By restraining proliferation and promoting apoptosis, resveratrol (RES) has anti-tumor effect in various cancers. Here, we examine whether RES exerts similar effect in drug-resistant renal cell carcinoma (RCC). To this end, Caki-1 cells derived from renal carcinoma are subjected to escalated doses of paclitaxel (PTX) to produce the PTX resistant Caki-1PTX cells. Both Caki-1 and Caki-PTX cells are sensitive to PTX, in a dose dependent manner. RES, dose-dependently, suppresses the expression of survivin, a molecular biomarker of cancer and a member of the inhibitor of apoptosis (IAP) family and its co-administration with PTX inhibits the effect of this drug on cell viability. To decipher whether survivin expressed by cancer cells is involved in rendering cells PTX sensitive, survivin is overexpressed. This dramatically counteracts the effects of RES on cell survival in the presence of PTX. Furthermore, decrease of survivin by inhibition of PI3K/AKT pathway significantly inhibits the effect of PTX in Caki-1PTX cells. These data show that RES increases the sensitivity of PTX resistant renal cells to drug treatment.
Renal cell carcinoma (RCC) is one of the most common and fatal malignant tumors in adult kidney (1). The prognosis of patients with distant metastasis of RCC is very poor, and the 5-year survival rate is less than 20% (2). At present, due to the high resistance of RCC tumor cells to conventional chemotherapeutic drugs, the standard treatment of RCC has not yet been established (3, 4). Therefore, it is urgent to find new drugs to improve the chemical sensitivity of RCC tumor cells to chemotherapeutic drugs.
In recent years, the role of natural products in tumors has become more and more prominent. Resveratrol (RES, trans-3, 4, 5-trihydroxystilbene) is a natural polyphenol antioxidant extracted from many fruits. Previous studies show that RES has a variety of biological activities, such as chemical prophylaxis and anticancer activity (5-8). Numerous studies show that RES plays an anti-tumor role by inducing apoptosis and autophagy in cancer cells. For example, RES resists prostate cancer by inducing apoptosis of reactive superoxide species-independent cells in mouse prostate cells (9). Kim et al. reports that RES induces apoptosis of oral squamous cell carcinoma cells and inhibits epithelial-mesenchymal transition (10). In addition, RES also inhibits the chemical resistance of cancer cells. Vinod’s study indicates that RES enhances docetaxel sensitivity in breast cancer cells by inhibiting docetaxel-mediated activation of the HER-2-Akt axis (11). RES enhances the sensitivity of cholangiocarcinoma cells to 5-fluorouracil (12). However, the effect of RES on paclitaxel-resistant RCC cells remains unclear and needs further study.
Survivin is the smallest member of the Inhibitor of Apoptosis (IAP) protein family. Survivin is abnormally expressed in many types of cancer (13 - 15). In malignant tumors, abnormal expression of survivin is associated with apoptosis, metastasis, and resistance to chemotherapeutic drugs (16, 17). Survivin may play an important role in chemotherapy resistance of RCC cells.
In this study, we investigate the role of RES in the chemosensitivity of RCC. The results indicate that RES enhances the sensitivity of RCC cells to PTX by inhibiting PI3K/AKT pathway to down-regulate survivin level. Our results suggest that RES combined with PTX may be a new chemotherapy strategy for the treatment of RCC.
The RCC cell line Caki-1 is purchased from the American Type Culture Collection (ATCC) and is stored in RPMI 1640 medium (Gibco, Grand Island, NY) supplemented with 10% FBS and 1% antibiotic-antimycotic solution. The cells are cultured at 37 ° C containing 5% CO2 in a humid atmosphere. Caki-1 cells against PTX (Caki-1 PTX) are established by stepwise exposure to PTX at a gradient concentration as previously described (18).
MTT assay is used to detect cell viability. The cells are seeded in 96-well plates with the final concentration of 5 × 103 cells / well. After the corresponding treatment, the cells are incubated with the MTT solution (1 mg / ml) for 4 hours. Formazan crystals are dissolved in DMSO and measured by absorbance at 570 nm using a microplate reader (SpectraMax M5, Molecular Devices). Cell viability is expressed as a percentage of the vehicle control.
