miR-139 has a tumor suppressor effect in many tumors. Here, we examined the suppressive role of this miRNA and its target, ROCK1, in osteosarcoma (OS), a highly malignant bone tumor that mainly affects children and adolescents. The expression of miR-139 was down-regulated in OS. Overexpression of miR-139 significantly inhibited OS cell proliferation, migration, and invasion. Ectopic expression of miR-139 down-regulated ROCK1, a target of miR-139, by direct binding to its 3′ untranslated region (3’UTR). Direct siRNA-mediated silencing of ROCK1 exerted an inhibitory effect on OS cell proliferation and invasion similar to the effect of miR-139. ROCK1 transfection reversed the suppressive effect of miR-139 on OS cell proliferation and invasion. Both miR-139 and siRNA knockdown of ROCK1 significantly down-regulated β-CATENIN and p-AKT and up-regulated E-CADHERIN and p53. The data provided here show that miR-139 exerts suppressive effects on proliferation and invasion of OS cells by targeting ROCK1.
Osteosarcoma (OS) is the most common malignant bone tumor affecting children and adolescents (1) and is characterized by direct formation of immature bone or osteoid tissue by tumor cells. Despite extensive efforts during the past decades, the prognosis of advanced OS remains poor, with a 5-year survival rate of <30% and a 10-year survival rate of <50% (2). Considering that approximately 80% OS patients developed metastatic disease after surgical resection (3), immediate identification of biomarkers and therapeutic targets for OS patients is warranted.
MicroRNAs (miRNAs) are a class of small (22-nucleotide) noncoding RNA molecules that control gene expression and modulate miRNA stability and/or translation by binding to the 3’UTR of their target mRNAs (4). Many miRNAs play an important role in the growth and metastasis of tumor cells (5, 6). Some miRNAs act as oncogenes, whereas others act as tumor suppressors (7-9); for example, miRNA-449a expression was down-regulated in OS cells, and it promoted cell apoptosis by targeting BCL2 (10). miRNA-15a inhibits OS cell proliferation, migration, and invasion by targeting TNFAIP1 in humans (11). Abnormal expression of miRNAs is common in human tumors, and it plays a role in various pathological processes, such as oncogenicity or promotion of metastasis. Although recent studies on miRNAs have provided insight into our knowledge of human cancers, future studies are warranted to explore largely unknown details.
miR-139 is located within the second intron of the phosphodiesterase 2A (PDE2A) gene on chromosome 11q13.4. miR-139-5p is a common mature miRNA type that is produced from the miR-139 precursor (12). Its expression has been shown to be down-regulated in many cancers, including gastric cancer, breast cancer, and colorectal carcinoma (13, 14). miR-139-5p overexpression led to increased apoptosis, and miR-139 was regularly confirmed to have tumor-suppressing effects (15, 16). These data suggest that miR-139-5p plays a critical role in tumorigenesis. However, there is no research investigating the role of miR-139 in OS progression. The detailed regulating mechanism and the target gene of miR-139 largely remain unknown. Research on this would help in a better understanding of OS tumorigenesis and provide a new molecular therapeutic target for OS clinical therapy.
In the present study, we demonstrated that the ectopic expression of miR-139 significantly suppressed OS cell proliferation, migration, and invasion. Additionally, Rho-associated protein kinase 1 (ROCK1) was confirmed as a target gene of miR-139 in OS cells. Expression of ROCK1 was down-regulated by the expression of miR-139 in OS tissues, and restoration of ROCK1 expression in miR-139-overexpressing OS cells reversed the suppressive effect of miR-139. Therefore, we suggest that miR-139 is a promising therapeutic target for OS.
Twenty-five paired OS and 19 non-tumor tissue samples were obtained in our hospital. Informed consent was obtained from all participants, and approval was obtained from the ethics committee of Jinling Hospital (Nanjing, China). Human OS cell lines HOS, SAOS2, MG-63, U2OS, and OS732 and normal hFOB1.19 osteoblast cells were obtained from the American Type Culture Collection. All cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) at 37°C with 5% CO2.
A miR-139 mimic, si-ROCK1, and negative control (NC) were used (GenePharma Co., Ltd, Shanghai, China). Cell transfections were conducted using the Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions.
