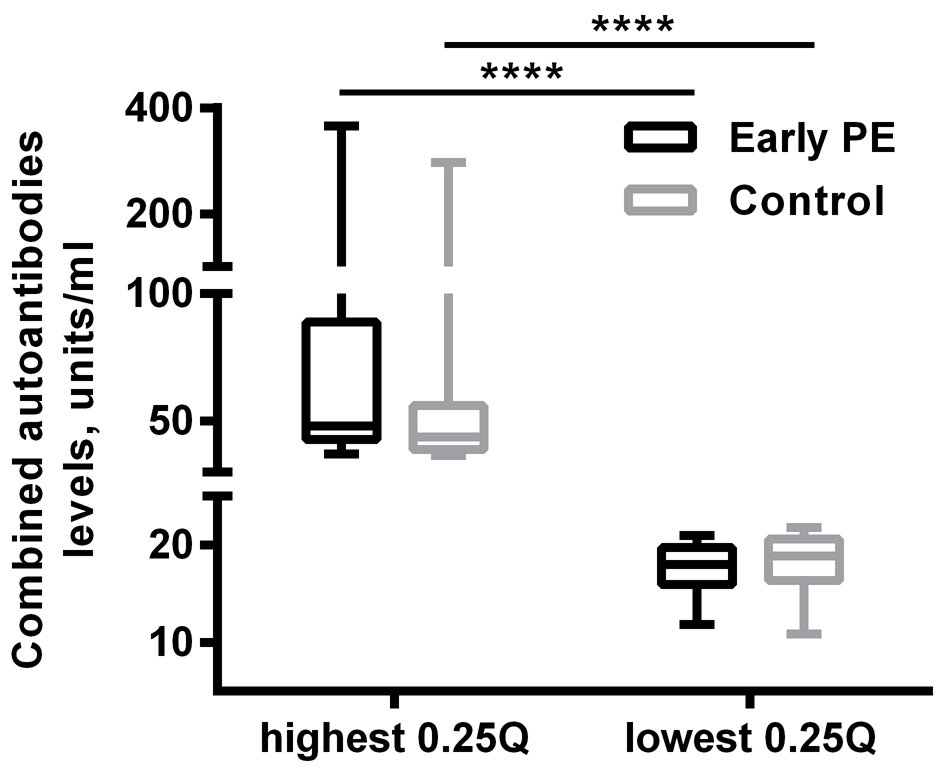 Figure 5.
Figure 5.
Combined autoantibodies levels, stratified by the highest vs lowest quantiles and diagnoses.
In our adjusted models, the association between regulatory autoantibodies and cardiovascular outcomes were rather poor, Table 2-Table 5. There was no association between autoantibodies and SBP, Table 2. Beta1AR-AA was significantly and negatively associated to DBP in the control group (adj. beta-coefficient (SEM): -0.470 (0.206), p=0.024, Table 3 C right panel), and CXCR3-AA correlated positively to DBP in the controls (adj. beta-coefficient (SEM): 0.322 (0.145), p=0.028, Table 3 C right panel). MAP did not correlate to any of the regulatory autoantibodies levels, Table 4. Considering the whole cohort, only ETAR-AA significantly contributed to HR, Table 5 A right panel.