Caki-1 PTX cells are seeded in 96-well plates with the final concentration of 2 × 105cells / well. After treated with different doses of PTX (0, 2, 4, 8, 16 nM) for 24 hours, the cells are incubated with 1 μg/ml Hoechst 33258 reagent at 37 ° C for 30 minutes in the dark. (Beyotime Institute of Biotechnology) Remove the Hoechst 33258 reagent and wash the cells with PBS three times (5 min × 3 times). The morphological changes of apoptotic cells are observed under an inverted fluorescence microscope and the images are captured.
Caki-1 PTX cells are seeded in 96-well plates with the final concentration of 2 × 105cells / well. After treated with different doses of PTX (0, 2, 4, 8, 16 nM) for 24 hours, the cells are stained with PI / AV-fluorescein isothiocyanate (BD Biosciences) and determined by flow cytometry FACSCalibur instrument (BD Biosciences) according to the manufacturer’s recommendations.
The cells are harvested and lysed with lysis buffer. The protein is extracted from cells, separated by 10% SDS-PAGE gel and transferred to PVDF membranes (Millipore, Boston, MA, USA). Then, the membrane is blocked with 5% skim milk and incubated overnight at 4 °C with the primary antibodies: survivin (No. 2808, 1:1000, Cell Signaling Technology, USA), PI3K (No. 4249, 1:1000, CST, USA), p-PI3K (No. 4228, 1:1000, CST, USA) AKT (No. 2920, 1:1000, CST, USA) and p-AKT (No. 4060, 1:1000, CST, USA). After that, the membrane is incubated with the corresponding HRP-conjugated secondary antibody for 1 hour at room temperature. Signals are detected using an Enhanced Chemiluminescence (ECL) commercial kit (Amersham Biosciences) according to the manufacturer’s instructions.
For overexpression of Survivin, the full-length Survivin sequence obtained from GenePharma is cloned into the pcDNA3.1-GFP vector. Caki-1 PTX cells are seeded into 96-well plates to achieve 60% confluence for transfection. Transfection is performed using Lipofectamine 2000 (Invitrogen, USA) according to the manufacturer’s protocol. Transfection efficiency is determined by western blotting.
All determinations are repeated in triplicate. All data are represented as the mean ± standard deviation (SD). Statistical software SPSS19.0 is used for the assessment. The Student’s t test is used to compare means of two groups and One-Way ANOVA is used for comparing means of multiple samples. Values of P< 0.05 are considered statistically significant.
First, Caki-1 cells and PTX-resistant Caki-1 PTX cells are treated with different doses of PTX (0, 2, 4, 8, 16 nM). The results show that PTX inhibits cell viability in Caki-1 and Caki-1 PTXcells in a dose-dependent manner, especially in Caki-1 cells. Besides, when the dose of PTX reaches 4 nM, the cell viability of Caki-1 PTX cells is significantly reduced, while no significant effect is observed at 2 nM. (Figure 1A, *P < 0.05, **P < 0.01, *** P< 0.001) Flow cytometry and Hoechst 33258 staining show that when the dose of PTX reaches 4 nM, the apoptosis rate of Caki-1 PTX cells is significantly increased, while no significant effect is observed at 2 nM. Taken together, these data indicate that PTX inhibits cell viability and inducts apoptosis in RCC cells. Caki-1 PTX cells in this study are not sensitive to 2 nM PTX. (Figure 1B-1D, *P < 0.05, **P < 0.01) Therefore, a 2 nM PTX dose is used for subsequent experiments.
