Primordial germ cell migration and homing within the gonadal ridge during early embryo development requires oocyte-secreted polypeptide, growth factors, growth and differentiation factors (GDFs), bone morphogenetic proteins, stem cell factor (SCF), and basic fibroblast growth factor (bFGF). During embryogenesis, the germ cells migrate into developing gonads and undergo intra-ovarian development which involves the contact of primordial germ cells with other cells. Further follicular development and differentiation is tightly regulated by hormones and by intraovarian regulators. Maturation of cumulus-oocyte complexes and ovulation are directly controlled by FSH and LH and requires activation of mitogen-activated protein kinase in granulosa cells. The selection of dominant follicles is driven by a series of proliferation and apoptotic events. Together, the available data suggests that follicular development is regulated both by systemic and local factors.
During embryogenesis, primordial germ cells (PGCs) migrate from the yolk sac through the dorsal mesentery of the hindgut to the genital ridge and the somatic cells derives from the mesenchyme of the genital ridge. Both germ cells and somatic cells proliferate at the genital ridge rapidly. Then each germ cell is enclosed by one layer of somatic cells to form the primordial follicle. After mitosis occurred in the somatic cells, the germ cells undergo the first meiotic division and are called primary oocytes. The primary oocytes become arrested in diplotene stage of meiosis, until the primordial follicles start to grow and finally reach the ovulatory stage (1–3). During onset of primordial follicle growth, flattened pre-granulosa cells become cuboidal and begin to proliferate, and the enclosed oocyte begins to grow at the same time. It is interesting to know why and how some primordial follicles are capable of starting to grow, while their neighbor sisters remain quiescent. At the earliest stage of primordial follicle growth, because of the absence of receptors for the gonadotropin (FSH) in the follicular cells, the initiation of primordial follicle growth is independent of FSH regulation. Crosstalk and interaction between follicular cells and oocyte may be important for the onset of the primordial follicle growth. During both mitosis and meiosis, large numbers of the germ cells are culled from the ovary for as yet unknown reasons, resulting in less than one-third of the total number of potential germ cells being endowed in the ovary within primordial follicles shortly after birth. At birth, the number of germ cells in the primordial follicles has decreased markedly due to germ cell apoptosis occurring before the formation of ovarian follicles.
Folliculogenesis is a complex process involving dramatic morphological and functional changes in granulosa and theca cells. This process is sequential and dictated by specifically regulated responses to endocrine hormones and intraovarian regulators. Follicular selection dominantly depends on cell-cell interaction and apoptosis. A balance of cell proliferation and apoptosis plays an important role in the follicular dominate selection (4). Control of the primordial follicle development, differentiation and ovulation by the factors mentioned above are summarized in Figure 1.
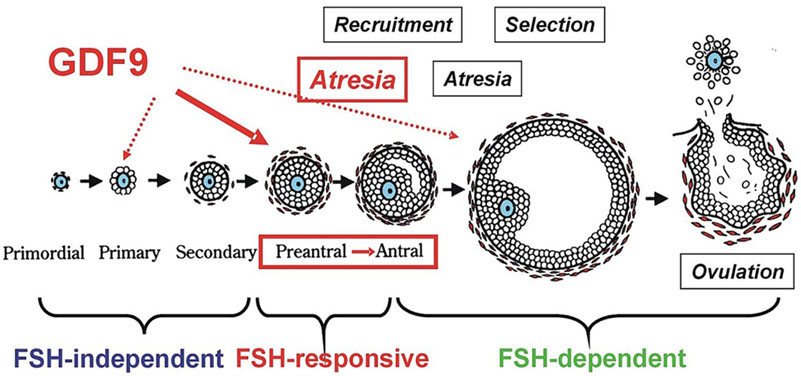 Figure 1.
Figure 1.A representative diagram to show the action of FSH and GDF9 on follicular development and atresia in a stage-dependent manner of follicular development. Growth of the follicle can be classified into distinct stages, each of which is influenced by a different subset of factors. From primordial to secondary follicle development FSH may be not needed. At this stage the follicular development may be termed gonadotropin-independent. Transition of the follicle from the preantral to early antral stage is primarily controlled by intraovarian regulators such as GDF9, the gonadotropin at this stage may be not required, termed as gonadotropin-responsive. The continual growth past antrum formation to the preovulatory stage may be FSH-dependent. (Reproduced with permission from (4)).