| Predictors of SBP | Adj. beta-coefficient (SEM) | p value | Predictors of SBP | Adj. beta-coefficient (SEM) | p value |
| A. Whole cohor | |||||
| Whole cohort, n = 485, adj. R2 = 0.195, p < 0.001 | Whole cohort, n = 485, adj. R2 = 0.198, p < 0.001 | ||||
| Intercept | 87.606 (12.526) | <0.001 | Intercept | 86.976 (12.428) | <0.001 |
| Age | 1.068 (0.183) | <0.001 | Age | 1.044 (0.182) | <0.001 |
| Gravidity | -0.714 (0.526) | 0.175 | Gravidity | -0.718 (0.523) | 0.170 |
| Years post index partus | -0.559 (0.258) | 0.031 | Years post index partus | -0.550 (0.257) | 0.033 |
| GA at index partus (wks) | -0.792 (0.130) | <0.001 | GA at index partus (wks) | -0.790 (0.129) | <0.001 |
| BMI | 0.688 (0.137) | <0.001 | BMI | 0.696 (0.136) | <0.001 |
| Waist-to-hip ratio | 13.401 (12.437) | 0.282 | Waist-to-hip ratio | 13.982 (12.381) | 0.259 |
| AT1R-AA | -0.065 (0.184) | 0.725 | |||
| ETAR-AA | 0.116 (0.162) | 0.714 | |||
| CXCR3-AA | -0.033 (0.098) | 0.737 | |||
| PAR1-AA | 0.015 (0.100) | 0.878 | |||
| Beta1AR-AA | -0.175 (0.183) | 0.338 | |||
| B. Early PE | |||||
| Early PE, n = 293, adj. R2 = 0.135, p < 0.001 | Early PE, n = 293, adj. R2 = 0.141, p < 0.001 | ||||
| Intercept | 57.323 (19.530) | 0.004 | Intercept | 53.720 (19.290) | 0.006 |
| Age | 3.833 (2.843) | <0.001 | Age | 1.225 (0.260) | <0.001 |
| Gravidity | -1.045 (0.756) | 0.158 | Gravidity | -1.065 (0.746) | 0.155 |
| Years post index partus | -3.346 (2.871) | 0.042 | Years post index partus | -0.744 (0.356) | 0.038 |
| GA at index partus (wks) | -0.496 (0.252) | 0.045 | GA at index partus (wks) | -0.491 (0.249) | 0.050 |
| BMI | 0.527 (0.197) | 0.008 | BMI | 0.516 (0.194) | 0.008 |
| Waist-to-hip ratio | 42.669 (19.462) | 0.032 | Waist-to-hip ratio | 44.085 (19.226) | 0.023 |
| AT1R-AA | -0.221 (0.285) | 0.439 | |||
| ETAR-AA | 0.198 (0.284) | 0.486 | |||
| CXCR3-AA | -0.071 (0.120) | 0.552 | |||
| PAR1-AA | -0.010 (0.123) | 0.936 | |||
| Beta1AR-AA | -0.124 (0.249) | 0.618 | |||
| C. Control | |||||
| Control, n = 192, adj. R2 = 0.130, p < 0.001 | Control, n = 192, adj. R2 = 0.124, p < 0.001 | ||||
| Intercept | 70.525 (9.133) | <0.001 | Intercept | 71.792 (9.092) | <0.001 |
| Age | 0.763 (0.226) | 0.001 | Age | 0.736 (0.226) | 0.001 |
| Years post index partus | -0.397 (0.350) | 0.258 | Years post index partus | -0.378 (0.347) | 0.278 |
| BMI | 0.770 (0.178) | <0.001 | BMI | 0.806 (0.177) | <0.001 |
| AT1R-AA | 0.098 (0.288) | 0.733 | Beta1AR-AA | -0.087 (0.160) | 0.558 |
| ETAR-AA | 0.084 (0.205) | 0.681 | |||
| CXCR3-AA | 0.216 (0.208) | 0.300 | |||
| PAR1-AA | 0.166 (0.191) | 0.385 | |||
| Beta1AR-AA | -0.594 (0.313) | 0.059 | |||
| Prediction was performed by multiple regression analysis in the whole cohort (A), early PE (B) and control (C) groups. Left panel: prediction models incl. regulatory autoantibodies as predictors. Right panel: prediction models without autoantibodies. SBP = systolic blood pressure, GA = gestational age, BMI = body mass index, AA = autoantibody. | |||||
| Predictors of DBP | Adj. beta-coefficient (SEM) | p value | Predictors of DBP | Adj. beta-coefficient (SEM) | p value |