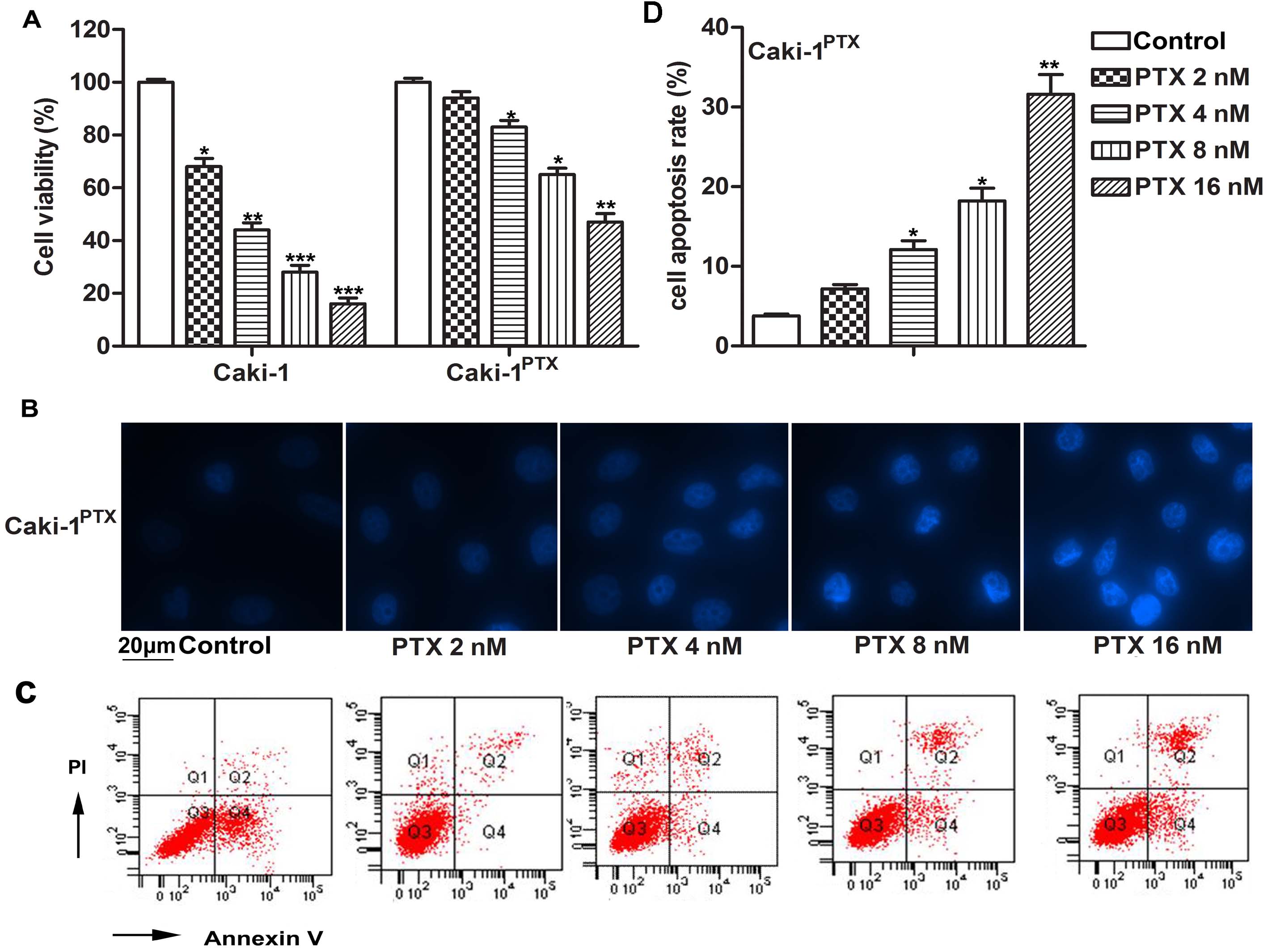 Figure 1.
Figure 1.
PTX inhibits cell viability and induces apoptosis in RCC cells. (A) Caki-1 cells and Caki PTX cells are treated with different doses of PTX (0, 2, 4, 8, 16 nM) for 24 h. Cell viability is measured by MTT assay. (B-D) CakiPTX cells are treated with different doses of PTX (0, 2, 4, 8, 16 nM) for 24 h. Cell apoptosis are measured by Hoechst 33258 staining and flow cytometry analysis. (B) Hoechst 33258 staining. (C) Flow cytometry. (D) Cell apoptotic rate in each group. The bars showed means ± SD of three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001 compared with control group.