The OS cell lines MG63 and U2OS were seeded in 96-well plates with 5000 cells/well. Cell proliferation was assessed using a Cell Counting Kit-8 (CCK-8, Dojindo Molecular Technologies, Gaithersburg, MD). Every 24 h after seeding, 10 μL CCK-8 solution was added to the culture medium, and the cultures were incubated for 30 min at 37°C with 5% CO2. The absorbance was measured at 450 nm using a Microplate Reader (Bio-Rad, Hercules, CA, USA).
Cell invasion was determined via the transwell assay using 24-well Millicell Hanging Cell Culture inserts with 8-mm PET membranes (Millipore, Bedford, Massachusetts, USA); 4×104 cells in 200 μl serum-free DMEM medium were plated onto invasion chambers (BD Biosciences), and complete medium containing 10% FBS was added to the lower chamber. After processing the invasion chambers for 24 h (37°C with 5% CO2), non-invading cells were washed with PBS, and invading cells were stained with crystal violet solution and enumerated microscopically. The data are presented as the mean number of cells attached to the bottom surface from five randomly chosen fields.
MG63 and U2OS cells (5×105) were seeded into a 6-well culture plate containing medium with 10% FBS till monolayer cells were nearly confluent. Then a scarification was wounded by a by a 200μL sterile pipette tip, and the floating cells were removed by washing with PBS. Then, the wounded monolayers were maintained in serum-free medium for 24 h and then photographed under a phase-contrast microscope (Olympus, Japan).
Total RNA was extracted using the RNeasy kit (Qiagen AG, Hombrechtikon, Switzerland) according to manufacturer’s protocol. The RT-PCR protocol implemented was as follows: 37˚C for 1 h and 85˚C for 15 s. Specific RNA transcripts were quantified using SYBR Green qPCR using the ABI Prism 7700 Sequence Detection system (Applied Biosystems; Thermo Fisher Scientific, Inc.). Specific amplification was confirmed by the dissociation curve analysis. β-actin mRNA was used as an internal control. The primer sequences used in the study are presented in Table 1.
| Name | Forward 5′-3′ | Reverse 5′-3′ |
| miR-139-5p (Reverse-transcribed primer) | GTCAGAAGGAATGATGCACAGCCACTGGAG | |
| miR-139-5p | TCTACAGTGCACGTGTCTCCAG | ACCTGCGTAGGTAGTTTCATGT |
| U6 (Reverse-transcribed primer) | AACGCTTCACGAATTTGCGT | |
| U6 | CTCGCTTCGGCAGCACA | AACGCTTCACGAATTTGCGT |
| ROCK1 | ACCTGTAACCCAAGGAGATGTG | CACAATTGGCAGGAAAGTGG |
| β-ACTIN | GGGACCTGACTGACTACCTCA | TGACTCGTCATACTCCTGCTTG |
| ROCK1 (3′ UTR) | CCCTCGAGCAAACTACCTGCTACA | TTGCGGCCGCAGATACCCATCCATAA |
miR-139 and control mimics were obtained from RiboBio (Guangzhou, China). For conducting the luciferase reporter assay, the 3′ UTR of ROCK1 was amplified with primers shown in Table 1.
The mutation was performed using the QuikChangesite directed mutagenesis kit (Stratagene, La Jolla, CA, USA). ROCK1 siRNA (siROCK1) was obtained from Santa Cruz Biotechnology (Sangon, China)
Human embryonic kidney-293 cells were seeded into a 24-well plate and co-transfected with wild-type (WT) 3′ UTR or mutant (Mut) 3′ UTR of ROCK1 and miR-139 or the control mimics. Cells were incubated for 48 h and collected for the Renilla and firefly luciferase activity assays, for which the Dual Luciferase® Reporter Assay System (Promega, Madison, WI, USA) was used.
The protein samples were extracted using the RIPA lysis buffer (Beyotime Biotechnology, Shanghai, China) supplemented with protease inhibitors (Roche, Guangzhou, China). The proteins were quantified using the BCA™ Protein Assay Kit (Pierce, Appleton, WI, USA). Primary antibodies (ROCK1, catalog no., 21850-1-AP; dilution, 1:1000; β-actin, catalog no., 20536-1-AP; dilution, 1:2000) were incubated with the membrane at 4°C overnight; this was followed by incubation with Western Chemiluminescent HRP Substrate (Millipore, MA, USA). The signals were captured, and the band intensity was quantified using the Image Lab™ Software (Bio-Rad, Shanghai, China).
