1 Interventional Cardiology, ZNA Cardiovascular Center Middelheim, 2020 Antwerp, Belgium
2 Department of Cardiology, Ziekenhuis Oost-Limburg, 3600 Genk, Belgium
3 Interventional Cardiology Unit, San Raffaele Scientific Institute, 20132 Milan, Italy
4 Interventional Cardiology Unit, GVM Care & Research, Maria Cecilia Hospital, 48010 Cotignola (RA), Italy
5 Department of Cardiology, IRCCS Policlinico San Donato, 20097 San Donato Milanese-Milan, Italy
6 Department of Cardiovascular Medicine, Universitaire Ziekenhuizen Leuven, 3000 Leuven, Belgium
7 Cardiovascular Center, OLV Hospital, 9300 Aalst, Belgium
8 Cardiovascular Research Center, 04012-070 São Paulo, Brazil
9 Department of Biomedical Sciences, Humanitas University, 20090 Pieve Emanuele-Milan, Italy
10 Humanitas Clinical and Research Center IRCCS, 20089 Rozzano-Milan, Italy
Abstract
Background: The DynamX Novolimus-Eluting Coronary Bioadaptor System
(DynamX
Keywords
- coronary artery disease
- bioadaptor
- drug-eluting stent
- novolimus
- target lesion failure
- vessel motion
- pulsatility
- vasomotion
- thrombosis
While restenosis rates in percutaneous coronary interventions have been significantly reduced following the use of drug-eluting stents (DES), the permanent implant prevents normal vessel movement and function (expansion, contraction, rotation, vasomotion) due to permanent caging of the vessel [1].
Drug-coated balloons (DCBs) were developed to avoid a permanent implant, but
they don’t support the scaffolding of the vessel, particularly to prevent early
recoil, and come with a set of challenges such as drug delivery restricted to the
time of inflation, and that only
Fully bioresorbable scaffolds aim to provide scaffolding to the vessel during the initial healing phase and to be resorbed thereafter, ultimately allowing vascular restoration including lumen enlargement and restoration of vascular physiology and motion [1, 7, 8]. However, they have fallen out of favor through their intrinsic mechanical properties and as outcomes have not been comparable to contemporary DES, in particular in terms of higher restenosis and device thrombosis rates [2, 7], albeit optimized treatment strategies might improve outcomes [7].
The DynamX
The DynamX Mechanistic study was initiated to evaluate the safety and performance of the DynamX Bioadaptor System in patients with de novo coronary artery lesions by assessing both clinical and imaging outcomes. Clinical and imaging outcomes out to two years have been published previously [9, 11], we herein report the final 36-month data.
In addition, two preclinical studies (porcine animal studies) were conducted to obtain further insights into the consequences of the uncaging of the bioadaptor. The first study assessed positive remodelling by optical coherence tomography (OCT) imaging. Another animal study assessed the levels of gene expression of the smooth muscle cells (SMC) in the device implanted neointimal tissue that is indicative of SMC phenotype switching during the arterial injury and subsequent healing upon peripheral coronary intervention [12, 13]. The results are included in this manuscript to provide insights into the biological response seen in the DynamX Mechanistic study.
The study design has been described in detail previously [9]. The DynamX Mechanistic study is a prospective, multi-centre, non-randomized trial with consecutive enrollment. It was conducted at six sites in Belgium and Italy. Imaging follow-up with quantitative coronary angiography, intravascular ultrasound (IVUS) and OCT was performed at either 9 or 12 months and clinical follow-up was performed at 1, 6, 12, 24 and 36 months.
Eligible patients had single de novo coronary arteries measuring
between 2.5 and 3.5 mm in diameter and
Sources of bias were minimized through monitoring of electronic records (case report forms) with source document verification, imaging review through a core laboratory, and independent event adjudication by a clinical events committee. The study adhered to the Declaration of Helsinki, ISO14155:2011, and local regulations.
