- Academic Editor
†These authors contributed equally.
This is an open access article under the CC BY 4.0 license.
Background: Patients with acute myocardial infarction (AMI) complicated with arrhythmia are not uncommon. Insertion of temporary pacemakers (tPMs) in patients with arrythmia during acute myocardial infarction (AMI) is imperative support therapy. Arrhythmias include high-degree atrioventricular block (AVB), sinus arrest/bradycardia, and ventricular arrythmia storm. To date, no study has evaluated the prognosis of tPMs in patients with AMI complicated with arrhythmia. Especially in the era of thrombolysis or emergency percutaneous coronary intervention (PCI) for coronary artery revascularization, our study was designed to investigate the value of tPMs implantation in cases of AMI complicated with various arrhythmias. Methods: From January 2009 to January 2019, 35,394 patients with AMI, including 62.0% (21,935) with ST-segment elevation myocardial infarction (STEMI) and 38.0% (13,459) with non-ST-segment elevation myocardial infarction (NSTEMI) in four hospitals, were reviewed. A total of 552 patients with AMI associated with arrythmia were included in the cohort. Among the 552 patients, there were 139 patients with tPM insertions. The incidence trend of myocardial infarction complicated with various arrhythmias in the past 10 years was analysed, and the clinical characteristics, in-hospital mortality, postdischarge mortality, composite endpoints of modality, and independent risk factors were compared in patients with and without tPM in the era of coronary artery revascularization. Results: In patients with AMI-associated arrythmia, high-degree AVB was the major cause of tPM insertion (p = 0.045). In the past 10 years, the number of patients with high-degree AVB, tPM implantation, ventricular arrythmia storm, and in-hospital mortality has decreased year by year in the era of coronary artery revascularization. In the tPM group, the culprit vessel was the left main artery, and cardiogenic shock, acute renal injury and high brain natriuretic peptide (BNP) levels were independent risk factors for patients with AMI complicated with arrhythmia. The in-hospital mortality in the tPM group was higher than that in the non-tPM group. The patients with tPM insertion showed better postdischarge survival than patients without tPM insertion. Conclusions: In the era of emergency thrombolysis or PCI, coronary revascularization can ameliorate the prognosis of patients with AMI complicated with various arrhythmias. Temporary pacemaker insertion in patients with AMI complicated with arrhythmia can reduce the postdischarge mortality of these patients.
Insertion of transvenous temporary pacemakers (tPMs) in patients with bradycardiac arrythmia during acute myocardial infarction (AMI) is imperative support therapy, which was widely studied in the era before the generalization of intravenous fibrinolysis and primary percutaneous coronary revascularization. The majority of tPM insertions in patients with AMI were due to high-degree or complete atrioventricular block (AVB). In the era before coronary revascularization, the average in-hospital mortality for inferior wall AMI without AVB was 9%, compared with 23% in patients with high-degree AVB and 29% in patients with third-degree AVB. Recently, a large retrospective analysis from the Global Registry of Acute Coronary Events reported that high-degree AVB had a strong association with in-hospital but not late mortality, while tPMs therapy was not associated with improved in-hospital survival [1].
Compared with the 1990s, in the last 10 years, we observed a perceptible decrease in the incidence of temporary pacemaker therapy in patients with AMI in clinical practice. The aim of this study was to determine the incidence of tPM insertion, death and permanent PM implantation associated with tPM insertion and risk factors associated with death in patients with tPM during either ST-segment elevation myocardial infarction (STEMI) or non-ST-segment elevation myocardial infarction (NSTEMI) in the era of fibrinolysis and primary percutaneous coronary revascularization.
This is a retrospective multicentre study that enrolled patients with AMI at 4
hospitals (Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese
Academy of Medical Sciences and Peking Union Medical College, Linyi People’s
Hospital of Shandong Province, University-Town Hospital of Chongqing Medical
University, Chongqing University Center Hospital) in China between 2009 and 2019.
Eligible patients were
ECGs were interpreted at each enrolling centre by 2 experienced cardiologists
and not centrally adjudicated. ST-segment elevation of ECGs was defined as
Indication for transvenous tPMs insertion was defined as: (1) new high-degree
AVB due to acute myocardial ischaemia or complications during percutaneous
coronary intervention (PCI) (such as no reflow, or iatrogenic coronary
dissection); (2) new sinus arrest
Standardized case report forms were completed by study physicians to document patient data, clinical history, clinical manifestation, medication use (before and during hospitalization), in-hospital treatment (medical and invasive therapies), and in-hospital clinical events (death, permanent pacemaker, cardiogenic shock, sustained ventricular tachycardia, or stroke). The primary endpoints were the occurrence of in-hospital and postdischarge death. Secondary endpoints were in-hospital and postdischarge permanent pacemaker implantations. The follow-up consisted of a clinical visit every 6 months and contacts by telephone every 12 months to identify vital status and new clinical events.