The precursors of spermatozoa and oocytes derive from a small number of pluripotent epiblast cells under the influence of inductive signals during gastrulation. Following specification, the PGCs actively migrate across the embryo to reach the developing genital ridge at approximately embryonic day (E) 10.5. (1). Once the PGCs reach the genital ridge, they continue to proliferate rapidly and maintain sexually undifferentiated state. In vivo, both transcriptional repression and chromatin remodeling block PGCs from acquiring a fully pluripotent stem cell ability. Although the developmental potency of PGCs is restricted to the germ lineage, PGCs can dedifferentiate into embryonic germ (EG) cells which possess pluripotency similar to that of embryonic stem (ES) cells in vitro. When exposed to exogenous signaling molecules such as leukemia inhibitory factor (LIF), stem cell factor (SCF) and basic fibroblast growth factor (bFGF), PGCs at bipotential stage show propensity to form EG cells. The balance between pluripotency and differentiation of fetal germ cells is crucial for the generation of a sufficient number of spermatogonial stem cells in males (2–4). However, how somatic signals regulate this balance, and which signaling pathways are involved remain unknown. The Wt1 is specifically expressed in germ-cell supporting somatic cells in fetal testes. Using a tamoxifen-inducible Cre-loxP mouse model (Wt1-/flox, Cre-ERTM), we demonstrate that germ cells fail to activate the male-fate driving gene Nanos2 in Wt1-deletion testes. Pluripotent embryonic germ (EG) cell colonies can be derived from embryonic day (E) 13.5. PGCs from Wt1-deletion testes, which is no longer possible from wild-type differentiated testes, moreover, the expression of pluripotent regulators (such as Oct4, Sox2, Nanog and Dppa4) can be still detected after E14.5. In the absence of Wt1 function, no signs of G1/G0 arrest and de novo methylation of the genome are detected. We observed that fibroblast growth factor (FGF) signals are ectopically activated in Wt1-/flox, Cre-ERTM testes. We further prove that Wt1 in Sertoli cells acts to directly repress FGF2 expression and hence FGFs production by those cells (4–6). Inhibition of the FGF signals by a FGFR-specific tyrosine kinase inhibitor SU5402 rescues the abnormal germ cell differentiation in Wt1-/flox, Cre-ERTM testes. In addition, this effect of FGFs on germ cell differentiation in Wt1-deletion testes also depends on Nodal signaling, because it is blocked by a specific inhibitor of type I ALK 4/5/7 receptors SB431542 (7–10).
Stem cell factor (SCF) is essential for development of primordial follicles. Using cultured ovaries from neonatal rats, the effect of SCF on early follicular development was investigated (11, 12). Expression of the three key protein factors, SF-1, StAR and P450arom in steroidogenesis were measured. SF-1 and StAR proteins were expressed in all ovarian cells. P450arom mRNA was localized exclusively in oocytes, implying that estrogen might be synthesized by oocytes at this stage. SCF up-regulated the mRNA and protein expression of these proteins. To study the differentiation status of follicular cells, the expression of FSHR and its response to SCF treatment was examined. The results showed that SCF inhibited FSHR expression and stimulated oocyte basic fibroblast growth factor (bFGF) expression. Inactivation of bFGF by its antibody resulted in a reversal of the inhibitory effect of SCF on the expression of FSHR. Therefore, the FSHR inhibitory effect of SCF could be mediated by bFGF, and SCF might stimulate oocyte to produce estrogen, while inhibit granulosa cell differentiation at the early stages of follicular development (13–15).
A primordial follicle is characterized by an undifferentiated oocyte surrounded by a single layer of squamous pregranulosa cells. During onset of primordial follicle growth, flattened pre-granulosa cells become cuboidal and begin to proliferate. The Oocyte factors direct growth of primordial follicles (1, 2). Evidence has been shown that oocytes isolated from secondary follicles of the rat ovary at 12-day-old co-cultured with somatic cells isolated from the primordial follicles of newborn ovaries could form re-aggregated ovaries, suggesting that the oocytes may produce factors to initiate primordial follicular growth by affecting the surrounding somatic cells.
