Frontiers in Bioscience-Landmark (FBL) is published by IMR Press from Volume 26 Issue 5 (2021). Previous articles were published by another publisher on a subscription basis, and they are hosted by IMR Press on imrpress.com as a courtesy and upon agreement with Frontiers in Bioscience.
1 Department of Biology, College of Science, Sultan Qaboos University, Muscat, Oman
2 Department of Food Science and Nutrition, Ageing and Dementia Research Group, College of Agricultural and Marine Sciences, Sultan Qaboos University, Muscat, Oman
3 Research and Policy Department, World Innovation Summit for Health (WISH), Qatar Foundation, P.O. Box 5825, Doha, Qatar
4 Department of Biomedical Sciences, Faculty of Medicine and Health Sciences, Macquarie University, Sydney, Australia
5 Department of Biochemistry, Faculty of Science, Annamalai University, Tamilnadu, India
6 Department of Behavioral Medicine, College of Medicine and Health Sciences, Sultan Qaboos University, Muscat, Oman
7 Department of Biology, College of Sciences, Shiraz Branch, Islamic Azad University, Shiraz, Iran
Abstract
Attention Deficit Hyperactivity Disorder (ADHD) is a common neurodevelopmental disorder among children and adults. Impulsivity, inattention, and hyperactivity are hallmark of ADHD. While ADHD is not on the autism spectrum, they are related in several ways as they have some overlapping symptoms. The pathogenesis of ADHD has so far remained enigmatic, however, there is some evidence suggesting critical association among ADHD and the level of oxidative stress which trigger cell membrane damage, changes in inner structure and function of proteins, as well as structural damage to DNA which eventually culminate into development of ADHD. Although stimulants as well as some classes of non-stimulants are used to ameliorate symptom of ADHD, various adverse effects have been associated with such compounds. To date, treatment of ADHD is done with a combination of medications, behavior modifications, psycho-education, family therapy and life-style changes. The American Academy of Pediatrics officially promote stimulant medications and/or behavior therapy as ‘first line of therapy’. In addition to the presently therapeutic armamentarium, evidences are emerging on relevancy of natural products. There has been an interest on the therapeutic role of antioxidants in the treatment of ADHD. The present review aims to highlight the beneficiary role played by different antioxidants in mitigating the symptoms of ADHD.
Keywords
- Attention Deficit Hyperactivity Disorder
- ADHD
- Antioxidants
- Oxidative stress
- Oxidation
- Antioxidant therapy
- Review
Attention Deficit Hyperactivity Disorder (ADHD) is a common cognitive and behavioral disorder that owes its onset during childhood development (1). Studies have reported various ratios of ADHD among adults (2-5 %) and children (5-7%) (2). The estimated prevalence of ADHD is around 6% to 8% (3). ADHD is believed to be more common in boys, as compared with girls (4). Roughly two-thirds of kids with ADHD have at least one co-existing condition, and autism is among those that commonly occur with ADHD. Based on the Diagnostic and Statistical Manual of Mental Disorders (DSM-V), ADHD is characterized by three symptoms including impulsivity, inattention, and hyperactivity (5). These symptoms may cause serious problems to the individual, family, as well as the society. Various studies have indicated some associations between ADHD and disorders related to attention seeking and impaired impulse control (6).
The pathogenesis related to ADHD has remained unknown (7). Some associations among ADHD and the dopamine (DA) level in brain have been found, highlighting the fact that subjects with ADHD manifest attenuated dopaminergic activity (8), a notion that has wide support in both preclinical and clinical studies and constitute the basis existing mainstay pharmacology approaches (9). Thus, pharmacology of neuro-stimulant such as methylphenidate is partly related to its affinity to DA neuron. Emergence of ADHD symptoms goes hand in hand with the reduction of DA levels in the brain (7). The present study aimed at reviewing the antioxidant therapies in ADHD.
Reactions related to reduction of oxidation in the body result in the production of waste materials known as oxidants. Human’s body has a complicated antioxidant defense system with the main duty of preventing the formation of oxidants, as well as the harmful effects associated with them. The concept of OS tends to describe the biological damage derived from the lack of balance among oxidants and antioxidants. OS is a biological condition where excess accumulation of oxidants occurs as a resultant of either, production of excessive amounts of oxidants, decreased level of anti-oxidants that scavenge them, or culmination of both events (10).
Humans are constantly exposed to free radicals created by electromagnetic radiation from the man-made environment like pollutants and cigarette smoke. Natural resources like radon, cosmic radiation, and cellular metabolisms (respiratory burst, enzyme reactions) may also add free radicals to the environment (11). Oxidants could be harmful, as they can be destructive to the protein structure of cell membranes. Excessive amount of free radicals and/or inefficiency of anti-oxidant system lead into detrimental impacts such as changes in inner structure as well as in the function of proteins, membrane damage and structural damage to DNA (12). It is believed that oxidants might inhibit uptake of enzymes and/or neurotransmitters which are involved in the physiological functioning of cells and could possibly be regarded as a predisposing factor for ADHD (13).
Oxidative Stress, as a common cause of ADHD, has recently gained a significant deal of attention among researchers. ADHD is usually treated by stimulant medications like methylphenidate and amphetamines, as well as non-stimulants like atomoxetine (2). However, these compounds are known to have various adverse effects on patients. Several studies have tended to highlight the potential roles of antioxidants in regulation of ADHD (14, 15).
Although the roles of factors related to biochemistry, genetics, psychology, and environment have already been accepted in the etiology of ADHD, the relationship among ADHD and OS, both in adults and children have been continuously studied (10). Changes in oxidative metabolism have been reported as a significant factor in the etiology of ADHD (16).
Kul et al. (13) have shown higher total antioxidant status (TAS) and lower OS index values among children with the ADHD inattentive type, as compared to those subjects with ADHD combined and hyperactivity/impulsivity types. However, similar levels of total oxidant status (TOS) among all subtypes were reported, suggesting that OS could possibly have a significant role in the pathogenesis of ADHD combined type (13). Sezen et al. (17) have also found an increase of TOS and oxidative stress index (OSI) in children with ADHD.
Structural and functional impairment in the prefrontal cerebral cortex, caudate, and cerebellum have been associated with ADHD symptoms (18, 19). In addition, studies have reported lower levels of neurotrophins like brain-derived neurotrophin factor (BDNF) in patients with ADHD (20). BDNF has a significant role in the function of hippocampus, long-term potentiation for learning and remembering, synaptic plasticity, neurogenesis, and neuroprotection (21). O&NS could be partly responsible for the damage to relevant structures of the brain which causes a reduction of neurotrophin concentrations.