| A. Whole cohort | |||||
| Whole cohort, n = 485, adj. R2 = 0.244, p < 0.001 | Whole cohort, n = 485, adj. R2 = 0.245, p < 0.001 | ||||
| Intercept | 69.073 (5.540) | <0.001 | Intercept | 69.465 (5.513) | <0.001 |
| Age | 0.594 (0.102) | <0.001 | Age | 0.592 (0.101) | <0.001 |
| Gravidity | -0.819 (0.357) | 0.022 | Gravidity | -0.867 (0.356) | 0.015 |
| GA at index partus (wks) | -0.661 (0.086) | <0.001 | GA at index partus (wks) | -0.675 (0.086) | <0.001 |
| BMI | 0.621 (0.091) | <0.001 | BMI | 0.624 (0.090) | <0.001 |
| AT1R-AA | -0.149 (0.126) | 0.238 | Beta1AR-AA | -0.137 (0.081) | 0.089 |
| ETAR-AA | 0.176 (0.111) | 0.115 | |||
| CXCR3-AA | 0.057 (0.067) | 0.397 | |||
| PAR1-AA | 0.047 (0.069) | 0.496 | |||
| Beta1AR-AA | -0.237 (0.126) | 0.060 | |||
| B. Early PE | |||||
| Early PE, n = 293, adj. R2 = 0.148, p < 0.001 | Early PE, n = 293, adj. R2 = 0.151, p < 0.001 | ||||
| Intercept | 42.776 (12.939) | 0.001 | Intercept | 40.504 (12.801) | 0.002 |
| Age | 0.753 (0.173) | <0.001 | Age | 0.754 (0.172) | <0.001 |
| Gravidity | -0.708 (0.498) | 0.156 | Gravidity | -0.720 (0.495) | 0.147 |
| Years post index partus | -0.370 (0.237) | 0.120 | Years post index partus | -0.379 (0.236) | 0.110 |
| GA at index partus (wks) | -0.383 (0.166) | 0.022 | GA at index partus (wks) | -0.380 (0.165) | 0.022 |
| BMI | 0.437 (0.130) | 0.001 | BMI | 0.438 (0.128) | 0.001 |
| Waist-to-hip ratio | 25.088 (12.846) | 0.052 | Waist-to-hip ratio | 25.735 (12.759) | 0.045 |
| AT1R-AA | -0.250 (0.189) | 0.186 | |||
| ETAR-AA | 0.218 (0.188) | 0.248 | |||
| CXCR3-AA | 0.032 (0.079) | 0.684 | |||
| PAR1-AA | 0.024 (0.081) | 0.765 | |||
| Beta1AR-AA | -0.203 (0.165) | 0.219 | |||
| C. Control | |||||
| Control, n = 192, adj. R2 = 0.210, p < 0.001 | Control, n = 192, adj. R2 = 0.200, p < 0.001 | ||||
| Intercept | 39.060 (6.878) | <0.001 | Intercept | 40.803 (6.866) | <0.001 |
| Gravidity | -0.618 (0.487) | 0.206 | Gravidity | -0.691 (0.493) | 0.159 |
| Age | 0.565 (0.170) | 0.001 | Age | 0.532 (0.170) | 0.002 |
| Years post index partus | -0.173 (0.264) | 0.514 | Years post index partus | -0.184 (0.264) | 0.486 |
| BMI | 0.772 (0.134) | <0.001 | BMI | 0.797 (0.133) | <0.001 |
| AT1R-AA | -0.075 (0.216) | 0.727 | Beta1AR-AA | -0.470 (0.206) | 0.024 |
| ETAR-AA | 0.204 (0.154) | 0.186 | CXCR3-AA | 0.322 (0.145) | 0.028 |
| CXCR3-AA | 0.266 (0.156) | 0.090 | |||
| PAR1-AA | 0.184 (0.143) | 0.200 | |||
| Beta1AR-AA | -0.567 (0.234) | 0.016 | |||
| Prediction was performed by multiple regression analysis in the whole cohort (A), early PE (B) and control (C) groups. Left panel: prediction models incl. regulatory autoantibodies as predictors. Right panel: prediction models without autoantibodies. | |||||
| Predictors of MAP | Adj. beta-coefficient (SEM) | p value | Predictors of MAP | Adj. beta-coefficient (SEM) | p value |
| A. Whole cohort | |||||
| Whole cohort, n = 485, adj. R2 = 0.239, p < 0.001 | Whole cohort, n = 485, adj. R2 = 0.239, p < 0.001 | ||||
| Intercept | 76.412 (6.573) | <0.001 | Intercept | 76.304 (6.534) | <0.001 |