After exposure to 2 nM PTX for 24 hours, Caki-1 PTX cells are then treated with RES (0, 10, 30, 50 μM) for another 24 hours. Flow cytometry shows that RES (30, 50 μM) significantly reduce cell viability in a dose-dependent manner, while increasing apoptotic rate compared to the control group (Figure 2A-2B, *P < 0.05, **P < 0.01). These results indicate that RES enhances the sensitivity of Caki-1 PTX cells to PTX.
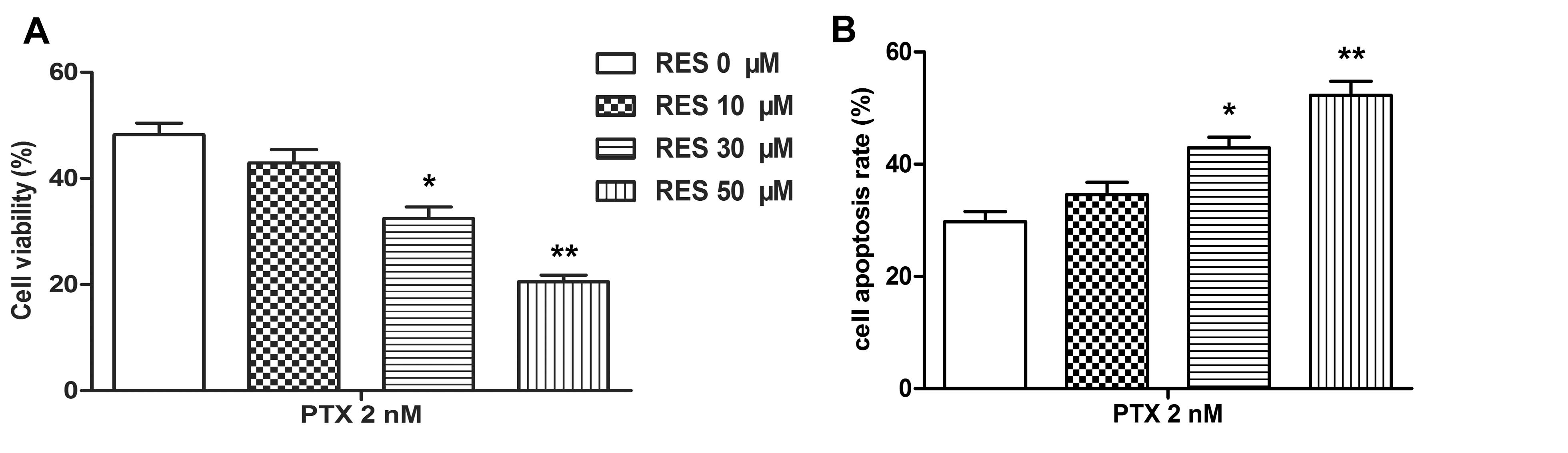 Figure 2.
Figure 2.
RES enhances the sensitivity of PTX resistant cells to PTX. Caki PTX cells are exposed to 2 nM PTX for 24 h, and then treated with different doses of RES (0, 10, 30, 50 μM) for another 24 h. (A) Cell viability is measured by MTT assay. (B) Cell apoptosis is measured by flow cytometry assay. The bars showed means ± SD of three independent experiments. *P < 0.05, **P < 0.01 compared with control group.
To investigate the role of survivin in Caki-1 PTX cells, western blot, MTT assay and flow cytometry are used. After exposure to 2 nM PTX for 24 hours, Caki-1 PTX cells are then treated with RES (0, 10, 30, 50 μM) for another 24 hours. As shown in Figure 3A, RES treatment (30, 50 μM) downregulate survivin level in a dose-dependent manner. (Figure 3A-3B, *P < 0.05, **P < 0.01) Besides, Caki-1PTX cells are transfected with pcDNA3.1-GFP or pcDNA3.1-GFP-Survivin after exposure to 2 nM PTX to determine transfection efficiency. As shown in Figure 3C-3D, transfection with pcDNA3.1-GFP-Survivin significantly increases survivin level, whereas there is no significant change after transfection with pcDNA3.1-GFP compared to the control. (* P <0.05, **P <0.01, ## P <0.01) In addition, western blotting also shows that treatment with RES (50 μM) significantly reduces survivin level, whereas overexpression of survivin has opposite effects. Furthermore, elevated survivin in Caki-1PTX cells significantly attenuates RES (50 μM)-induced cell viability inhibition and apoptosis promotion. (Figure 3E-3F, ## P <0.01, $P <0.05) Our results demonstrate that survivin is involved in the regulation of RES in RCC cells.