In the cases where the antioxidant defense mechanisms are impaired, DA is very sensitive to auto-oxidation. Increase in OS may suppress striatal dopamine secretion, which ends in degeneration of dopaminergic neurons, dysfunction of dopamine receptors, dopamine transporters inhibition, as well as decreased hippocampal dopamine levels (22, 23). In addition, increased reactive oxygen species (ROS) may decrease the synthesis of dopamine through destroying 5,6,7,8-tetrahydrobiopterin (BH4), an enzyme with a rate-limiting responsibility and the function of facilitating the biosynthesis of dopamine, serotonin, adrenalin, and noradrenalin (24). Impaired dynamic thiol/disulfide homeostasis might have impacts on the pathophysiology of ADHD (25). This could be mediated through impairing the nature and activity or release of DA, as well as damaging the brain structures which are related to ADHD. However, a causal relationship among ADHD and dynamic thiol/disulfide homeostasis has not yet been confirmed and more studies are needed to establish this link (25).
O&NS could also have an impact on DA, which is known as the primary neurotransmitter associated with ADHD. Increase in hydrogen peroxide can suppress the striatal dopamine release (22), and excessive O&NS is known to have a role in the degeneration dopaminergic neurons (26). O&NS also have an impact on the function of dopamine receptor (27, 28). In addition, nitric oxide has an inhibitory impact on dopamine transporters by decreasing dopamine levels in the hippocampus (29, 23). 5,6,7,8- tetrahydrobiopterin (BH4) is a rate-limiting enzyme which is associated with the biosynthesis of serotonin and the catecholamines dopamine, adrenaline, and noradrenaline.
Increasing the output of ROS in macrophages over an extended period can be destructive to BH4. This leads to capacity reduction for dopamine and other neurotransmitter synthesis (24). Reduced BH4 activity could therefore be regarded as another factor responsible for impaired dopaminergic and noradrenergic associated with symptom of ADHD.
Several pharmacotherapies have been developed to ameliorate the symptom of ADHD (30). However, around 30% of children treated through pharmaceuticals either do not respond to the medications (31), or have been found to suffer from adverse effects such as weight loss and insomnia (32). As a result, there is a considerable interest in developing complementary and alternative approach for ADHD (33).
To date, there is no agreement upon the antioxidant levels in patients with ADHD, as findings of the related studies vary significantly. While some studies have shown increased antioxidant levels in patients with ADHD, some highlighted a decreased level. For instance, Selek et al. (34) argued that the total antioxidant status (TAS) levels were higher in adult patients with ADHD than those in the control group. Similarly, Spahis et al. (35) reported higher levels of the antioxidant vitamin E in children with ADHD. Oztop et al. (36) claimed that thiol levels were higher in children with ADHD, although the results of their study was not statistically significant. On the other hand, Sezen et al. (17) have reported a decreased paraxonase-1 (PON-1), arylesterase (ARE) activity and TAS in children suffering from ADHD. Similarly, Ceylan et al. (37) found a significantly lower glutathione peroxidase activity, which is an antioxidant defense system, in children with ADHD as compared to the controls. Hence, more studies are needed in this area to establish a direct link between altered antioxidant status and the outcomes in ADHD patients. Further, a thorough investigation on the beneficial effects of dietary antioxidants supplementation in relieving the symptoms of ADHD is of paramount importance due to less toxicity and high affordability associated with modifying dietary regimens.
The diet and nutraceuticals are referred to as two of the most well-known and effective antioxidant therapeutic choices. The fact that they have less side and adverse effects, and are less expensive along with long-term retention capacity in the body have turned diet and nutraceuticals into effective and practical ways of treating ADHD. Oxidative and nitrosative stress (O&NS) may occur due to various factors and therapies related to these factors can also be categorized as antioxidant therapies. For instance, antioxidants could influence improvement in sleep, increase physical activities, as well as being helpful in the treatment of comorbid medical and psychiatric disorders. Hence, these approaches should be considered in the treatment of ADHD, especially in ADHD patients, where oxidative stress is high and their TAS have been significantly altered. Life style and dietary modifications may greatly influence these metabolic complications. In fact, previous studies have highlighted the significant roles of exercise (38) as well as sleep interventions (39) to potentially improve some of the symptoms associated with ADHD.
Vitamin C is a free radical scavenger that displays an antioxidant activity (40). Even in the case of marginal deficiency of other antioxidants, the redox homeostasis in the brain are known to be successfully maintained by vitamin C (41). Hence, unlike other tissues like the liver, a small negative change in vitamin C status in the brain may cause a significant increase in oxidative damage. This fact highlights the pivotal and significant role of vitamin C as the primary redox modulator in the protection of reactive oxygen species (ROS)-mediated neuronal damage in the Central Nervous System (CNS) (42).