| Age | 0.829 (0.139) | <0.001 | Age | 0.810 (0.138) | <0.001 |
| Gravidity | -0.729 (0.400) | 0.069 | Gravidity | -0.747 (0.399) | 0.062 |
| Years post index partus | -0.350 (0.196) | 0.075 | Years post index partus | -0.341 (0.196) | 0.082 |
| GA at index partus | -0.687 (0.099) | <0.001 | GA at index partus | -0.693 (0.098) | <0.001 |
| BMI | 0.657 (0.100) | <0.001 | BMI | 0.667 (0.099) | <0.001 |
| AT1R-AA | -0.117 (0.140) | 0.405 | |||
| ETAR-AA | 0.153 (0.123) | 0.215 | |||
| CXCR3-AA | 0.025 (0.074) | 0.740 | |||
| PAR1-AA | 0.034 (0.076) | 0.653 | |||
| Beta1AR-AA | -0.215 (0.139) | 0.122 | |||
| B. Early PE | |||||
| Early PE, n = 293, adj. R2 = 0.151, p < 0.001 | Early PE, n = 293, adj. R2 = 0.156, p < 0.001 | ||||
| Intercept | 47.625 (14.644) | 0.001 | Intercept | 44.909 (14.474) | 0.002 |
| Age | 0.914 (0.196) | <0.001 | Age | 0.911 (0.195) | <0.001 |
| Gravidity | -0.827 (0.564) | 0.144 | Gravidity | -0.835 (0.560) | 0.137 |
| Years post index partus | -0.491 (0.268) | 0.068 | Years post index partus | -0.500 (0.267) | 0.062 |
| GA at index partus (wks) | -0.424 (0.188) | 0.025 | GA at index partus (wks) | -0.417 (0.187) | 0.027 |
| BMI | 0.467 (0.147) | 0.002 | BMI | 0.464 (0.145) | 0.002 |
| Waist-to-hip ratio | 30.641 (14.539) | 0.036 | Waist-to-hip ratio | 31.852 (14.427) | 0.028 |
| AT1R-AA | -0.240 (0.214) | 0.261 | |||
| ETAR-AA | 0.211 (0.213) | 0.322 | |||
| CXCR3-AA | -0.002 (0.090) | 0.980 | |||
| PAR1-AA | 0.013 (0.092) | 0.889 | |||
| Beta1AR-AA | -0.177 (0.186) | 0.344 | |||
| C. Control | |||||
| Control, n = 192, adj. R2 = 0.196, p < 0.001 | Control, n = 192, adj. R2 = 0.173, p < 0.001 | ||||
| Intercept | 48.932 (7.172) | <0.001 | Intercept | 50.111 (7.214) | <0.001 |
| Age | 0.622 (0.177) | 0.001 | Age | 0.590 (0.179) | 0.001 |
| Years post index partus | -0.278 (0.275) | 0.313 | Years post index partus | -0.264 (0.276) | 0.340 |
| BMI | 0.775 (0.140) | <0.001 | BMI | 0.807 (0.141) | <0.001 |
| AT1R-AA | -0.030 (0.226) | 0.895 | Beta1AR-AA | -0.092 (0.127) | 0.467 |
| ETAR-AA | 0.175 (0.161) | 0.279 | |||
| CXCR3-AA | 0.255 (0.164) | 0.121 | |||
| PAR1-AA | 0.184 (0.150) | 0.220 | |||
| Beta1AR-AA | -0.579 (0.246) | 0.020 | |||
| Prediction was performed by multiple regression analysis in the whole cohort (A), early PE (B) and control (C) groups. Left panel: prediction models incl. regulatory autoantibodies as predictors. Right panel: prediction models without autoantibodies. | |||||
| Predictors of HR | Adj. beta-coefficient (SEM) | p value | Predictors of HR | Adj. beta-coefficient (SEM) | p value |
| A. Whole cohort | |||||
| Whole cohort, n = 485, adj. R2 = 0.019, p = 0.030 | Whole cohort, n = 485, adj. R2 = 0.025, p = 0.003 | ||||
| Intercept | 64.846 (3.177) | <0.001 | Intercept | 64.492 (3.098) | <0.001 |
| Gravidity | -0.405 (0.425) | 0.341 | Gravidity | -0.428 (0.422) | 0.312 |
| Smoking | 2.872 (1.498) | 0.056 | Smoking | 2.988 (1.488) | 0.045 |
| BMI | 0.205 (0.108) | 0.058 | BMI | 0.205 (0.107) | 0.055 |
| AT1R-AA | -0.078 (0.151) | 0.605 | ETAR-AA | 0.158 (0.062) | 0.011 |
| ETAR-AA | 0.237 (0.133) | 0.075 | |||
| CXCR3-AA | 0.020 (0.080) | 0.798 | |||