We have demonstrated that treatment of cultured ovaries from neonatal rats with SCF for 8 days is capable of inducing primordial follicle development, initiating folliculogenesis by inhibiting oocyte apoptosis and up-regulating anti-apoptosis protein Bcl-2 and Bcl-Xl expression (14, 15), PI-3K/AKT pathways may be involved in the regulatory process. We also demonstrated that the early developing oocytes expressed SF-1, StAR and cytochrome P450 aromatase (P450arom). SCF up-regulated SF-1 and StAR expression and increased P450arom mRNA level, but suppressed GC FSHR mRNA expression.
Mammalian oocyte development occurs through tight coordination and interaction between all ovarian structures. Gap junctions between oocyte and granulosa cells appear to be important in cross-talk between the two cell types. Gap junctions are intercellular membrane channels composed of connexins (Cx), a family of integral membrane proteins, in which Cx43 and Cx37 may play the most important role in ovarian folliculogenesis. The oocyte is an effective modulator of granulosa-theca interactions. Oocyte control of granulosa and theca cell function may be mediated by several growth factors via a local feedback loop (s) between these cell types (4, 5).
The oocyte derived BMP15, also known as GDF9B, has also been shown to be essential for ovarian follicular development. However, the action of BMP15 and GDF9 varied with respect to the species of origin and the stages of follicle development. Moreover, the effects of GDF9 and BMP15 together were often co-operative and not always the same as those observed for these growth factors alone. Oocyte derived GDF9 stimulates rat granulosa cell proliferation, cumulus expansion, and prenatal follicle growth in vitro, whereas down-regulation of GDF9 by intra-oocyte injection of a GDF9 antisense morpholino attenuates both the basal and FSH-induced follicle growth, while addition of recombinant GDF9 enhances the basal and FSH-induced follicular growth (4–6).
We have carried out various experiments to comparatively investigate the interaction and communication of granulosa cells with oocyte in rat and monkey (18, 19). The regulation of steroidogenesis by granulosa cells of monkey infant ovaries was somehow different with the adult monkey granulosa cells. The signal pathways of autocrine regulation of steroidogenesis by FSH in the cultured rat granulosa cells were also investigated (18). We observed that a factor (s) produced by rat granulosa cells could stimulate oocyte tPA activity in vitro. The interaction and signal transduction between and oocyte and somatic cells in rat ovary have been discussed in the review (4, 5).
Excess androgen may be a main cause of polycystic ovary syndrome (PCOS). However, the mechanism is unclear. To investigate the possible impacts of androgen on early follicular development, neonatal mouse ovaries mainly containing primordial follicles were cultured with testosterone (20). We demonstrated that the number of primary follicles was increased after 10 days’ culture with testosterone via phosphatidylinositol 3-kinase/Akt pathway. Androgen induced Forkhead box (Foxo)-3a activation, and translocation of Foxo3a protein from oocyte nuclei to cytoplasm, which might be a key step for primordial follicle activation. Testosterone was also capable of down-regulating growth and differentiation factor-9 expression in granulosa cells via its receptor. Therefore, we suggest that intraovarian excessive androgen in PCOS might result in excess early follicles by inducing oocyte Foxo3a translocation and follicular arrest at the very early stages by down-regulating growth and differentiation factor-9 expression, as shown in Figure 2.
 Figure 2.
Figure 2.Testosterone regulated localization and phosphorylation of Foxo3a. A, Testosterone rapidly and transiently promoted Foxo3a export from oocyte nuclei. a and b, Ovaries were untreated or stimulated with testosterone (T) (105 M) for 30 min; c and d, pretreated with or without LY294002 (25 M) or wortmannin (50 nM) for 1 h and then stimulated with T (105 M) for 30min. The ovaries were then fixed and probed with Foxo3a antibody (green) and Hoechst33342 (blue). Arrows indicate the Foxo3a localization in the region of oocyte cytoplasm. B, The cell lysates were analyzed by Western blot using antibodies to phospho-Foxo3a or Foxo3. C, The bar graph represents the results of densitometric analysis. The statistical evaluation of the data were from three independent experiments. *, P <0.0.5. (Repruduced with permission from (1)).