For the nude mouse xenograft tumor assay, 2×106 U2OS cells stably expressing miR-139 or NC cells were injected subcutaneously into the two sides of the 4-week-old BALB/c nude mice. Every week after the injection, the tumor volume was measured and quantified.
All data are expressed as the mean±SD values obtained through at least three independent experiments. The Student’s t-test was used to compare between-group differences. All statistical analyses were performed using SPSS 17.0 software. A P value of <0.05 was considered to be statistically significant.
The expression of miR-139 in 25 OS tissues and 19 non-tumor tissues was examined by qRT-PCR. The result showed that the expression of miR-139 was significantly decreased in OS tissues compared with that in non-tumor tissues (P<0.001) (Figure 1A). In addition, miR-139 expression was also significantly decreased in the five human OS cell lines HOS, SAOS2, MG-63, U2OS, and OS732 compared with that in the normal hFOB1.19 osteoblast cells (Figure 1B).
 Figure 1.
Figure 1.
miR-139 expression is down-regulated in OS tissues and cell lines. (A) The expression of miR-139 in 25 OS patient tissue samples and 19 normal tissue samples was determined using qRT-PCR analysis. (B) The relative expression level of miR-139 in HOS, SAOS2, MG-63, U2OS, and OS732 cell lines and the normal hFOB1.19 cell line was determined by qRT-PCR analysis. Data are presented as the mean ± standard deviation (SD), **P<0.01 and ***P<0.001.
To investigate the role of miR-139 in OS tumorigenesis, we overexpressed miR-139 in the OS cell lines MG63 and U2OS to detect cell proliferation and invasion. The expression of miR-139 was confirmed by qRT-PCR (Figure 2A). The results revealed that overexpression of miR-139 significantly inhibited proliferation of MG63 and U2OS cells (Figure 2B and 2C). Moreover, transwell assays showed that miR-139 overexpression suppressed the invasive ability of MG63 and U2OS cells significantly (Figure 2D and 2E). Figure The nude mouse xenograft tumor assay was performed to detect the effect of miR-139 on OS growth in vivo (Figure 2F and 2G). The weight of the tumors from miR-139 overexpressing nude mice was significantly lower than that of the NC group (Figure 2H). The results showed that miR-139 markedly inhibited the growth of U2OS cells in nude mice. Western blot analysis revealed that the protein levels of p-AKT and β-CATENIN were decreased in cells overexpressing miR-139 compared with the control cells (Figure 2I and 2J). Knowing that β-CATENIN and E-CADHERIN are associated with cell invasion, we measured levels of β-CATENIN and E-CADHERIN and found that the former was decreased, whereas the latter was increased (Figure 2I and 2J). These data suggest that miR-139 inhibited OS cell proliferation and invasion by regulating the AKT and E-CADHERIN/β-CATENIN pathways.
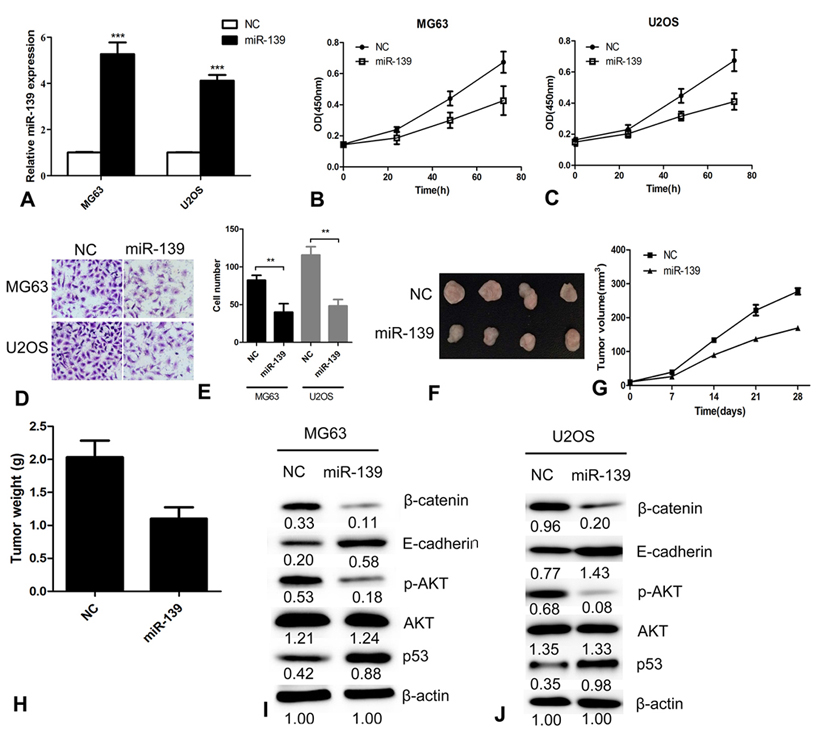 Figure 2.