| PAR1-AA | 0.053 (0.083) | 0.521 | |||
| Beta1AR-AA | -0.104 (0.150) | 0.488 | |||
| B. Early PE | |||||
| Early PE, n = 293, adj. R2 = 0.041, p = 0.011 | Early PE, n = 293, adj. R2 = 0.037, p = 0.003 | ||||
| Intercept | 62.566 (4.027) | <0.001 | Intercept | 64.244 (3.867) | <0.001 |
| Gravidity | -0.794 (0.557) | 0.156 | Gravidity | -0.887 (0.556) | 0.112 |
| BMI | 0.282 (0.135) | 0.037 | BMI | 0.293 (0.134) | 0.029 |
| Smoking | 4.791 (1.996) | 0.017 | Smoking | 4.737 (1.984) | 0.018 |
| AT1R-AA | -0.025 (0.215) | 0.907 | |||
| ETAR-AA | 0.208 (0.213) | 0.330 | |||
| CXCR3-AA | 0.048 (0.090) | 0.594 | |||
| PAR1-AA | 0.075 (0.93) | 0.422 | |||
| Beta1AR-AA | -0.101 (0.187) | 0.590 | |||
| C. Control | |||||
| Control, n = 192, adj. R2 = -0.003, p = 0.496 | Control, n = 192, adj. R2 = -0.002, p = 0.442 | ||||
| Intercept | 50.568 (21.664) | 0.021 | Intercept | 54.231 (21.334) | 0.012 |
| GA at index partus (wks) | 0.490 (0.543) | 0.368 | GA at index partus (wks) | 0.411 (0.533) | 0.442 |
| AT1R-AA | 0.007 (0.299) | 0.982 | |||
| ETAR-AA | 0.166 (0.214) | 0.439 | |||
| CXCR3-AA | -0.214 (0.215) | 0.322 | |||
| PAR1-AA | -0.061 (0.195) | 0.754 | |||
| Beta1AR-AA | 0.077 (0.323) | 0.813 | |||
| Prediction was performed by multiple regression analysis in the whole cohort (A), early PE (B) and control (C) groups. Left panel: prediction models incl. regulatory autoantibodies as predictors. Right panel: prediction models without autoantibodies. HR = heart rate, GA = gestational age, BMI = body mass index, AA = autoantibody. | |||||
One of the interesting findings in our study is the high prevalence of hypertension ca. 10 years after the index pregnancy, with nearly half of the former preeclamptic women being hypertensive. The levels of single regulatory autoantibodies against GPCR were not different between women after preeclamptic pregnancies and women without any birth complications 8- 11 years postpartum. When we combined all autoantibodies levels, women with the lowest levels of regulatory antibodies (lower 0.25 quantile) and a history of early PE had significantly higher BP compared to the control group in the lower quantile. In the higher quantile, BP was not different between the groups. We thus hypothesize that, in accordance with our primary hypothesis, regulatory autoantibodies are not sufficient alone to cause pathologic conditions, such as hypertension, but together with other risk factors (e.g., history of PE) might drive the normal balance towards pathophysiological conditions.
Intercorrelations between AT1R-AA, beta1AR-AA, ETAR-AA, PAR1-AA and CXCR3-AA contributed to the multicollinearity in the prediction models. However, when the autoantibodies were removed from the models, no multicollinearity could be detected anymore. In our adjusted models, ETAR-AA significantly and positively associated to HR in the whole cohort, beta1AR-AA correlated negatively and CXCR3-AA positively to DBP in the controls. No further relationships between the single autoantibodies and SBP, DBP, MAP or HR could be detected in any of the sub-groups.