The canonical Wnt/β-catenin signaling is an evolutionarily conserved pathway, which regulates cell fate determination, differentiation, proliferation, motility and apoptosis in embryogenesis, as well as homeostasis in adult tissues. By using cultured secondary follicles with two layers of granulosa cells. We found that the development of the cultured follicles was repressed by either canonical Wnt signaling activators, LiCl and Wnt3a. LiCl-treated follicles were of smaller size with fewer granulosa cells surrounding the oocytes compared to the control follicles. Meanwhile the development of follicles was completely stopped after Wnt3a treatment and most of the granulosa cells were detached. In addition, the secretion of estradiol was also reduced by LiCl treatment. Further studies demonstrated that the proliferation and apoptosis of granulosa cells of the follicles were dependent on the Wnt signaling. LiCl treatment inhibited the proliferation of in vitro cultured follicular granulosa cells and promoted their apoptosis (12). Moreover, by activating the transcription factor Forkhead box (Foxo)-3a and its downstream targets Bim, PUMA and p27, LiCl accelerated apoptosis and suppress proliferation of the granulosa cells. On the other hand, inhibition of Wnt/β-catenin signaling by IWR-1 exerted the opposite effect, leading to better developed follicles via restricted function of Foxo3a and its targets (Figure 3). In conclusion, canonical Wnt/β-catenin signaling is involved in follicular development by regulating granulosa cell proliferation and apoptosis through modulating the function of Foxo3a and its downstream targets (12).
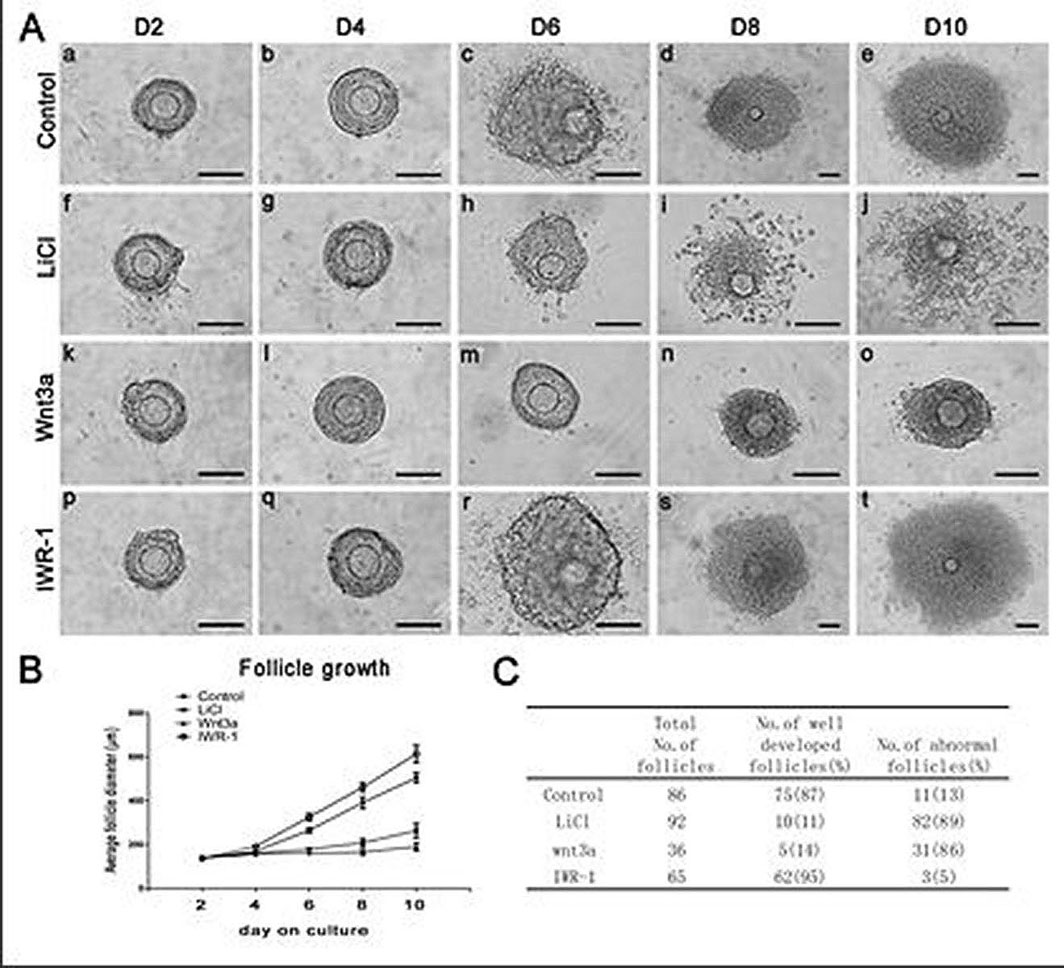 Figure 3.