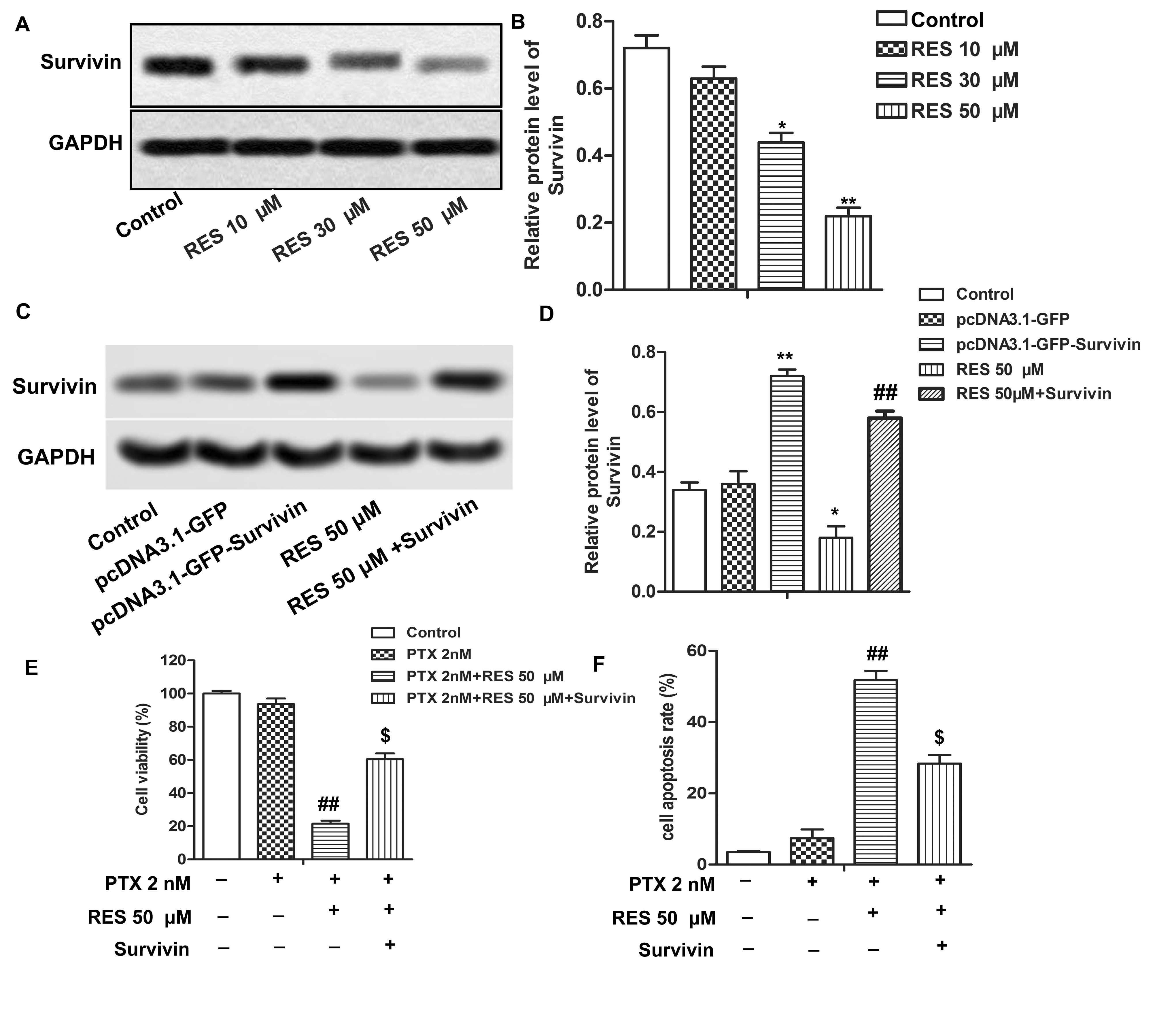 Figure 3.