The DynamX Bioadaptor (Fig. 1) combines three 71 µm cobalt-chromium
helical strands that are temporarily linked together by unique uncaging elements
coated with a bioresorbable polymer. This creates a highly conformable scaffold
with high acute compression resistance similar to DES. The thin polymer coating
resorbs over six months allowing the cobalt-chromium helical strands to initiate
the triple mechanism of unlocking the bioadaptor, uncaging the artery, and
continue providing dynamic scaffolding after uncaging while uniquely adapting to
vessel biomechanical forces and providing the necessary vessel reinforcement. The
device has a crossing profile of
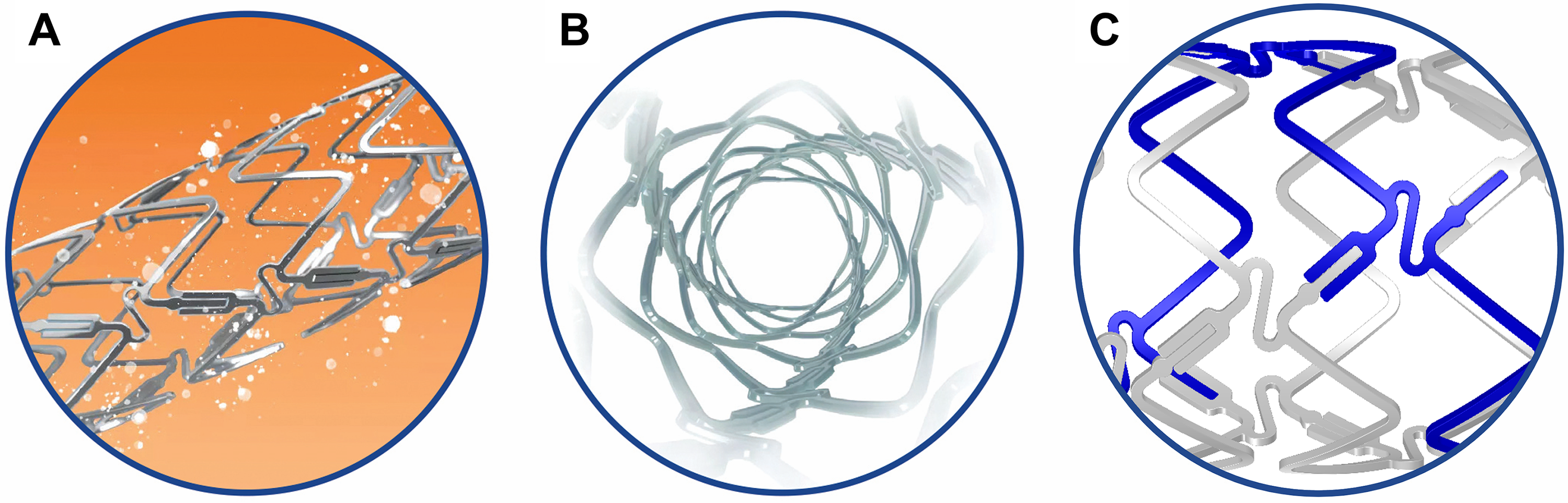 Fig. 1.
Fig. 1.DynamX Bioadaptor. The drug elutes over 3 months (A). The polymer coating resorbs over 6 months and uncages the device circumferentially (B) at the U-shaped uncaging elements (C) while maintaining the longitudinal continuity of the three cobalt chromium strands in a helical pattern. Re-used with permission of Elixir Medical Corporation.
The delivery system is comprised of standard materials. Two radiopaque balloon markers indicate the working length of the balloon and reflect the expanded bioadaptor length aiding to accurately position the bioadaptor and delivery system during implantation. The delivery system is designed to accommodate 0.014-inch or smaller diameter guide wires.
Implantation of the bioadaptor follows standard procedures for DES; dual antiplatelet therapy was recommended for at least 12 months.
The primary safety endpoint was target lesion failure (TLF, defined as a composite of cardiac death, target-vessel myocardial infarction, and clinically-driven target lesion revascularization) at 6 months, and the primary efficacy endpoint was change in the mean in-device area and lumen area at 9–12 months by IVUS.
Secondary endpoints at 3 years are TLF at other time points, target-vessel failure (TVF), defined as a composite of cardiac death, target-vessel myocardial infarction (TV-MI) and clinically-driven target vessel revascularization (CD-TVR); mortality, myocardial infarction (target-vessel and overall); clinically-driven target lesion revascularization (CD-TLR); overall TLR; CD-TVR; overall TVR; and definite or probable device thrombosis. Imaging endpoints have been reported previously [9, 11]. Myocardial infarction was defined as enzyme elevation of 2-times upper normal limit of creatinine kinase (CK) with an elevation of CK-MB; cardiac death, revascularization and device thrombosis were adjudicated according to Academic Research Consortium criteria [14].