Figure 3.Follicle growth, diameter and survival treated by LiCl, Wnt3a or IWR-1. A,Morphological observation of follicle growth over 10 days of culture. The up panel represents the control follicle on day 2 (a), day 4 (b), day 6 (c), day 8 (d) and day 10 (e) of culture. The second panel represents the LiCl (15mM) treated follicle on day 2 (f), day 4 (g), day 6 (h) , day 8 (i) and day 10 (j) of culture. The third panel represents the Wnt3a (100ng/ml) treated follicle on day 2 (k), day 4 (l), day 6 (m) , day 8 (n) and day 10 (o) of culture. The low panel represents the IWR-1 (10μM) treated follicle on day 2 (p), day 4 (q), day 6 (r), day 8 (s) and day 10 (t) of culture. Scale bar, 100μm. B,LiCl, Wnt3a and IWR-1effected on diameters of cultured follicles. Data are presented as the mean±S.E.M. The statistical evaluation of the data was from three independent experiments. C, Numbers of total cultured follicles, follicles of well developed and abnormal follicles from different treatment were counted. Percentage of each pattern was shown in parentheses (Reproduced with permission from reference (22)).
Notch signaling is also an evolutionarily conserved pathway, which regulates cell proliferation, differentiation and apoptosis. The members of Notch signaling are expressed in mammalian ovaries, by treating the primary follicles with Notch signaling inhibitors, L-658, 458 and DAPT (22), we found that the cultured follicles completely stopped developing, most of the granulosa cells were detached, and the oocytes were also degenerated with condensed cytoplasma. Further studies demonstrated that the proliferation of granulosa cells was dependent on the Notch signaling. Treatment with L-658,458 and DAPT inhibited proliferation of in vitro cultured primary granulosa cells and decreased the expression of c-Myc. Lentivirus-mediated overexpression of NICD2 and c-Myc could promote the proliferation of granulosa cells and rescued the growth inhibition induced by L-658,458 and DAPT. In conclusion, Notch signaling may be involved in follicular development by regulating granulosa cell proliferation (4, 21, 22).
Inhibin has long been considered as a FSH secretion suppressor from the anterior pituitary through pituitary-gonad negative feedback to regulate follicle development. We demonstrated that exogenous inhibin in cultured rat GCs could dramatically decrease FSH-induced P450arom and P450scc level, in GC observed in the same section of the early developing follicle may prevent the oocyte tPA mRNA translation. Once inhibin expression decreases in GC of the developing follicles an increasing oocyte tPA activity was observed, indicating that the translated oocyte tPA activity may induce its certain morphological changes similar to GVBD, leading to the oocyte apoptosis and the follicle atresia (23, 24). Based on this finding, we have proposed a hypothesis of follicular atresia originating from oocyte apoptosis (Figure 4).
 Figure 4.
Figure 4.Effect of inhibin emanated from GC on tPA mRNA translation in oocyte (Reproduced with permission from (4)). Inhibin originating from the GCs inhibits the oocyte maturation by inhibiting tPA mRNA translation in the oocyte. Once inhibin expression in GCs is decreased, the oocyte tPA mRNA starts to translate into its active protein, the subsequently increased tPA activity induces the oocyte GVBD in the dominant follicle leading to the oocyte maturation and ovulation; On the other hand, decreases in GC inhibin expression in the developing follicle, the oocyte tPA mRNA is triggered to translate tPA protein which is capable of inducing its certain morphological changes similar to GVBD in the developing follicle, subsequently leading to the oocyte and/or the follicle apoptosis.