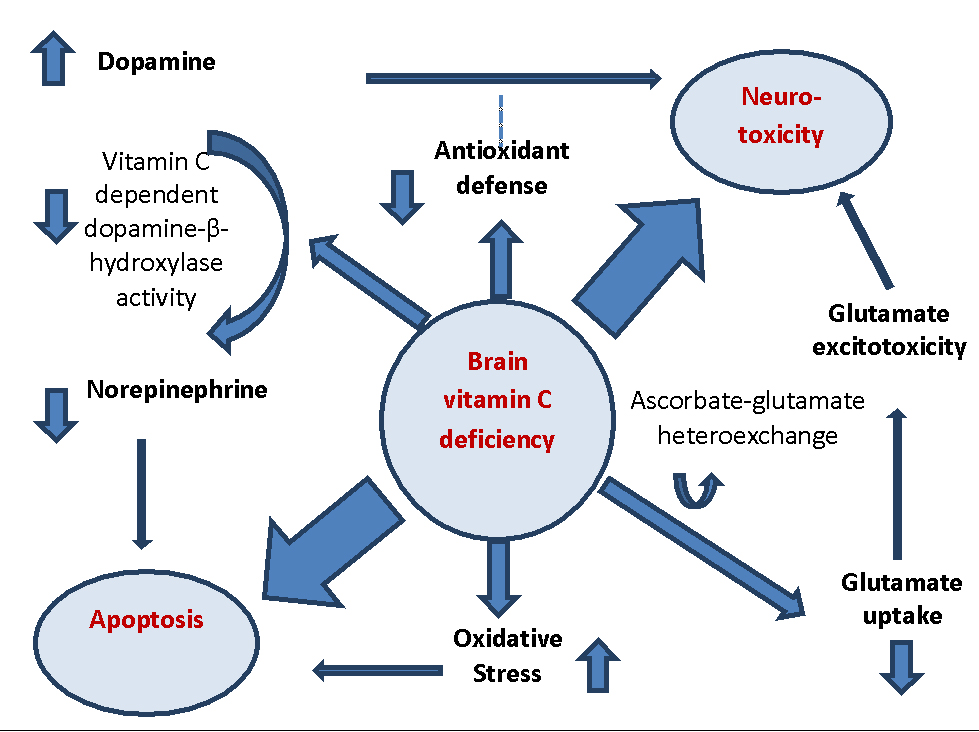
Vitamin C has the responsibility of maintaining the CNS function through different molecular pathways. In cases of vitamin C deficiency, the conversion of dopamine to norepinephrine which is usually catalyzed by vitamin C-dependent dopamine hydroxylase is impaired, which in turn results in increased concentrations of dopamine. Similarly, this excessive amount of dopamine results in ROS generation which could normally be removed by antioxidants (42). In the case of vitamin C deficiency, however, the antioxidant defense is improbable to function in an optimal manner to prevent neurotoxicity. Decrease in norepinephrine could make the neurons unable to stop the signals triggering apoptotic pathways. Similarly, vitamin C deficiency can have a role in inducing apoptosis by generally increasing the oxidative stress. In addition, the ascorbate–glutamate hetero-exchange system might possibly be affected. This process would result into decreased glutamate uptake, which in turn, allows extracellular accumulation and excito-toxic neural injury, conducted by an increase of intracellular Ca2+, which may evoke apoptotic pathways (42) (fig1).
Few studies have been carried out on the effects of vitamin C on Attention Deficit Disorder (ADD) and ADHD. In a study carried out by Haslam et al. (43), the effectiveness of a megavitamin regimen in 41 subjects with ADD was investigated. Findings revealed no significant difference (P > 0.0.5) in most behavior scores among children receiving vitamin on the one hand, and placebo on the other. Similarly, finding revealed no significant difference in serum pyridoxine and vitamin C levels among subjects and control group. Therefore, it was concluded that megavitamins including vitamin C were ineffective in the management of ADD.
Another study on the effect of vitamin C on ADHD was conducted by Joshi et al. (44), which aimed at evaluating the impact of alpha linolenic acid (ALA)-rich nutritional supplementation in the form of flax oil and Vitamin C emulsion on blood fatty acids composition and behavior in children with ADHD. Flax oil supplementation corresponding to 200 mg ALA content along with 25 mg Vitamin C twice a day was given to the subjects of the study for three months. This was the first study to show that supplementation with flax oil-based omega-3 precursors in combination with Vitamin C was an effective method for significantly improving the symptoms of ADHD including impulsivity, restlessness, inattention and self-control.
Considering these facts, few more studies are needed to be conducted in establishing vitamin C supplementation as an effective treatment option in patients with ADHD. Further studies are also needed to see if vitamin C alone or in combination with other antioxidants is highly effective against the symptoms of ADHD.
The CNS is rich in omega-3 (n-3) and omega-6 (n-6) polyunsaturated fatty acids (PUFAs). The n-3 PUFA docosahexaenoic acid (DHA) could be found more than n-6 PUFA arachidonic acid (AA) in the CNS (45). The activities of DHA are mostly in the retina and in synapses, where the synthesis, transport and release of neurotransmitters are modulated through DHA. In addition to that, DHA has a primary role in neurite growth, membrane fluidity, neurotransmission, endothelial function, neuronal survival and attenuating neurodegeneration (45). Humans cannot manufacture PUFAs; hence, they must be obtained from dietary sources (46).
Omega-3 EFA supplementation, especially formulations which contain higher doses of eicosapentaenoic acid has been confirmed to have a modest effectiveness in the treatment of ADHD. The anti-inflammatory impacts of fish oil are well-recognized, and in some clinical experiments, some associations among this supplementation and reduction of oxidative stress have been found (47, 48).
Lower omega-3 fatty acid concentrations in children with neuro-development disorders (NDD) have been reported, as compared with healthy controls (49, 50). Although associations among maternal omega-3 fatty acid intake and cognitive development are in conflict (51, 52) the overall significance of adequate maternal and childhood dietary intake of omega-3 fatty acids from both animal and human studies are highly suggestive.
From a physiological as well as a biochemical point of view, there are at least three well-known pathways which provide a solid physiological and biochemical rationale in order to include omega-3 fatty acids as a complementary nutritional component in the treatment of NDD. These include:
1) structural modifying components interacting with membrane phospholipids,
2) precursors to eicosanoid or oxidatively modified products, and
3) effectors of nuclear transcription factors and/or receptor agonists or antagonists (53).
An experimental study has highlighted the fact that supplementing antioxidants with flax oil-based omega-3 precursors is a useful way of improving ADHD symptoms in a significant way, including restlessness, impulsivity, self-control, and inattention (44).
Stevens et al. (50) were not able to find a significant relationship among utilization of omega-3 (LC-PUFAs) supplementation in children with ADHD and their skin/thirst problems. However, the findings might not be generalizable to a higher population of subjects with ADHD, as its specificity might have acted as a confounding variable. Using olive oil as a placebo might mask the useful clinical impacts of essential fatty acids, as an active constituent of olive oil is converted into oleamide, which is known to affect the brain function (54). In addition, in some studies, the essential fatty acids utilized might not be adequate to result in long-term changes in neuronal membrane structure required for clinical improvement, as they have been mostly used for short durations and in low doses.
Milte et al. (55) investigated the effects of DHA and eicosapentaenoic acid (EPA) on literacy, attentions, and behavior in children with ADHD. In this regard, 90 children were randomly selected to consume supplements high in EPA, DHA, or linoleic acid (control) for 4 months each in a crossover design. Erythrocyte fatty acids, attention, cognition, literacy, and Conners’ Parent Rating Scales (CPRS) were measured at 0, 4, 8, 12 months. All in all, 53 children completed the treatment. Findings of the study revealed no significant differences among the 3 treatments. However, in children with blood samples, increased erythrocyte EPA + DHA was associated with improved spelling and attention and reduced oppositional behavior, hyperactivity, and cognitive problems (55).