The study was designed to confirm the performance and safety of the DynamX Bioadaptor and to generate hypotheses for future studies. Since there is no hypothesis testing, the sample size was not calculated based on the endpoint hypothesis. However, the sample size requirement was determined by assessing the minimal number of patients required to provide reliable and non-trivial results.
The analysis is based on the intention-to-treat principles. Qualitative data are
presented as counts and percentages. Quantitative variables are presented as
means and standard deviations. The results are based on the data available.
Clinical data were analysed using Excel
The study protocols of both studies were approved by the Institutional Animal Care and Use Committee (IACUC) and both studies were conducted in accordance with the Guide for the Care and Use of Laboratory Animals.
A total of 5 DynamX Bioadaptors (3.0
OCT imaging was performed at post-implant, and at 3, 12, and 24-month timepoints using the C7XR imaging system (LightLab, Westford, MA, USA). The OCT catheter was advanced distally to the implanted device and a motorized pullback was performed at a rate of 15 or 20 mm/sec to acquire images at a rate of 100 frames/sec. Images were acquired free of occlusion using a continuous flush of the contrast media. Three cross-sectional frames were chosen (proximal, mid and distal) to measure mean lumen diameter and mean device diameter to derive mean lumen area and mean device area at various time points. The analysis was performed with computer-assisted methods with the Image Pro Premier software (Version 9.2, Media Cybernetics, Rockville, ML, USA).
A total of 8 pigs were implanted in the coronary arteries with 2 devices each of
the DynamX Bioadaptor (test) and the Xience everolimus-eluting DES (control,
Abbott Vascular, Santa Clara, CA, USA). Two pigs were euthanized at time points of 3,
6, 9, and 12 months post device implantation, the device implanted vessel segment
was explanted, and the neointimal tissue harvested from the luminal side of the
implanted DynamX Bioadaptor and the control Xience DES was isolated and stored in
RNAlater solution. Total RNA purified from the neointimal tissue samples was
reverse transcribed using First Strand cDNA Synthesis Kit (Syd Labs, Hopkinton, MA, USA) and
quantified using qRT-PCR (Agilent MX3000P) at 3, 6, 9 and 12 months.
Supplementary Table 1 provides the target genes evaluated and the PCR
primers employed in the qRT-PCR assay. The
The 50 patients enrolled were 66.3
Pre-dilatation was performed in 96% of lesions and post-dilatation in 62%. One additional device was used to cover a dissection.
Four patients were lost to follow-up at 36 months and 3 patients died during the course of the study. All 3 patients had an uneventful procedure and index-hospital stay and all cardiac deaths were classified by the site as being not device- or procedure-related. The first death involved a 59-year-old male with multiple medical co-morbidities and Wernicke-Korsakoff syndrome. During the follow-up period, the patient had several hospital visits with only one cardiac-related visit on day 55 for atypical chest pain which spontaneously resolved. At day 255 he was found dead at home. The second death involved a 78-year-old male with hypertension and multiple comorbidities who was admitted to a non-study hospital for heart failure, where he died as a result of multi-organ failure on study day 267. Communication between the investigational site principal investigator and the cardiologist at the non-study hospital indicated that the patient did not experience any ischemic symptoms during the final hospitalization. The third cardiac death was reported on day 972 in a patient with a complex cardiac history not related to the index procedure (hospitalization for non-target vessel revascularization on post-procedure day 152, transcatheter aortic valve replacement on day 217, heart failure on day 553). On day 972, the patient died at home.
One clinically-driven TLR was reported in a patient on day 952 (Table 1). This patient underwent a protocol-required angiogram at 12 months which showed some narrowing of the target lesion (46% diameter stenosis and 0.91 fractional flow reserve, FFR) but did not warrant treatment at that time. On day 952, the patient was hospitalized for recurrent angina (nightly episodes) as well as rheumatic symptoms. Angiography showed severe in-stent restenosis (70% visual estimation, FFR 0.81) that was treated with a DES.
| N = 46 | |
| Target lesion failure | 8.7% (4/46) |
| Cardiac death | 6.5% (3/46) |
| Target-vessel myocardial infarction | 0.0% (0/46) |
| Clinically-driven target lesion revascularization | 2.2% (1/46) |
| Target vessel failure | 8.7% (4/46) |
| Clinically-driven target vessel revasclarization |
2.2% (1/46) |
| Device thrombosis, definite or probable | 0.0% (0/46) |
Data are displayed as % (n/N).