Figure 3.
Survivin participates in the regulation of RES in RCC cells. (A-B) Caki PTX cells are exposed to 2 nM PTX for 24 h, and then treated with different doses of RES (0, 10, 30, 50 μM) for another 24 h. Protein level of survivin is measured by western blot. P < 0.05, **P < 0.01 compared with control group (C-D) Caki PTXcells are exposed to 2 nM PTX for 24 h, and then transfected with pcDNA3.1-GFP or pcDNA3.1-GFP-Survivin or RES 50 μM or the combination of RES 50 μM and pcDNA3.1-GFP-Survivin. Protein level of survivin is detected by western blot. *P < 0.05, **P < 0.01 compared with control group, ##P < 0.01 compared with RES 50 μM group (E) Cell viability is measured by MTT assay. (F) Cell apoptosis is measured by flow cytometry analysis. The bars showed means ± SD of three independent experiments. ##P < 0.01 compared with PTX 2 nM group, $P < 0.05 compared with PTX 2 nM + RES 50 μM group.
Western blotting analysis is performed to investigate the molecular mechanism of RES on RCC cells. Caki-1PTX cells are treated with RES (0, 10, 30, 50 μM) for another 24 hours after exposure to 2 nM PTX. As shown in Figure 4A-4B, RES treatment (10, 30, 50 μM) inhibit phosphorylation of PI3K and ATK in a dose dependent manner. (* P <0.05, ** P <0.01) These results indicate that the PI3K/AKT pathway is involved in the regulation of RES in RCC cells.
 Figure 4.
Figure 4.
PI3K/AKT pathway is involved in the regulation of RES in RCC cells. Caki PTXcells are exposed to 2 nM PTX for 24 h, and then treated with different doses of RES (0, 10, 30, 50 μM) for another 24 h. (A) Phosphorylation level of PI3K is monitored by western blot. (B) Phosphorylation level of AKT is monitored by western blotting. The bars showed means ± SD of three independent experiments. *P < 0.05, **P < 0.01 compared with control group.
To further confirm the role of RES in the PI3K / AKT pathway, the pathway activator IGF-1 is used. Western blotting shows that RES (50 μM) treatment significantly inhibits the phosphorylation levels of PI3K and AKT. However, the opposite result is observed by adding IGF-1 (50 ng / ml). (Figure 5A, *P < 0.05, **P < 0.01, #P < 0.05) Besides, the decrease in p-PI3K and p-AKT further reduces survivin level, while IGF-1 has the opposite effects. (Figure 5B, * P< 0.05, **P < 0.01, #P < 0.05) Furthermore, down-regulation of survivin ultimately inhibits cell viability and promotes apoptosis compared to Caki-1 PTX cells treated with 2 nM PTX. (Figure 5C-5D, ##P < 0.01, $P < 0.05) All these results indicate that RES inhibits cell growth and induces apoptosis by inhibiting the PI3K / AKT pathway to downregulate survivin level in PTX resistant cells.