Several studies have been conducted to investigate the roles of omega-6 in patients with ADHD. As the most significant issue on omega-3 and omega-6 fatty acids is the ratio of these two substances, nearly all recent studies have investigated both in patients with ADHD. A systematic literature review by La Chance, et al. (56) has identified original articles measuring blood n6/n3 or AA/EPA ratio in children and youth suffering from ADHD, compared to controls without ADHD. Several articles met inclusion criteria for the meta-analysis. It was argued that both groups of subjects with ADHD have increased ratio of both blood n6/n3 and AA/EPA fatty acids, as compared to healthy controls. This could possibly mean that in patients with ADHD, an increased n6/n3 and particularly AA/EPA ratio might represent the underlying disturbance in essential fatty acid levels (56).
However, not all studies have shown positive effects of n3/n6 compounds in patients with ADHD. For example, in a study conducted by Raz et al. (57), the impact of short-chain EFA supplementation on ADHD children was examined through parent and teacher questionnaires, as well as a computerized continuous performance test. In doing so, the total number of 73 unmedicated children which were between 7 and 13 years of age with a diagnosis of ADHD were studied. Sixty three (63) children completed the study successfully. The EFA supplement used in the study contained 480?mg of linoleic acid and 120?mg of α-linolenic acid, and the placebo contained 1000?mg of vitamin C (daily amounts). Both substances were given for a 7-weeks supplementation period. The findings of the study revealed some improvements in the symptoms. However, no statistically significant differences were found among the groups in any of the treatment effects.
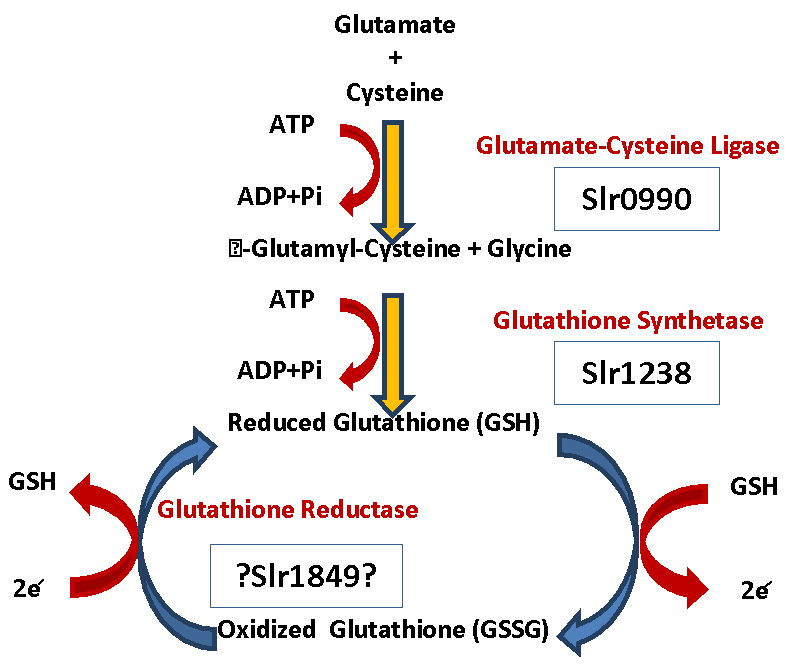
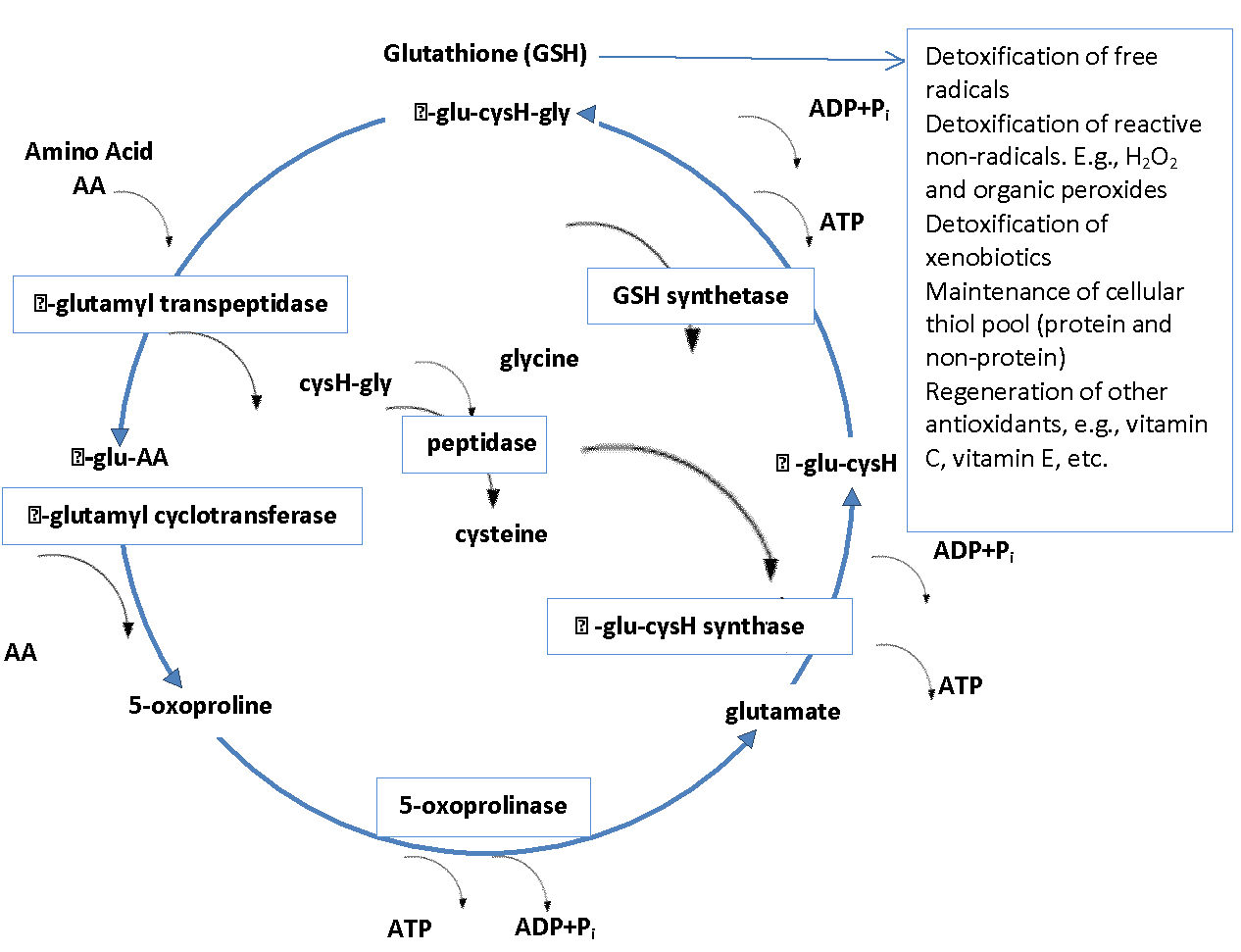
The most important and abundant antioxidant that a body makes is Glutathione (GSH). Glutathione is a powerful built-in defense system that modulates and stimulates the immune system (e.g., increases levels of white blood cells) and eliminates free-radicals, metabolic waste as well as other toxic substances. It plays a role in detoxification, maintaining cell membrane (curtails lipid peroxidation), DNA synthesis and repair, apoptosis, amino acid transport and the regeneration of other antioxidants such as vitamins C and E to name a few (58). Glutathione is a tripeptide synthesized from three L-amino acids-cysteine, glycine and glutamic acid. Sufficient quantities of glycine and glutamic acid are available in our foods, however, L-cysteine is a limiting factor in cellular GSH biosynthesis since it is relatively rare in foods and is destroyed by food cooking and processing (59).