No non-clinically driven TLR or TVR occurred, but 5 non-TVR were reported (10.9%, on days 126, 152, 274, 535, and 1071).
OCT images of the test DynamX Bioadaptor were performed serially at multiple
time points demonstrating good strut apposition to the vessel wall for the test
and control devices (Fig. 2A). Since multiple follow-up imaging procedures were
planned in the study, not all the animals were imaged at all follow-up time
points in order to avoid multiple procedures in the animals as per the IACUC
requirement. Hence an unpaired analysis of serial images was performed (Fig. 2B).
Following uncaging (~6 months), imaging measurements showed that
the mean device area had increased from 7.02
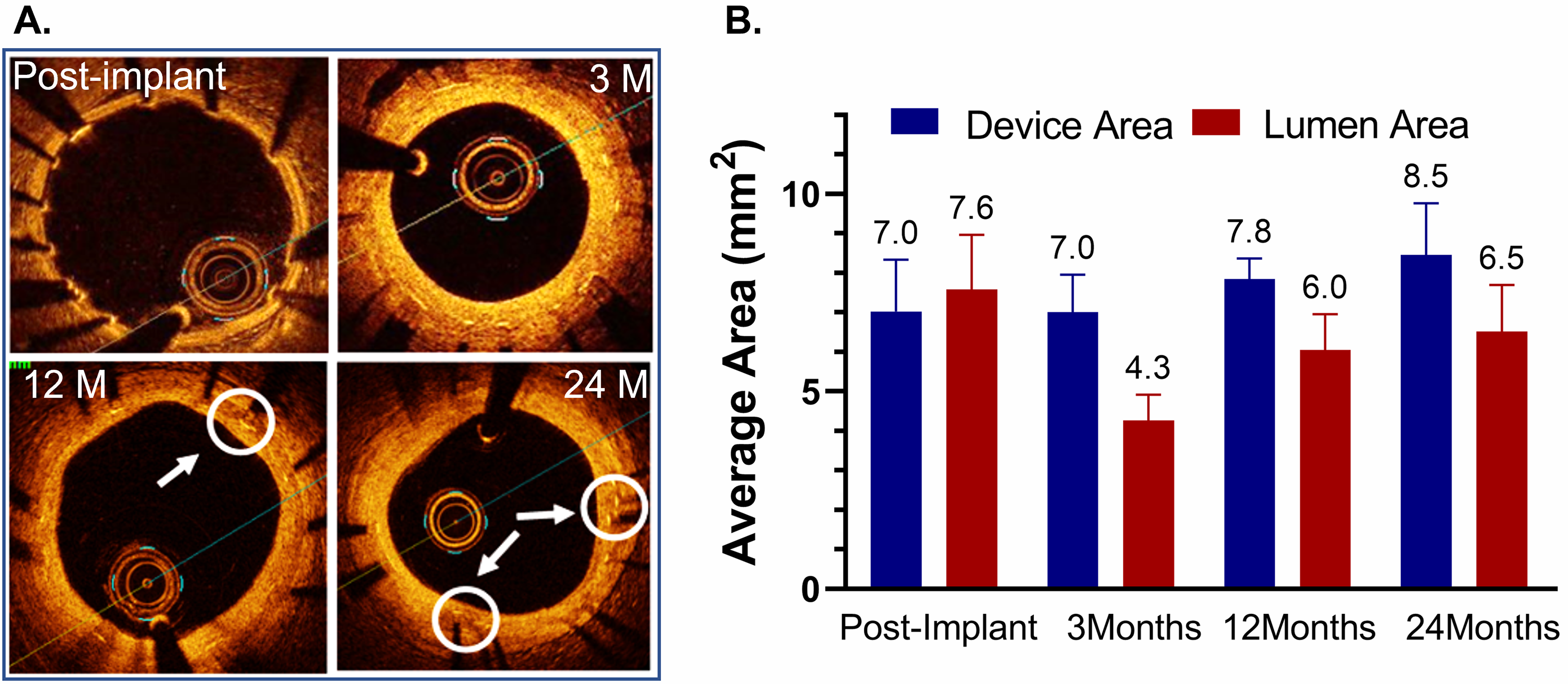 Fig. 2.