FSH-activated p38 MAPK signaling cascade is involved in the regulation of steroidogenesis in GCs (25). GCs were prepared from the ovaries of DES-treated immature rats and cultured in serum-free medium. Treatment of GCs with FSH (50 ng/ml) induced the phosphorylation of p38 MAPK rapidly with the phosphorylation being observed within 5 min and reaching the highest level at 30 min. Such activation was protein kinase A-dependent as indicated by the results using specific inhibitors. FSH stimulated the production of progesterone and estradiol as well as the StAR expression in a time-dependent manner, with a maximum level at 48 h. Moreover, the potent p38 MAPK inhibitor SB203580 (20 μM) augmented FSH-induced steroid and StAR production, while reduced FSH-induced estradiol production at the same time. Inclusion of SB203580 in the media enhanced FSH-stimulated StAR mRNA production, while decreased FSH-stimulated P450arom mRNA expression. FSH treatment together with the inhibition of p38 MAPK activity resulted in a higher expression of StAR in mitochondria than FSH treatment alone. FSH also significantly up-regulated the protein level of LRH-1, a member of the orphan receptor family that activates the expression of P450arom in ovary and testis (25). Using the cultured GCs, we also examined whether FSH-activated ERK1/2 was involved in the regulation of the proliferation-related gene proliferating cell nuclear antigen (PCNA) and steroidogenesis. The results showed that FSH activated ERK1/2 in a time-dependent manner. Similarly, StAR and the steroid levels increased as FSH treatment time extended. ERK1/2 inactivation by UO126 inhibited the stimulatory effects of FSH on both PCNA and StAR and steroid synthesis in the GCs. ERK1/2 inhibition led to a reduction of mitochondrial StAR in the GCs by FSH. These observations suggested that the stimulation of FSH on PCNA expression and steroidogenesis in GCs was mediated in part by ERK1/2.
Gonadotrophin-releasing hormone type 1 (GnRH-I) and type 2 (GnRH-II) have been demonstrated to inhibit FSH-induced GC steroidogenesis (26–32). A third type of GnRH (GnRH-III) had been purified from salmon. The actions of various GnRH or their antagonist on FSH-regulated GC function have been investigated. Granulosa cells obtained from the ovaries of DES-treated immature rats were cultured in the presence or absence of FSH (100ng/ml) and GnRH-III (10-6 M) with androstenediol (10-7 M). The FSH-induced estrogen and progesterone production was significantly inhibited by the GnRH. The FSH-stimulated expression of steroidogenic acute regulatory protein (StAR), HSD3B2,aromatase (CYP19) and cytochrome P450 side-chain cleavage enzyme (CYP11A) were also significantly suppressed by this peptide (8, 9) The inhibitory action of the peptide on FSH-induced steroidogenesis was demonstrated via Akt and p38 mitogen-activated protein kinase (MPAK) signaling pathways through suppressing its own receptor (FSHR) expression (9). Further studies indicated that FSH could stimulate NR5A2 and upstream stimulatory factor (USF) activation, and their induction was significantly suppressed by the GnRH-III. Therefore, it is suggested that the inhibition of FSH-induced steroidogenesis by GnRH-III in granulosa cells might be due to the suppression of FSH-induced its own receptor expression via NR5A2 and USF transcriptional factors.
The demonstration of GnRH II expression, specific high affinity receptors for GnRH II and its potent bioactivity in human and baboon tissues led us to hypothesize that GnRH II is a bioactive peptide in primates. We demonstrated its contraceptive activity in rhesus monkey. We further found that there were dose–related actions of this analog on parameters of ovarian luteal function and conception (26, 27). GnRH II analog (0- 32 ug/day) or saline was administered via osmotic mini-pumps for six days (Day 1-6 post ovulation) to regularly cycling rhesus monkeys mated with fertile males around the time of ovulation. Progesterone production (Day 3 to 11) was significantly less (p<0.0.5.) for animals treated with 2, 4 or 8 ug/ml GnRH II analog than for controls, yet with higher doses of GnRH II analog (16 or 32 ug/day) luteal progesterone production was not different from saline-treated controls. Length of the luteal phase in all treated groups was similar to controls. In the eighteen animals, mated at the time of ovulation and then treated with GnRH analog (dose of 2 to 32 ug/day), no pregnancies resulted; but in the saline-treated controls five of eight animals (62.5%) became pregnant. Thus, the contraceptive activity of this GnRH analog did not correlate with luteal progesterone inhibition (Figure 5). These data demonstrate a dose-related action of GnRH II analog on luteal progesterone and establish the contraceptive activity of GnRH II analog at 2 to 32 ug/day administered just post ovulation.