GSH is used as a cofactor by the following: 1) peroxidases, to detoxify peroxides generated from oxygen radical attack on biological molecules; 2) transhydrogenases, to reduce oxidized centers on DNA, proteins, and other biomolecules; and 3) glutathione S-transferases (GST) to conjugate GSH with endogenous substances, exogenous electrophile, and diverse xenobiotics. Low GST activity may increase risk for disease (59, 58).
High levels of GSH are found in liver (highest concentration) and other organs frequently exposed to toxins. Glutathione exists in two forms in our body: the reduced form (GSH) and the oxidized form (GSSG) (59). Over 90% of the total glutathione pool is in the GSH form and less than 10% exists in the GSSH form. The ratio of reduced glutathione (GSH) to oxidized glutathione (GSSG) within cells is often used as a biomarker of cellular oxidative stress. GSSG conversion to GSH is carried out by Glutathione reductase. GSH is under tight homeostatic control both intracellularly and extracellularly. A dynamic balance is maintained between GSH synthesis, recycling from GSSG, and its utilization (58) (fig2).
Daily intake of glutathione from food averages 100-150 mg. A healthy adult has about 10g of glutathione circulating in the body tissues. Thus, dietary intake comprises only 1-1.5% of circulating GSH (62). The rest of glutathione is manufactured inside the body. Food sources that increase glutathione do so by providing the precursors of glutathione, or enhance its production by some other means. Glutathione could be found in both plant and animal sources. Cruciferous and dark leafy greens are considered rich sources for glutathione. Fresh fruit and vegetables, cooked meat and fish contain about 25–750 mg per pound. Processed foods, dairy products, most nuts, grains and legumes are not substantial sources (63).
Glutathione supplement has a poor bioavailability, i.e., poorly absorbed since it is a substrate for proteases and is destroyed by the stomach acidity. The most effective way to elevate hepatic glutathione is to administer its dietary precursors, like cysteine, glycine or methionine (62). For instance, the glutathione precursor N-acetylcysteine (NAC) is quickly metabolized to GSH and is more effective in increasing glutathione levels in the blood. NAC is capable of helping to regenerate glutathione levels, in particular, it is commonly used to treat overdose of acetaminophen (Tylenol), which is harmful in part due to severe depletion of glutathione levels. Other antioxidants like ascorbic acid (vitamin C) may also work synergistically with glutathione, preventing depletion of either (63).
Autism and ADHD are not the same though they do share many similarities. As of December 2017 based on our own search there are 167 studies on PubMed that investigated glutathione levels, and antioxidant and detoxification activity in Autism compared to 19 for ADHD. We do not know for sure if low glutathione is the cause or the consequence of Autism/ADHD disorders. However, there is mounting evidence that a low or suboptimal level of GSH is associated with deficiency in glutathione antioxidant activity. A few studies now show that a deficit in GSH mostly occurs prior to neuropathological abnormalities in these diseases (e.g., autism, schizophrenia, bipolar disorder, Alzheimer's disease, and Parkinson's disease) (64).
To illustrate the potential role of glutathione we will describe three recent studies that examined GSH concentration and ratio levels (GSH/GSSG) in children with autism spectrum disorders (ASD) and controls to draw similarities to ADHD.
In the first investigation, Chauhan, Audhya and Chauhan (65) found that GSH levels were significantly lower in the ASD - by 34.2% (in the cerebellum) and by 44.6. % (in the temporal cortex), with an associated increase in the levels of GSSG by 38.2% and 45.5.% respectively, as compared to the control group. A significant decrease in the levels of total GSH was also observed - by 32.9% in the cerebellum, and by 43.1% in the temporal cortex of ASD. The GSH/GSSG ratio was also significantly decreased - by 52.8% in the cerebellum and by 60.8% in the temporal cortex of ASD, suggesting glutathione redox imbalance in the brain of individuals with autism.
The second investigation, (66) showed that the serum level of glutathione in ASD was significantly lower than that of controls, while levels of homocysteine and S-adenosyl-homocysteine (SAH) were elevated, indicative of oxidative stress and decreased methylation capacity. At the same time mercury levels were markedly elevated in the hair of ASD compared to control subjects, consistent with the importance of glutathione for mercury detoxification. Mercury is a well-documented neurotoxin. Significant nutritional deficiencies in serum folate and vitamin B12 were observed as well– these are crucial glutathione cofactor vitamins, also involved in methylation. In a Kuwaiti study, hair mercury levels averaged 15 times higher in 40 children with ASD in Kuwaitas compared to 40 typically developing children (4.5.0 versus 0.3.0 µg/g hair, p < 0.0.01) (67).
The third investigation (68), assessing transsulfuration pathways and oxidative metabolites indicated that homocysteine levels and oxidized glutathione levels were significantly higher in children with autism (clinical severity of autism behavior was determined by Childhood Autism Rating Scale (CARS) and by the Autism Behavior Checklist (ABC)), while cysteine levels, total glutathione, reduced glutathione and the GSH/GSSG ratio showed remarkably lower values in ASD compared to the control group leading to the conclusion that abnormal transsulfuration metabolism, reduced antioxidant capacity and increased oxidative stress appear to have a potentially negative impact on clinical severity of ASD.