Fig. 2.Optical coherence tomography (OCT) after implantation of the DynamX Bioadaptor in Yucatan minipigs. (A) Serial OCT images at various timepoint intervals show optimal strut apposition at post-implantation and uncaged elements at 12- and 24-month time points (circles & arrows). (B) The in-device area showed that there was only a marginal difference between post-implant and three months, indicating the absence of device recoil. The vessel lumen decreases at 3 months due to neointimal growth but eventually increases as the device area increases due to uncaging suggesting adaptive positive remodelling (n = 2–5). Used with permission of Elixir Medical Corporation.
As seen in the heatmap (Fig. 3), at early time points of 3 and 6 months, before
and at the time of uncaging of the DynamX Bioadaptor, there is minimal difference
in the expression of known synthetic and contractile genes in the neointimal
tissue of vessels treated with either the DynamX Bioadaptor or the control Xience
DES. At 9 months, 3 months after uncaging of the bioadaptor, the mRNA expression
of well-known differented SMCs marker genes, namely,
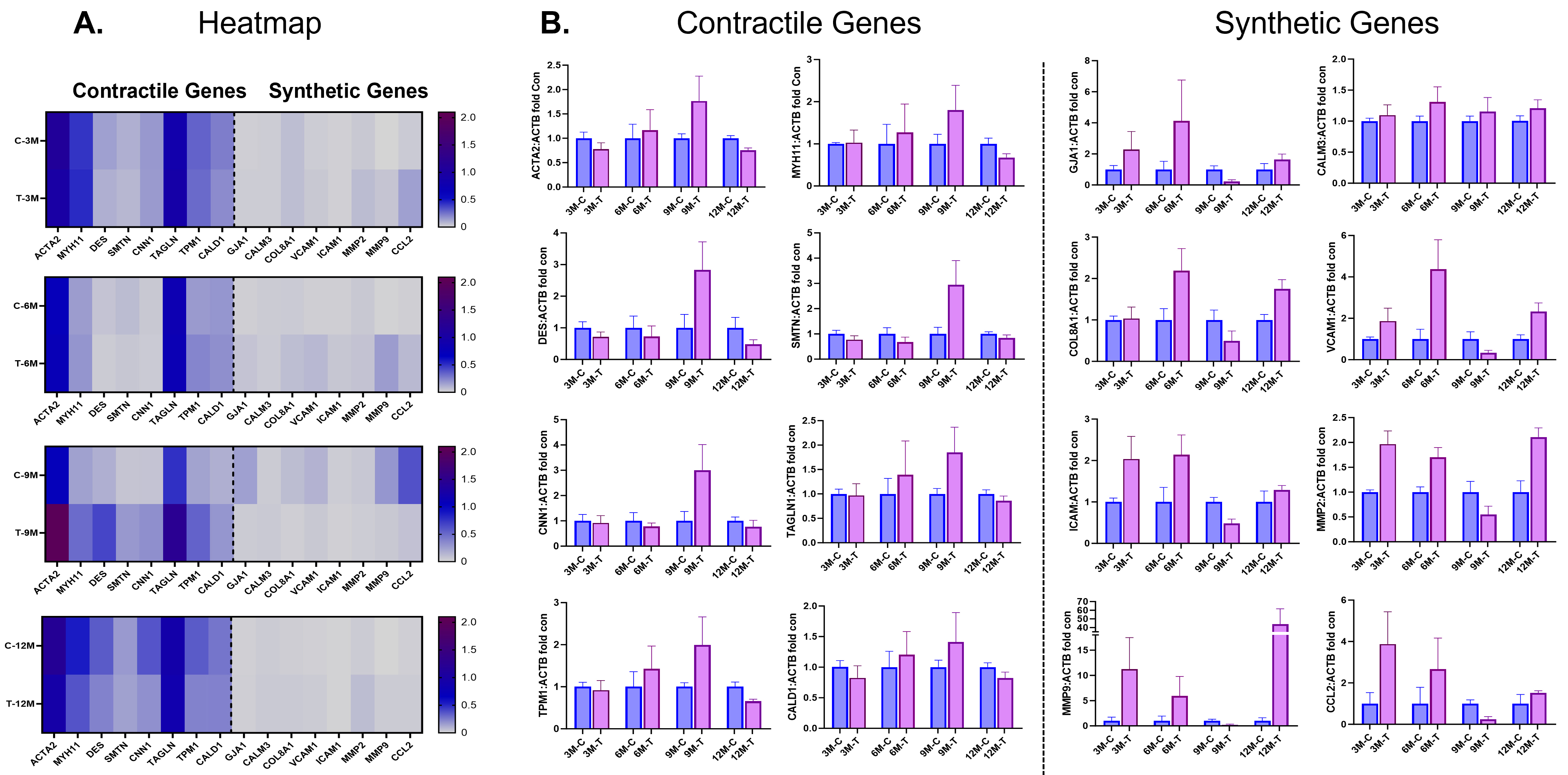 Fig. 3.