Figure 2.
miR-139 inhibits OS cell growth in vitro and in vivo. (A) Overexpression of miR-139 was detected by qRT-PCR. (B) MG63 cell proliferation was measured by the CCK-8 assay at different time points. (C) Cell proliferation was measured by the CCK-8 assay at different time points. (D) Representative images of transwell invasion assay. (E) Quantification of transwell invasion assays of MG63 and U2OS cells. (F) Representative images of the wound healing migration assay. (G) Quantification of the wound healing migration rate of MG63 and U2OS cells. (H) Images of nude mice tumors. (I) Growth curve of xenograft tumors and the mean tumor weight were plotted every week. (H) The weight of nude mice tumors. (K) Western blot detection of miR-139 overexpression in MG63 cells. (L) Western blot detection of miR-139 overexpression in U2OS cells. **P<0.01 and ***P<0.001.
To elucidate the underlying mechanisms by which miR-139 exerts its function, we explored its targets using the TargetScan bioinformatics algorithm. Our analysis revealed that ROCK1 was a potential target of miR-139 based on the presence of putative target sequences at position 538–545 of the ROCK1 3′ UTR (Figure 3A). Then, we engineered luciferase reporter constructs containing the WT or Mut 3′ UTR of the ROCK1 gene to confirm whether ROCK1 was a direct target of miR-139. The results showed that miR-139 significantly decreased the luciferase activity of the WT but not that of the mutant ROCK1 in MG63 and U2OS cells (Figure 3B). Western blot analyses showed that miR-139 overexpression significantly down-regulated the expression of ROCK1 in U2OS cells (Figure 3C). The association of miR-139 and ROCK1 was further examined by analyzing the relative expression levels of ROCK1 in OS tissues, and the result showed that the level of ROCK1 in OS tissues was 3 times as high as that in normal tissues (Figure 3D). Next, we correlated expression of ROCK1 with miR-139 expression in the same OS specimens using Spearman’s correlation analysis and found a significant inverse correlation between the mRNA level of miR-139 and ROCK1 (Figure 3E, r2=0.9985, P=0.008). These data suggest that ROCK1 is a direct target of miR-139 in OS cells.
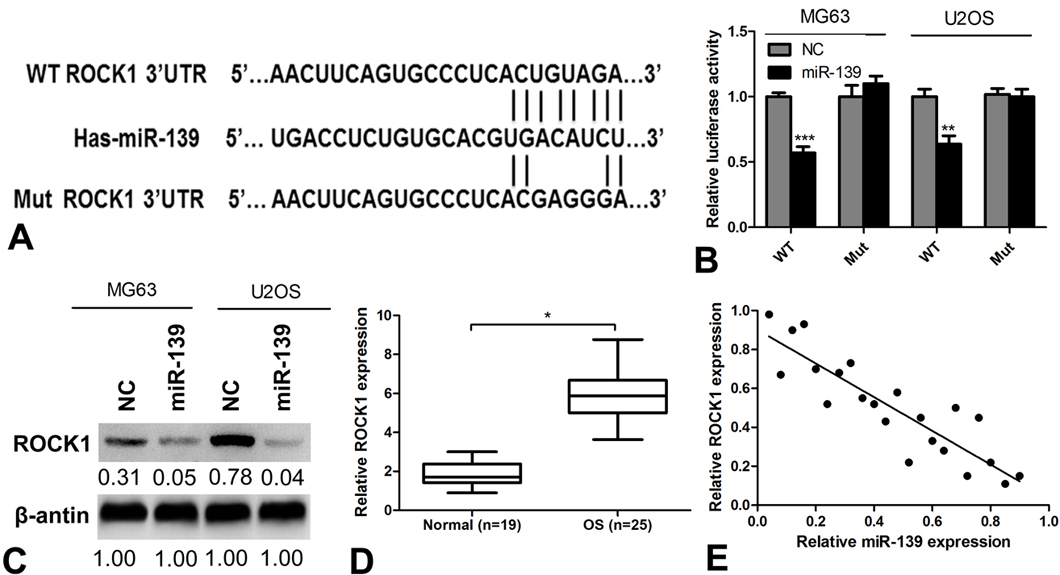 Figure 3.