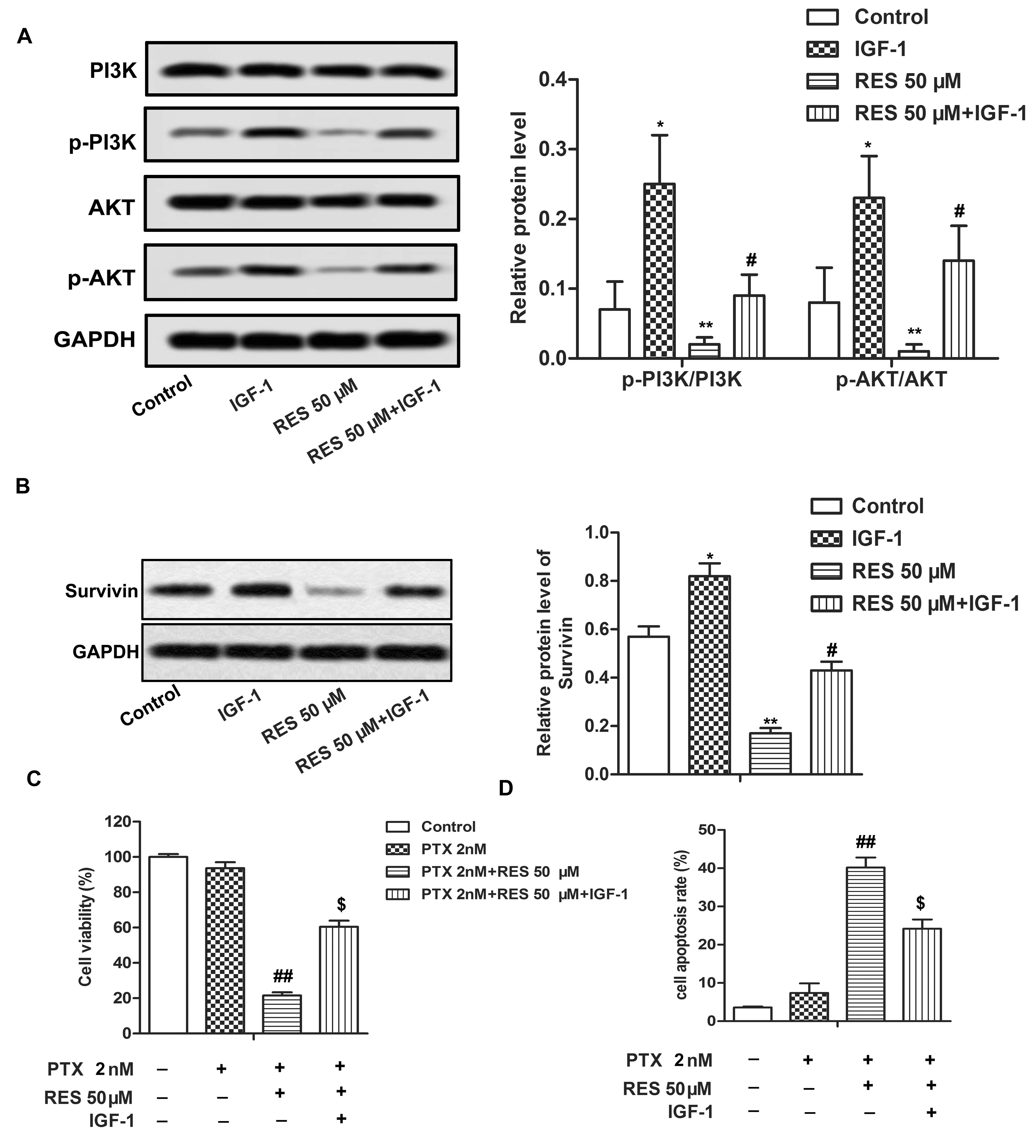 Figure 5.
Figure 5.
RES inhibits cell growth and induced apoptosis by inhibition PI3K/AKT pathway to downregulate Survivin level in PTX resistant cells. Caki PTXcells are exposed to 2 nM PTX for 24 h, and then treated with RES (50 μM) or/and IGF-1 (50 ng/ml). (A) Phosphorylation level of PI3K is monitored by western blotting. (B) Survivin is detected by western blotting. *P < 0.05,**P < 0.01 compared with control group, #P < 0.05 compared with RES 50 μM group (C) Cell viability is measured by MTT assay. (D) Cell apoptosis is measured by flow cytometry analysis. The bars showed means ± SD of three independent experiments. ##P < 0.01 compared with PTX 2 nM group, $P < 0.05 compared with PTX 2 nM + RES 50 μM group.