Fig. 3.Contractile and synthetic gene expressions at follow-up. (A)
Heatmap and (B) individual gene expression data showing comparison of smooth
muscle cell (SMC) contractile and synthetic genes in neointimal samples between
the test (DynamX Bioadaptor) and control (Xience stent) treatment. C-control,
M-month, T-test. Used with permission of Elixir Medical Corporation. ACTA2,
These are the first 36-month outcomes presented for the novel DynamX Bioadaptor device platform. Only one TLR occurred and no definite or probable device thrombosis were reported.
The DynamX Bioadaptor shares some advanced features with contemporary second-generation DES, i.e., it consists of ultrathin (71 µm) cobalt-chromium struts covered with a biodegradable polymer that elutes novolimus [9]. Its innovative design, however, includes uncaging elements within the bioadaptor pattern that unlock the implant and permit the improvement in vessel movement and function after in-vivo resorption of the bioresorbable polymer coating.
Thus, the DynamX Bioadaptor is the first permanent coronary artery implant designed to improve vessel function and physiology. The ability to improve the vessel function through disengagement of the uncaging elements was demonstrated at 9- to 12-month imaging follow-up [9, 10, 11] through:
(a) Positive arterial remodelling. Unlike DES, where the lumen is expected to
decrease over time, the late vessel and bioadaptor expansion, measured by IVUS,
compensates for the increase in neointima that occurs with DES implantation and
allows for the maintenance of the lumen area that was achieved with the initial
deployment (increase in in-device area from 7.39
(b) Improved vessel pulsatility. The vessel pulsatility in the treated segment increased in response to the cardiac cycle (46% improvement in maximum lumen area change at follow-up by IVUS), resulting in a reduction of the segmental mismatch in area compliance between the treated and untreated adjacent segments.
(c) Improved vasomotion. Vasomotion in response to nitroglycerine increased from
0.03 mm
(d) Restoring angulation. There was a return towards baseline angulation at
follow-up (from a mean of 137.6
(e) Reduced stress. The peak stress within the bioadaptor was reduced by 70% after uncaging, evaluated by finite element analysis.
Likewise, in a preclinical animal study with serial OCT assessment at multiple timepoints, the mean device area did only marginally change from post-procedure to 3 months, indicating the absence of acute or subacute vessel recoil. But after uncaging, at 12 and 24 months, the mean device area increased, further demonstrating the ability for positive adaptive remodelling that compensates for the neointimal growth, as seen for the lumen diameter that decreased at three months, but increased again after uncaging.
Another preclinical study showed that the neointimal covering is majorly
constituted of SMCs that are not terminally differentiated and may modulate their
phenotype in response to local microenvironmental changes such as vascular injury
[12, 13]. An initial proliferative and dedifferentiated state of neointimal SMCs
is characterized by upregulation of synthetic or activation marker genes, which
eventually reverses into a low proliferative and differentiated state
characterized by upregulation of contractile genes, e.g., ACTA2, DES, smooth
muscle myosin heavy chain (SM-MHC), smooth muscle protein 22-
Acknowledging the limitations of the small patient population enrolled in the
DynamX Mechanistic study, outcomes are at least comparable to commercially
available second-generation DES systems. Twelve-month data have been presented
previously and reported low in-device late lumen loss (0.12
At 36 months, only one CD-TLR and no additional CD-TVR occurred. The absence of any non-target lesion CD-TVR is possibly associated with the improvement in segmental compliance mismatch, reducing the irritation at the stent edges. Certainly, the CD-TLR rate of 2.2% at 36 months compares well to other contemporary DES. In the BIONYX trial, the 36-month CD-TLR rate was 4.7% for the zotarolimus-eluting Resolute Onyx stent (Medtronic, Santa Rosa, CA, USA) and 4.6% for the Orsiro sirolimus-eluting DES (Biotronik AG, Buelach, Switzerland) [21]; in the BIOFLOW-V trial, it was 3.2% for Orsiro versus 6.7% for the everolimus-eluting Xience DES (Abbott Vascular, Santa Clara, California) [22]; in the BIO-RESORT trial the 36-month CD-TLR rate ranged from 2.9% to 3.8% for the Orsiro DES, the Resolute Integrity zotarolimus-eluting stent, and the Synergy everolimus-eluting stent (Boston Scientific, Marlborough, Massachusetts) [23]; and in the TALENT trial, it was 5.0% for the Supraflex sirolimus-eluting stent (Sahajanand Medical Technologies, Surat, India) and 5.9% for Xience [24].