Figure 3.
ROCK1 is a direct target of miR-139. (A) A diagram of the ROCK1 3′ UTR-containing reporter construct. Schematic representation of the miR-139 sequences, putative miR-139 targeting site in the 3′ UTR of ROCK1, and the generated mutant ROCK1 3′ UTR. (B) Luciferase reporter assay result of the inhibitory effect of miR-139 on the ROCK1-3′ UTR luciferase activity in MG63 and U2OS cells. (C) ROCK1 protein expression was analyzed in MG63 and U2OS cells after transfection with miR-139. (D) The relative expression level of ROCK1 in OS tissues and normal tissues detected by qRT-PCR. (E). Correlation of ROCK1 expression to miR-139 expression in OS samples using simple linear regression analysis.
The above-mentioned results indicate that miR-139 inhibited OS cell growth by suppressing ROCK1 expression. Furthermore, the effect of ROCK1 in OS cell proliferation and invasion was detected using siRNA against ROCK1 or NC. Western blot analysis confirmed that ROCK1 expression was significantly knocked down by siROCK1 in MG63 and U2OS cells (Figure 4A). ROCK1 silencing significantly inhibited cell proliferation (Figure 4B and 4C) and invasion (Figure 4D and 4E) in MG63 and U2OS cells, which is similar to the effect of miR-139. Western blot analysis also revealed that ROCK1 knockdown decreased the expression of p-AKT and p53 in MG63 and U2OS cells (Figure 4F). Simultaneously, the expression of β-CATENIN was decreased, whereas that of E-CADHERIN was increased in ROCK1 knockdown cells (Figure 4F). These results demonstrate that ROCK1 was essential for OS cell proliferation and invasion.
 Figure 4.
Figure 4.
ROCK1 knockdown inhibits OS cell proliferation and invasion. (A) Knockdown of ROCK1 by siRNA confirmed by Western blot analysis in MG63 and U2OS cells. (B, C) CCK-8 cell proliferation assays performed after infection with siROCK1 or NC. (D) Representative images of transwell invasion assay. (E) The quantification of transwell invasion assays of MG63 and U2OS cells. (F) Western blot detection after siROCK1 transfected in MG63 and U2OS cells. **P<0.01 and ***P<0.001.
To further verify the relationship between miR-139 and ROCK1, MG63 and U2OS cells were transfected with miR-139 and co-transfected with ROCK1-expressing plasmids (Figure 5A). As shown in Figure 5B and 5C, overexpression of ROCK1 markedly reversed the inhibitory effect of miR-139 on proliferation and invasion (Figure 5D, 5E, and 5F). Western blot analysis also revealed that miR-139 overexpression decreased the expression of p-AKT and β-CATENIN in MG63 and U2OS cells. Simultaneously, the expression of β-CATENIN was decreased, whereas that of E-CADHERIN and P53 was increased in miR-139 overexpression cells, while, on this basis, when ROCK1 is overexpressed, this change in expression is reversible (Figure 6). Taken together, these results indicate that ROCK1 is a functionally important target of miR-139 involved in the suppression of OS cell proliferation and invasion.