The results we report here are partially inconsistent with our previously published studies (13, 14, 16, 17), where we have shown higher titers of AT1R-AA in preeclamptic women during pregnancy and at 18 months postpartum compared to women with uncomplicated pregnancies. A possible explanation is that in the previous studies, we used the bioassay developed by Wallukat and Wollenberger (13, 14, 16, 17, 26), which documented the changes in the beating rate of spontaneously beating neonatal cardiomyocytes exposed to agonistic autoantibodies. Whereas we used a functional assay in previous studies, a commercially available ELISA kit from CellTrend was used to determine the titers of autoantibodies in the present study. This ELISA is already used in clinical practice in transplantation medicine to detect non-HLA mediated graft rejection (27, 28). The ELISA is further used in scleroderma to detect patients who are at risk for pulmonary hypertension and vascular complications (29, 30). The vascular pathology of scleroderma, graft rejection due to anti-endothelial antibodies and preeclampsia share several homologies. They all involve small vessels with a heavy inflammatory reaction. Unfortunately, it is not known what the permissive factor is for causing the pathology in the lung, kidney or placenta. Furthermore, it was shown that the AT1R-AA in a kidney rejection patient bind to two epitopes of the AT1-receptor (31, 32), whereas only one binding site is seen in preeclamptic patients (13). This might be an important difference for the different assays used. Nevertheless, AT1R-AA were detected in preeclamptic patients during pregnancy compared to controls (33). Further, the present sub-study is a post-hoc analysis of all available serum samples, and the original study was not designed and powered to detect differences in the autoantibodies levels.
The functional activity of regulatory antibodies and the direction of their immunological function remain obscure and need to be further evaluated. While some of the studies report an aggravation of a disease with detectable or increasing levels of autoantibodies (9, 13, 28-31, 34, 35), no association between autoantibodies and classic cardiovascular outcomes is found in another study (36). Kreienbring et al. detected a negative relationship between PAR1-AA levels and histological grading in primary epithelial ovarian cancer, where the autoantibody levels were also significantly reduced in the patient group as compared to the healthy controls (37). A study on CXCR3- and CXCR4-AA in scleroderma patients showed contradictory results, where high antibody levels were positively associated with signs of lung fibrosis and simultaneously reduced progression of lung fibrosis (38). Low anti-CXCR3/4 levels predicted progressive deterioration of lung function (FVC values) and suggested a protective effect of the antibodies in that study (38). Further, patients with acute coronary syndrome displayed likewise lower levels of beta1AR-AA compared to controls (39). Here, we show that higher levels of anti-GPCR antibodies might have a similar protective effect in post-preeclamptic patients by ameliorating the increase in blood pressure.
In conclusion, regulatory autoantibodies are not sufficient alone to cause hypertension or other pathologic conditions, but together with other risk factors, such as pathological pregnancy, might induce pathophysiological conditions. One hypothesis is that they may rise secondary to an altered immune reaction due to the preeclamptic cause. The presence of the regulatory autoantibodies may be limited after the pathological event. No data are available if they are present before a preeclamptic pregnancy. From kidney transplantation cohorts we know that AT1R-AA are present before the transplantation when the patients have been in renal failure for some time (31, 40). Again, we do not know, if they are present before the kidney disease started. However, with the robust and established ELISA we will solve these important questions. Our data also indicate that individual regulatory autoantibodies against GPCR are alone not predicting hypertension, but together with other risk factors, such as pathological pregnancy, might be able to identify patients at risk. Further studies are needed to investigate the potential of regulatory autoantibodies against GPCR as pre-symptomatic biomarkers identifying women at particular cardiovascular risk. Combination of several autoantibodies could prove to be a more promising approach in future studies.
We thank May-Britt Köhler, Ilona Kamer and Juliane Anders for their excellent technical assistance. The Deutsche Forschungsgemeinschaft (DFG) supported Dr. Herse (HE 6249/4-1, HE 6249/5-1). The German Centre for Cardiovascular Research (DZHK) supported Drs. Müller, Dechend and Haase, as well as Mss. Kräker and Birukov.