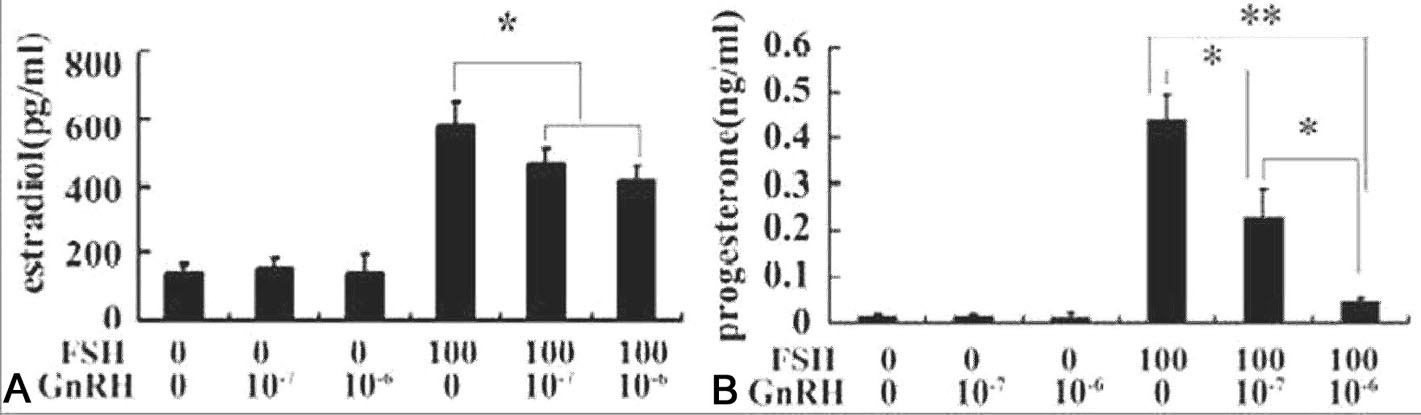 Figure 5.
Figure 5.GnRH-III inhibited FSH-induced estrogen and progesterone production in cultured primary granulosa cells. Granulosa cells obtained from DES-primed immature rat ovaries were cultured in the serum-free McCoy’s 5a containing androstendione (10-7M) alone (control) or supplemented with 100 ng/ml FSH and/or GnRH-III (10-7M and 10-6M) for 48 hrs. The contents of estradiol (Fig 1A) and progesterone (Fig 1B) in the media were measured by standard RIA procedures. Data are presented as mean±S.E.M. (n=3). Bars with * among the groups indicated significant different. *, significant different at P<0.0.5.; **, P<0.0.1. (Reproduced with permission from (28)).
It is well known that granulosa cells contain enzymes required for progesterone synthesis, and aromatases for androgen conversion into estrogens. However, GCs do not contain any enzymes for androgen production from progesterone. Therefore, GC itself cannot produce any estrogen. On the other hand, theca-interstitial cells are capable of forming androgens from progesterone, but no aromatase is present in the cells. Neither granulosa nor theca-interstitial cells alone are capable of producing estrogens. A synergistic interaction between granulosa and theca cells is a prerequisite for estrogen biosynthesis. Our early experiments demonstrated that granulosa cells obtained from preovulatory follicles under the action of FSH and LH produce high level of progesterone, which can be used by the theca- interstitial cells to synthesize high level of androgens under the influence of LH (19), as shown in Figure 6, the coordinated interaction between these two cell types under the action of both FSH and LH may be responsible for the granulosa cell estrogen production.