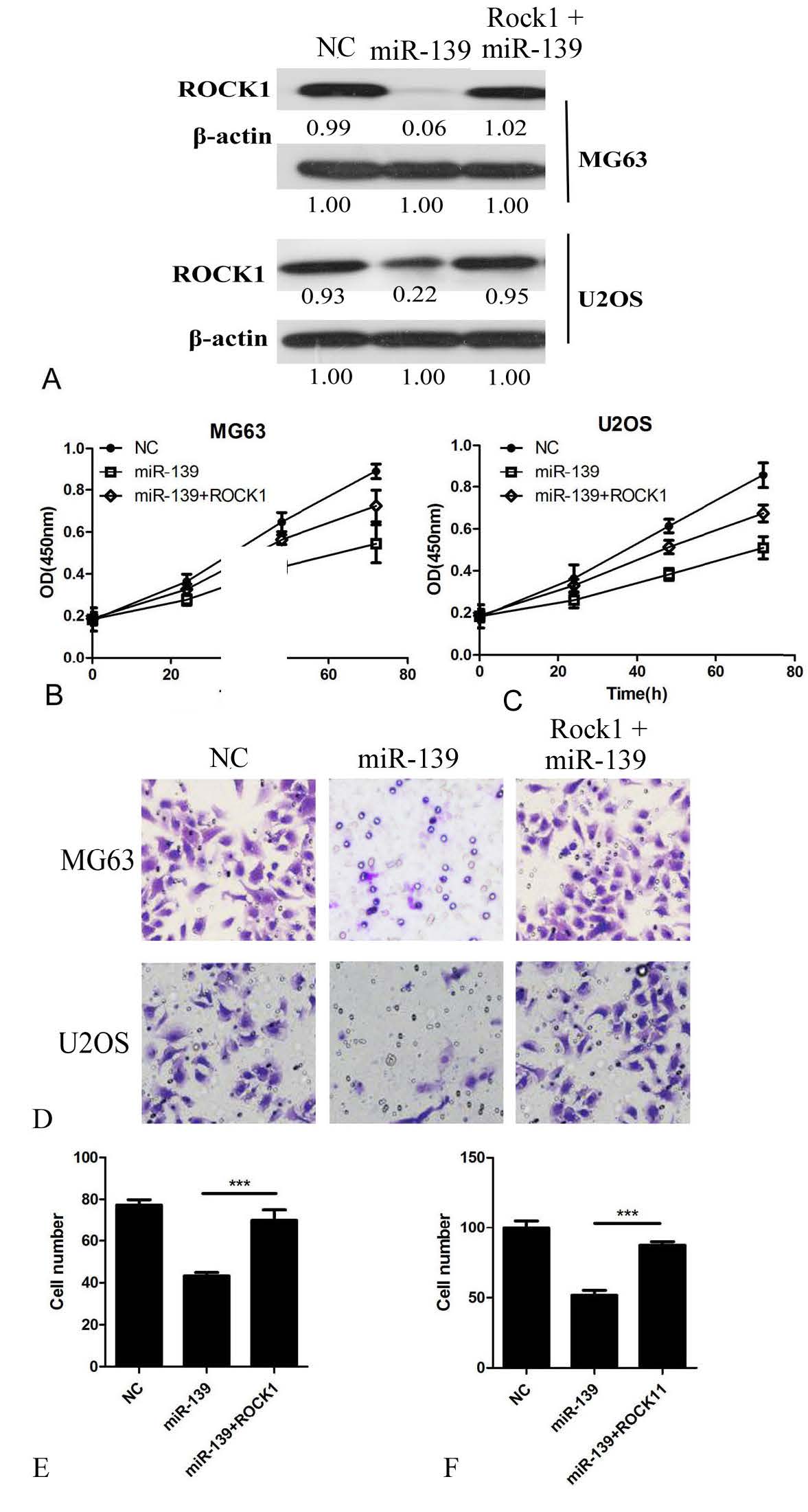 Figure 5.
Figure 5.
ROCK1 rescues cell proliferation and invasion in miR-139 overexpression OS cells. (A) ROCK1 overexpression in miR-139 transfected cells confirmed by Western blot analysis in MG63 and U2OS cells. (B, C) CCK-8 assays were performed to detect the effect of ROCK1 in miR-139 overexpression cell proliferation. (D) Representative images of transwell invasion assay. (E) The quantification of transwell invasion assays of MG63 cells. (F) The quantification of transwell invasion assays of U2OS cells. ***P<0.001.
 Figure 6.
Figure 6.