Genetic research on the levels of glutathione in autism has also identified certain genetic abnormalities as a risk factor for the development of autism, including mutations in the genes responsible for regulating the activity of the glutathione antioxidant enzymes. Similarly, recent results imply that the polymorphisms in the GST genes may affect ADHD (69). This was a case–control designed study in 243 ADHD children and 327 controls in Korean children (fig3).
Children and adults with ADHD generally have increased O&NS biomarkers in urine, blood, and saliva. Examination of antioxidant biomarker Glutathione S-transferase (GST) in ADHD patients yielded conflicting results that is discrepancy in GST levels when compared to controls (15, 2). N-acetylcysteine (NAC), a precursor to the antioxidant glutathione, was effective for the treatment of ADHD symptoms in adults with systemic lupus erythematosus (2). Rucklidge et al. (71) cited a therapy study that included amongst other things injection 1-3 times per week with glutathione and vitamin B12. While there has been an average improvement in the ADHD condition, it is difficult to attribute to one treatment due to carried multi-therapies offered.
Search of NIH engine (https://clinicaltrials.gov/) as of December 2017 lists 317 drug intervention dietary supplements studies (last update of the website is June 2017). It also reveals a total of 10 studies on autism and oxidative stress. Only one completed study with the intervention being glutathione or a concoction of glutathione, vitamin C and NAC was found. This was “Study of Glutathione, Vitamin C and Cysteine in Children with Autism and Severe Behavior Problems”; although the completion date for the trial was 2012, no results were posted on the website.
Ahn et al. (72) reviewed clinical trials evaluating safety and efficacy of natural products (botanical products, nutritional supplements or combination therapy thereof) in ADHD. However, on the NIH’s site (https://clinicaltrials.gov/) the only listed clinical trial on ADHD that uses a dietary supplement is the Phase 3 clinical trial “Effect of Pycnogenol® on ADHD”. This trial aimed at examining the impacts of a commercially available nutritional supplement on the behavior of patients with ADHD, as well as on their physical and psychiatric co-morbidities, and level of oxidative stress and immune activity, as compared to placebo and standard pharmaceutical treatment for ADHD (estimated enrollment of 144 participants). Estimated study completion date is March 2020 (fig4).
N-acetylcysteine (NAC) is acetyl derivative of the amino acid cysteine (74). NAC is available as an over-the-counter (OTC) nutritional supplement, holding antioxidant properties (75). According to the preclinical studies, NAC can be effective in regulation of pathophysiological processes which are mainly present in multiple psychiatric and neurological disorders such as oxidative stress, mitochondrial dysfunction, neurogenesis and apoptosis, neuroinflammation and dysregulation of glutamate and dopamine neurotransmitter systems (76, 77).
The etiology of psychiatric disorders is known to be of a multifactorial mode which involves inflammatory pathways, glutamatergic transmission, oxidative stress, GSH metabolism, mitochondrial function, neurotrophins, apoptosis, dopamine pathway, and intracellular Ca modulation (76). NAC has a role in most of these pathways; therefore, its impacts on psychiatric disorders have mostly been investigated in clinical setting. In fact, more than twenty clinical studies have utilized NAC as a supplementation in regards to psychiatric disorders. Methamphetamine and cannabis dependence, nicotine and cocaine addiction, pathological gambling, obsessive–compulsive disorder, trichotillomania, nail biting and skin picking, schizophrenia, bipolar disorder, autism, and AD are some examples. Most of these studies have highlighted the positive effects of NAC through clinical results (78, 77).
The ability of a compound to cross the blood–brain barrier (BBB) is challenging and a critical issue. The ability of NAC to cross the BBB has turned into a debating issue. Most probably, this controversy stemmed from the variability shown by NAC to cross BBB, which might be attributed to variability in its doses and administration. Currently, N-deacetylation and a carrier-mediated active transport are the only known mode of this compound crossing the blood vessel wall. Different forms of NAC and different routes of administration might result in different concentrations and variable utilization of these mechanisms. For instance, after 2 h following oral administration of S-NAC to rats, the highest concentration was seen in the kidney and liver, followed by the adrenal gland, lung, spleen, blood, muscle and brain (79).
A study aimed at examining the impact of NAC (2.4 g/day or 4.8 g/day), in comparison with placebo on ADHD. The findings of the study which were derived from an ADHD Self-Report Scale Symptom Checklist (ASRS) revealed that, NAC had reduced the ADHD symptoms. With one LOE2b study, a GOR of C is assigned but this evidence must be tempered with the fact that the study population suffered from SLE and thus cannot be generalized as a treatment for typical ADHD (80).
Polyphenolic extract of pine bark, commercially known as Pycnogenol (Pyc) is a standard bark extract of the French maritime pine (Pinus pinaster). It consists mainly of procyanidins, catechin and phenolic acids (81). Pyc has a strong free radical scavenging activity against reactive oxygen and nitrogen species and a wide range of positive effects in vitro and in vivo (82).
The Pyc extract includes procyanidine oligomers as its main components with a chain length of 2 to12 monomeric units. They might be in interaction with the DNA molecules attacked by free radicals, as well as reactive oxygen and nitrogen species, playing a significant role in the development and progression of NDD (83). It is believed that children with ADHD have disturbance in catecholamine metabolism (84).
Chovanova et al. (84) investigated the effect of Pyc on the level of oxidized purines represented by 8-oxo-7, 8-dihydroguanine (8-oxoG) and on the TAS in children with ADHD. Findings of their study highlighted an increase in DNA damage as opposed to healthy controls. It was also shown that after 1 month of Pyc administration, 8-oxoG was significantly lower in comparison to the beginning stage and to the placebo group. The findings also revealed that children with ADHD, the TAS was lower as compared with controls. After Pyc administration, TAS was increased; however, statistical significant increase was reported after 1 month of termination of Pyc application. After Pyc administration, the improvements in DNA damage and TAS were associated with attention improvement in children with ADHD. In conclusion, administration of Pyc resulted into the reduction of oxidative damage to DNA, as well as normalizing TAS and improving attention in children with ADHD (84).