Noteworthy, no thrombotic events occurred (0.0% target-vessel myocardial infarction and 0.0% definite or probable device thrombosis), as compared to definite or probable stent thrombosis rates that range from 0.5% to 1.4% in the BIONYX, BIO-RESORT and TALENT trials [21, 23, 24].
Our study has several limitations: It included a limited number of subjects and a selected patient population with relatively simple lesions; the outcomes need to be confirmed in larger trials with more complex patients. Furthermore, the single-arm design precludes a direct comparison to other DES. Nevertheless, these first 36-month outcomes of this innovative device are relevant and the trial was listed in the Advances in Clinical Cardiology Summary of Key Clinical Trials [25]. Several trials are currently ongoing to assess the device in larger patient populations with more complex lesions, and to compare it against contemporary DES in randomized settings. The animal studies have the inherent limitation that the vessels are not atherosclerotic.
In conclusion, the DynamX Bioadaptor demonstrated very good safety and performance outcomes. The 36-month TLF-rate was low with the absence of any target-vessel myocardial infarction, and only one target lesion revascularization. No definite or probable device thrombosis was reported. These outcomes are potentially related to an improved vessel function in the treated segment, as demonstrated through intracoronary imaging and as confirmed in preclinical studies with late lumen enlargement assessed by OCT and an increased expression of contractile genes around 9 months compared to a conventional DES which is indicative for vessel healing. Larger, randomized studies are necessary to corroborate these findings and to compare long-term outcomes against contemporary DES.
CD, clinically-driven; DES, drug-eluting stent; IVUS, intravascular ultrasound; OCT, optical coherence tomography; SMC, smooth muscle cells; TLF, target lesion failure; TLR, target lesion revascularization; TVF, target vessel failure; TV-MI, target-vessel myocardial infarction; TVR, target vessel revascularization.
Data are available from the corresponding author upon reasonable request, but with an embargo of 12 months.
SV, MV, MM, CD, FG, FB, BDB, and AC performed the research. SV and AC were coordinating investigators. RAC, DC, JRC, and AA were responsible for the core laboratory including the core laboratory statistical analyses. SV wrote the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript and agreed to be accountable for all aspects of the work.
The study was approved by the ethics committees of all participating sites and all patients provided written informed consent before any study procedure. Ethics committees were: Institutional Review Board-ZNA/OCMW Antwerpen Lindendreef I, 2020 Antwerpen, Belgium (EC 5001), Comite Medische Ethiek ZOL Schiepse Bos 6, 3600 Genk, Belgium (17/079L), Ethisch Comite OLV Aalst Moorselbaan 164; 9300 Aalst, Belgium (2018/12), Ethische Commissie Onderzoek UZ/KU Leuven Herestraat 49, 3000 Leuven, Belgium (S60976), Il Comitato Etico via Olgettina, 60 - 20132 Milan, Italy (220/2017), Il Comitato Etico Via Morandi, 30 -20097 San Donato Milanese, Milan, Italy (237/2017).
We thank Beatrix Doerr, Consultant Medical Writer, for her help in preparing this manuscript, reimbursed by Elixir Medical Corporation.
This study and the publication charges were funded by Elixir Medical Corporation.
The institutions of SV, MV, MM, FG, FB, CD, BDB, AC received research funding from Elixir Medical to conduct the study, RAC, DC, JRC and AA were involved in the core laboratory. SV reports consultancy fees from Neovasc outside the submitted work and speaker fees from Elixir Medical. BdB reports consulting fees from Abbott Vascular, Boston Scientific, Medyrial, and Xenter paid to his institution. AC reports honoraria for lectures/presentations. The other authors declare no conflict of interest.
Supplementary material associated with this article can be found, in the online version, at https://doi.org/10.31083/j.rcm2408221.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.