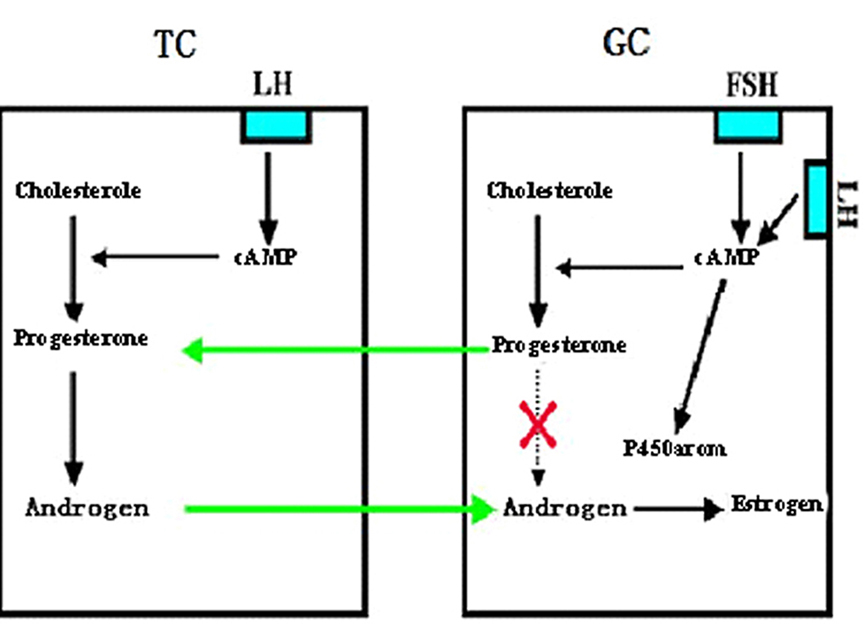 Figure 6.
Figure 6.A representative diagram showing involvement of interaction between granulosa cell (GC) and theca cell (TC) in estrogen production in the follicle. TC synthesizes androgen under the regulation of LH, but it cannot be converted into estrogen for lack of P450arom in TC. Under the action of FSH (also by LH at the late stage) GC synthesizes progesterone, the latter, however cannot be converted further into androgen for lack of the necessary converting enzymes in GC. GC cannot synthesize estrogen by itself, although it has the P450arom. TC used progesterone produced by GC to convert androgen, while the androgen produced by TC is used by GC to convert into estrogen. The interaction of TC and GC is a prerequisite for estrogen biosynthesis in the ovary (Reproduced from with permission from (4)).
Mammalian ovary and testis both originally differentiate from the very early primordial germ cells. The cell migration and homing within the gonadal ridge requires regulation of various integrated signal interaction produced by germ cells and somatic cells. Following gonadal specification, the PGCs actively migrate across the embryo to reach the developing genital ridge. The balance between pluripotency and differentiation of foetal germ cells is crucial for the generation of a sufficient number of spermatogonial stem cells. Therefore, the early regulation of oogenesis in female and SSCs proliferation in male may be the same. However, in adult mammalian animals, the regulation of oocyte proliferation and differentiation may be completed differently. The male spermatogenesis should be controlled in a lower temperature for SSCs proliferation and differentiation. These data suggest that regulation of male SSCs differentiation and meiosis may be more complicated (33–38).
This work was supported by Major Research Plan “973” Project (2006CB504001, 2006CB0F1002, 2007C B947502, 2012CB944702, 2011CB944302, G1999055901), National Technology Support Project (2012DA I131B08) and CAS “Chuangxi” (G1999055901, KSCX-2-SW-201), Natural Science Foundation of China (31501161, 31501953, 31471352, 31471400, 81270662 and 31171380), Clinical Capability Construction Project for Liaoning Provincial Hospitals (LNCCC-C09-2015, LNCCC-D50-2015) and Academician Workstation Support (Shenyang, Changsha and Shandong). The project was also supported by WHO/Rockefeller Foundation (RF96020#78) and CONRAD/CICCR Foundation (CIG-01-71).
The authors should thank Prof. Hsueh AJW, Ny T, Drs. Liu K, Feng Q, Peng XR, Hu ZY, Zou RJ, Chen YJ, Chen XL, Chen XX, Chen SR, Deng SL and Gao HJ for their contributions to the review data from the Lab.