In another study aiming at testing the impact of Pyc, 61 children (50 males and 11 females) aged 6 to 14 were selected as the participants. The mean age of the subjects in the Pyc group was 9.5 year, and for placebo group it was 8.8 years. The treatment dose included 1 mg/kg/day of Pyc. Prior to that, the symptoms were assessed through both self-assessment and external assessment by parents and teacher. In addition, Child Attention Prob-lems scale (CAP), the Conner’s Parents Rating Scale (CPRS) and the Conner’s Teacher Rating Scale (CTRS) were used. In addition, five sub-scales of the Prague Wechsler Intelligence test (PDW) were utilized for self-assessment. In addition, blood samples were taken at baseline, both after treatment and after wash-out periods. Findings of the study reported significant improvements in inattention and hyperactivity when assessed by teachers (CAP). However, the ratings related to CTRS revealed no significant improvements in inattention or hyperactivity. The reported improvements of hyperactivity and inattention during the intervention period rated by parents (CPRS) showed no significance. A relapse of symptoms was observed after the medication with Pyc was discontinued (85).
In another study on the effect of Pyc in patients with ADHD, 24 adults with the ages between 24 and 53 with Combined Type ADHD were studied, with Pyc and methylphenidate being the main issues of investigation. Participants of the study received Pyc, methylphenidate, and placebo, each for 3 weeks. This was carried out in a randomized and counterbalanced order. Although the symptoms of ADHD showed improvements during the treatment period, neither methylphenidate nor Pyc were found to outperform the placebo controls, as assessed by self-report rating scales, rating scales completed by the individual’s significant other, and a computerized continuous performance test. It was therefore concluded that the conservative dosage levels and the short length of treatment might have contributed to the lack of significant differences between treatment conditions (86).
Baicalin is a flavonoid purified from the plant Scutellaria baicalensis Georgi. It is the main medicinal component of the plant, with its highest concentration found in the radix scutellariae (87). According to Zhou et al. (7), Scutellaria Baicalensis has been long used in treatment of inflammation (88), fever (89), hepatitis (90), jaundice (91) and hypertension in traditional Chinese medicine. In addition, the safety of this substance has been proved clinically. The crystalline form of baicalin is pale yellow, odorless, and bitter and it has a melting point of 223 C. Its molecular formula is C21H18O11 and molecular weight is 446.3.5. Pharmacologically, baicalin has been used as an anticancer, anti-inflammatory and antioxidative agent. In addition, it has been used as an adjuvant drug in the clinical treatment of hepatitis in encephalitis (7).
An investigation (92) on the impact of baicalin and berberine on the transport of nimodipine (NMD) in primary-cultured, rat brain microvascular endothelial cells (rBMEC) revealed that baicalin has a bidirectional impact on the uptake of NMD by rBMEC. It was also shown that a low concentration of baicalin (2–5 lg/mL) could have positive impacts on the transport of NMD across the BBB, while a higher concentration had the opposite impacts. In addition, similar results have been obtained, highlighting the vascular barrier protective and nerve protective effects of baicalin (93, 20). The literature also shows that baicalin could pass through the BBB in either direction. These reports indicate that baicalin is able to pass into the brain through the BBB (7).
The literature shows that baicalin has the ability to pass through the BBB and is associated with the striatum and substantia nigra, which are enriched with DA neurons. In addition, baicalin has the ability of protection and regulation of DA system in numerous animal models such as models of Parkinson’s disease, depressive disorder, and cerebral ischemia (94). Baicalin can also improve the spatial learning ability in global ischemia/reperfusion rats (95). Baicalin distribution was very high in the DA system (96). In fact, the DA system is known as the target brain system for baicalin (96). In addition, the literature shows that children with ADHD have low levels of DA in the brain (8), as is the case in mice (9), while baicalin protects and regulates the DA system and increases the level of DA.
A study by Gong et al. (9) showed that baicalin has a dopamine neuroprotective effect to prevent methamphetamine (METH)-induced striatal damage in mice, and that baicalin may attenuate the loss of the DA transporter (DAT), playing a significant role in the pathogenesis of ADHD in the striatum. The study also provided evidence as to the fact that baicalin could significantly influence DA levels and DAT in the striatum. Baicalin might have a therapeutic impact in ADHD, making it meaningful and worthy of being investigated in prospective research (7).
In addition, Zhou et al. (97) studied the effect of baicalin on synaptosomal adenosine triphosphatase (ATPase) and lactate dehydrogenase (LDH), as well as its regulatory impact on the adenylate cyclase (AC)/cyclic adenosine monophosphate (cAMP) /protein kinase A (PKA) signaling pathway in rats with ADHD. In this regard, a total number of 40 SHR rats were randomly divided into five groups, including ADHD model, methylphenidate hydrochloride treatment (0.07 mg/mL), and low-dose (3.33 mg/mL), medium-dose (6.67 mg/mL), and high-dose (10 mg/mL) baicalin treatment (n=8 each). Eight WKY rats were selected as normal control group, as well. Percoll density gradient centrifugation was used to prepare brain synaptosomes and an electron microscope was used to observe their structure. Colorimetry was used to measure the activities of ATPase and LDH in synaptosomes. In addition, ELISA was used to measure the content of AC, cAMP, and PKA. The ADHD model group had a significant reduction in the ATPase activity, a significant increase in the LDH activity, and significant reductions in the content of AC, cAMP, and PKA. Both methylphenidate hydrochloride and baicalin were found to improve synaptosomal ATPase and LDH activities in rats with ADHD. The effect of baicalin is dose-dependent, and high-dose baicalin were found to have a significantly greater impact than methylphenidate hydrochloride. Baicalin exerted its therapeutic impact possibly by upregulating the AC/cAMP/PKA signaling pathway (97).
A free radical scavenger, Ginkgo biloba extract (EGb 761) might be useful in fighting oxidative stress related to aging (98). Although the exact pharmacological mechanism is not fully understood (99), a number of studies indicate positive effects in adults, particularly in the treatment of cognition in dementia (100-106), but findings on ADHD are rare (107). Additionally, electroencephalographic studies suggest that Ginkgo biloba has vigilance-enhancing and cognitively activating effects on performance and brain electrical activity (101,103,105).
A study aimed at comparing the herbal preparation of Ginkgo biloba with methylphenidate in the treatment process of ADHD was carried out by Salehi et al. (108). The mean age in the experimental groups was 9.1.2 years. Based on the subject’s body weights, the treatment doses were 80–120 mg/day of Ginkgo biloba and 20–30 mg/day of methylphenidate, respectively. Assessments of ADHD symptoms were done by both parents and teachers through the ADHD Rating Scale IV. The findings of this study within subject analysis revealed no significant changes over time in the assessed ADHD symptoms for the Ginkgo biloba group, neither if rated by parents, nor by teachers; whereas in the methylphenidate group, changes over time appeared significant for both assessment conditions. Furthermore, the comparison revealed significant differences in improving the symptoms of ADHD. Concerning the symptoms in the children treated with methylphenidate, considerable improvements were reported by both teachers and parents after the treatment period, but not in children treated with ginkgo biloba. Finally, findings of the study suggested that ginkgo biloba administration was less effective than methylphenidate in the treatment of ADHD (108).