The drug resistance of tumor cells is the main obstacle in the process of cancer treatment. Most cancer-related deaths are attributed to the resistance of tumor cells to chemotherapeutic drugs (19). The key to overcome tumor drug resistance is to find drugs that can increase the chemosensitivity of tumor cells. However, due to different pharmacokinetics, the effectiveness of combined drug use is usually unsatisfactory (20). In our current study, PTX and RES are combined to inhibit the growth of PTX-resistant RCC cells. The study also shows that RES inhibits the expression of survivin through PI3K / AKT pathway, thus enhancing the sensitivity of Caki-1 PTX cells to PTX.
RES can inhibit the occurrence of RCC and other tumors. Previous studies have shown that RES inhibits the viability and migration of RCC cells and promotes apoptosis of RCC cells through p53 / AMPK / mTOR-induced autophagy signaling pathway (21). Zhang et al. have shown that RES inhibits the growth of human nasopharyngeal carcinoma cells by blocking pAkt / p70S6K signaling pathway (22). RES also inhibits the migration and invasion of pancreatic cancer by inhibiting PI3K / AKT / NF- κ B signaling pathway (23). Recent studies have also shown that RES combines with radiotherapy and chemotherapy has a positive effect on the treatment of cancer. (24, 25) PTX is a common chemotherapeutic agent to inhibit the survival and proliferation of tumor cells. However, some tumor cells can develop resistance to PTX. RES, as a natural polyphenol antioxidant, has been found to enhance PTX sensitivity in PTX-resistant MDA-MB-231 breast cancer cells (26). Similarly, current studies have shown that PTX inhibits the activity of Caki-1 cells. When the dose of PTX is more than 4 nM, PTX shows obvious anti-tumor effect in Caki-1 PTX cells, but when the dose of PTX is 2 nM, Caki-1 PTX cells are insensitive to PTX. Interestingly, RES treatment enhances the sensitivity of Caki-1 PTX cells to 2nM PTX in a dose-dependent manner. These results suggest that RES enhances the anti-tumor effect of PTX in PTX-resistant RCC cells.
Survivin is a member of the inhibitor of apoptosis (IAP) family, which can inhibit the activation of caspase and thus inhibit apoptosis (27). The anti-apoptotic effect of survivin plays an important role in cancers intervention (28). Survivin is abnormally expressed in a variety of cancer cells. The high expression of survivin is associated with poor prognosis in cancer patients (29, 30). Chen et al. have found that RES treatment inhibits the expression of survivin and induces Sirt1-dependent apoptosis in preadipocytes (31). Zhao et al. reports that RES induces multidrug resistance (32) in human NSCLC SPC-A-1 / CDDP cells by reducing the level of survivin. Similarly, the current studies suggest that RES inhibits the expression of survivin in RCC cells in a dose-dependent manner. The overexpression of survivin eliminates the inhibitory effect of RES on the chemical resistance of Caki-1 PTX cells. In conclusion, our results suggest that RES increases the chemical sensitivity of RCC cells to PTX by inhibiting the expression of survivin.
PI3K / AKT signal pathway shows excessive activation in a variety of cancers. Therefore, the inactivation of PI3K / AKT pathway can be used as an effective means in the treatment of cancer (33). Numerous studies show that RES can inhibit PI3K / AKT signaling pathway in many cancers, including colon cancer, hepatocellular carcinoma and glioblastoma (34 - 36). Consistent with these previous studies, current studies have shown that RES can significantly inhibit the phosphorylation of PI3K and AKT, thereby inactivating the PI3K / AKT signal pathway in RCC cells. In order to verify this conclusion, PI3K / AKT activator IGF-1 is used. The results show that IGF-1 significantly increases the expression of survivin in Caki-1 PTX cells. In addition, the activation of PI3K / AKT signaling pathway inhibits the sensitivity of Caki-1 PTX cells to PTX.
In conclusion, this study suggests that RES enhances the sensitivity of RCC cells to PTX by inhibiting the PI3K / AKT pathway to down-regulate surviving level. Our results suggest that RES combines with chemotherapy may be a new treatment strategy for RCC.
Ying-jie Ke, Ling-wei Chen, and Min Zhou, equally contributed to this work.
Abbreviations: RES, Resveratrol; PTX, pacitaxel; RCC, renal cell carcinoma; SD, standard deviation.