Western blot detect the downstream molecules after transfected with miR-139 and co-transfected with ROCK1-expressing plasmids in MG63 and U2OS cells.
Recent study has demonstrated that miRNAs are involved in the progression of various tumors by regulating the expression of multiple target genes (17). Many studies have also reported on the aberrant expression and function of miRNAs in OS. They can function as oncogenes or tumor suppressors (18, 19); for example, miR-101 inhibits OC cell proliferation, migration, and invasion(18), and miR-335 suppresses OS cell migration and invasion by targeting ROCK1 (19).
miR-139-5p expression is down-regulated in many cancers, especially in colorectal cancer (CRC) and breast cancer (20, 21). Ectopic expression of miR-139 was found to suppress metastasis and progression of breast cancer (21), hepatocellular cancer (HCC) (22), and CRC (23). These findings indicate that miR-139 plays a vital role in cancer tumorigenesis. However, the target genes and a detailed regulating mechanism of miR-139 in OS remains unknown. Studies on the biological function and regulating mechanism of miR-139 in OS may provide a new target for its clinical therapy. We found that the level of miR-139 was significantly decreased in OS tissues and cell lines, and this finding is consistent with those of previous studies (22). miR-139 overexpression significantly inhibited OS cell proliferation, migration, and invasion. However, there are ongoing debates on the mechanisms underlying the regulatory effect of miR-139 in OS tumorigenesis. miR-139 functions as a metastatic suppressor in many human cancers. Several genes have been identified as targets of miR-139. The miR-139-5p target-binding sites were found to be located in the 3′ UTR of targeted mRNA, such as Rho-Kinase 2 and c-Fos in HCC (23-25) and IGF-1R in CRC (26).
ROCK1 is a serine/threonine kinase and has been implicated in cancer metastasis (27). The association of miRNAs and ROCK1 and their involvement in cancer have been well documented. ROCK1 was reported as a target for several miRNAs, such as miRNA148a in gastric cancer, miRNA-584 in human clear cell renal cell carcinoma, and miRNA-340 in OS (2, 28, 29).
TargetScan bioinformatics algorithm was applied to investigate whether ROCK1 is a target of miR-139, and the results showed that there is a large possibility that miR-139 binds to ROCK1 3′ UTR. Results of the luciferase report assay revealed that miR-139 could significantly decrease the luciferase activity of the ROCK1 3′ UTR but not that of the mutant, and miR-139 could suppress ROCK1 in OS cell lines, indicating that ROCK1 is a direct target of miR-139. Results of the proliferation and invasion assays revealed that knockdown of ROCK1 showed an inhibitory effect on OS cell proliferation and invasion, which was similar to that shown by miR-139 overexpression.
Previous report has found that that p53 is an upstream positive regulator of Notch raised the interesting possibility that p53 may also control ROCK1/2 expression in human keratinocytes (30). Reversely, ROCK1 can also interact with p53 and regulate the expression of p53 (31). In the other hand, ROCK1 could regulate cell EMT, and β-CATENIN/E-CADHERIN were reported as downstream of ROCK1 (32). So we speculate that p53 and E-CADHERIN/β-CATENIN pathway may also be involved in ROCK1 and miR-139 regulated biological process in OS. Our result showed that miR-139 overexpression and ROCK1 knockdown decreased the expression of p-AKT and increased that of p53. Simultaneously, the expression of cell invasion factors β-CATENIN decreased, whereas that of E-CADHERIN increased by miR-139 overexpression and ROCK1 knockdown, indicating that miR-139 exerted its inhibitory effect on OS cell proliferation by regulating the AKT pathway and on OS cell invasion by regulating the E-CADHERIN/β-CATENIN pathways. Furthermore, restoration of ROCK1 in miR-139-overexpressing OS cells reversed the suppressive effect of miR-139 on OS cell proliferation and invasion. Taken together, our results suggest that miR-139 is a tumor suppressor and plays a role in the metastasis and progression of OS by targeting ROCK1.
This work was supported by the National Natural Scientific Foundation of China for Guangxin Zhou (No. 81472508) and Jiangsu Province Six Talents Summit Project (2014-WSW-042).
Abbreviations: OS, osteosarcoma; miRNA, microRNA; ROCK1, Rho-associated protein kinase 1