Another study was conducted to compare the effectiveness of Ginkgo biloba and placebo as an add-on therapy to a methylphenidate treatment (109). The mean age of the subjects was 7.8.3 years. Treatment doses of 80–120 mg/day for Ginkgo biloba and 20–30 mg/day for methylphenidate were administered based on the body weights of the subjects, respectively. Assessments on ADHD symptoms were carried out by both teachers and parents by means of the ADHD Rating Scale IV. Measurements were performed at base line and 2 and 6 weeks after a 6-week medication period. The findings of the study revealed significant improvements in inattention rated by teachers and parents for the group of children treated with Ginkgo biloba along with methylphenidate, as compared to placebo (109).
There are rising tide of incidence of ADHD among children. The magnitude of ADHD are on the rise in both industrialized and emerging economies. Existing therapeutic armamentarium are been reported to be often impervious to symptoms of ADHD. Despite its wide occurrence, pathogenesis of ADHD has yet to be clearly charted. Indeed, there are no specific symptoms or biomarkers for early and precise diagnosis. However, some studies have highlighted the associations among ADHD and catecholaminergic neurotransmission (8). The compounds thought to have affinity to catecholaminergic neurotransmission are known to trigger unacceptable side effects. There is urgent call for finding alternative approach to come to grip with symptoms of ADHD. Hence in recent past studies related to identifying the potential regulating roles of antioxidants in mitigating the symptoms of ADHD has gained importance (Table 1).
| Antioxidant | Samples | Mean age (years) | Intervention | Duration (week) | Results | References |
|---|---|---|---|---|---|---|
| Vitamin C | ADD children, N=41 Control: Healthy children, N=75 | 9.5 | Megavitamin including Nicotinamide (NAA) Ascorbic acid (vitamin C) Pyridoxine (vitamin B6) | 6 | Megavitamins including vitamin C were ineffective in the management of ADD. | (43) |
| Omega3/Vitamin C | ADHD children, N=30 Healthy children control, N=30 | 7.25 | Flax oil supplementation with vitamin C | 12 | Significantly improved the symptoms of ADHD including impulsivity, restlessness, inattention and self-control | (44) |
| Omega-3 | ADHD with thirst/dry skin, N=25 Control: Healthy children with few thirst/dry skin symptoms N=25 | 9.5 | EFA supplement | 16 | Clear benefit from LC-PUFAs was not observed on major parent or teacher rating scales. | (50) |
| children with ADHD, N=87 | 8.9 | 16 | Increased erythrocyte DHA and EPA via dietary supplementation might improve behavior, attention, and literacy in children with ADHD. | (55) | ||
| Omega-3/Omeg-6 | ADHD, N= 39 control,N = 39 | 10 | LA, omega-6 and ALA, omega-3 Placebo: Vitamin C | 7 | No significant differences were found among the groups. | (57) |
| N-Acetylcysteine (NAC) | Participants with ADHD and SLE, N=24 Healthy adults as control, N=46 | 45.9 ± 1.8 | NAC :2.4 g/d NAC: 4.8 g/d placebo | 12 | In patients with SLE, elevated ASRS scores revealed previously unrecognized and clinically significant symptoms of ADHD that responded to NAC treatment. | (80) |
| Pycnogenol (Pyc) | ADHD, N= 61 Healthy children as control | 11.5 | Pyc: 1mg/kg/day Placebo: lactose (58mg) & cellulose (65mg) in the tablet | 4 | Pycnogenol administration reduced oxidative damage to DNA, normalized TAS and improved attention in children with ADHD. | (84) |
| ADHD, N=61 Healthy children as control | 9.5 | 1 mg/kg/weight Pycnogenol Placebo in same dosage | 4 | Results pointed to an option to use Pycnogenol as a natural supplement to relieve ADHD symptoms in children. | (85) | |
| Adults with ADHD, N=24 | 42 | Methylphenidate Pycnogenol placebo | 3 | ADHD symptoms improved during treatment, neither methylphenidate nor Pycnogenol outperformed the placebo | (86) | |
| Ginkgo biloba (EGb 761) | ADHD children, N=25 Healthy control, N=25 | 9.12 | Gingko biloba capsule 80–120 mg/day/weight Methylphenidate 20–30 mg/day/weight. | 6 | Administration of Ginkgo biloba was less effective than methylphenidate in the treatment of ADHD. | (108) |
| ADHD children, N=31 Control: ADHD children, N=60 | 7.83 | Gingko biloba 80 - 120 mg/day/weight Methylphenidate 20 -mg/ day/ weight | 6 | The Ginkgo biloba is an effective complementary treatment for ADHD. | (109) | |
| Baicalin | SHR Rat, N=40 | |||||
| WKY rats as normal control, N=8 | - | methylphenidate (0.07 mg/mL), baicalin low-dose: 3.33 mg/mL medium-dose: 6.67 mg/mL high-dose: 10 mg/mL | - | Both methylphenidate hydrochloride and baicalin could improve synaptosomal ATPase and LDH activities in rats with ADHD. | (97) | |
| Abbreviations: LA: Linoleic acid, ALA: Alpha-linolenic acid, SLE: Systemic lupus erythematosus, TAS: Total antioxidant status, EPA: Eicosapentaenoic acidEPA, DHA: Docosahexaenoic acid | ||||||
The present review has discussed the different antioxidants which might be potent against ADHD symptoms. These include vitamins, unsaturated fatty acids and other substances. In addition, the signaling networks modulated by these antioxidants and how they cross the BBB were also discussed. There are various theoretical studies and empirical investigations related to use of antioxidants against pathogenesis of ADHD and other common neurological disorders, which have been comprehensively compiled here. This review has also highlighted the gap in knowledge and the area where further investigations are required for establishing antioxidants as an effective intervention against ADHD.
The support provided by Sultan Qaboos University in the form of Ph.D. Bench Fees to Ms. Marzieh Moghadass is highly acknowledged. The authors declare no conflict of interest.