Patients with AMI and myocardial ischaemia-associated arrythmia (high-degree
arrythmia, sinus arrest or bradycardia, or ventricular shock with bradycardia
occurring at any time during AMI) were divided into two groups: those with tPM
insertion and those without tPM insertion at any time during AMI. Mean
A total of 35,394 patients with AMI hospitalized in 4 hospitals (Fuwai Hospital, Linyi People’s Hospital of Shandong Province, University-Town Hospital of Chongqing Medical University, Chongqing University Center Hospital) between 2009 and 2019 were continuously included: 62.0% (21,935) STEMI and 38.0% (13,459) NSTEMI. In the past 10 years, the incidence of AMI and the number of PCIs have increasing. Among the patients with STEMI, 10,723 (30.3%) patients with acute inferior infarction were identified. The overall tPM insertion rate of the cohort was 0.39% (139/35,394). In patients with cardiac ischaemia-associated arrythmias (552/35,394, 1.6%), high-degree AVB was the major cause of tPM insertion (p = 0.045, Fig. 1), and the rate of tPM insertion showed no significant difference in patients with STEMI compared with that in patients with NSTEMI (0.14% vs. 0.15%, p = 0.331). The median time of tPM usage was 103 (48–192) hours, and no significant difference was recorded between the STEMI and NSTEMI cohorts (p = 0.103).
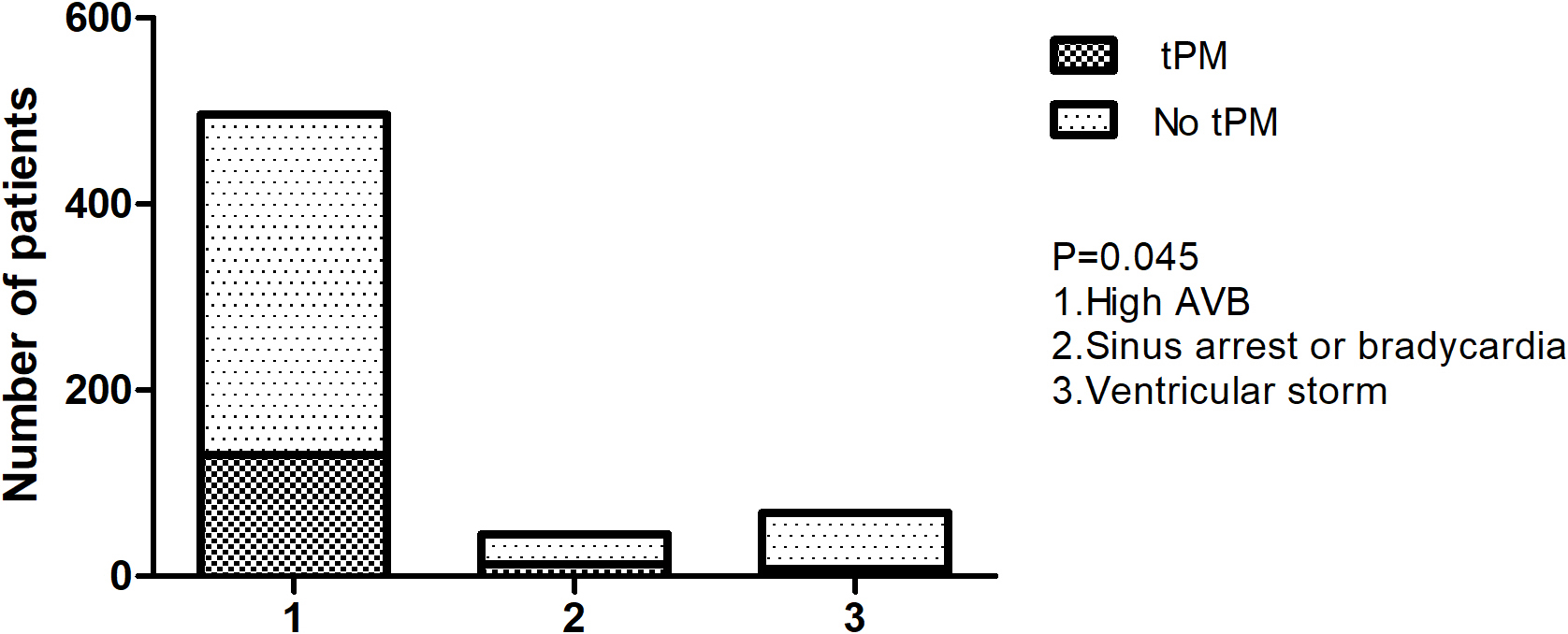 Fig. 1.
Fig. 1.Temporary pacemaker in different arrythmias due to AMI. tPM, temporary pacemaker; AVB, atrioventricular block; AMI, acute myocardial infarction.
This study observed the change trend of patients with AMI and high-degree AVB,
sinus arrest/bradycardia, ventricular arrythmia storm, the proportion of
temporary and permanent pacemaker insertion and in-hospital mortality in the last
10 years. AMI with high-degree AVB (linear trend = –0.1; p = 0.007,
Fig. 2), combined with ventricular arrythmia storm (linear trend = –0.1%;
p = 0.001, Fig. 3), proportion of tPM implantation (linear trend =
–0.1%; p
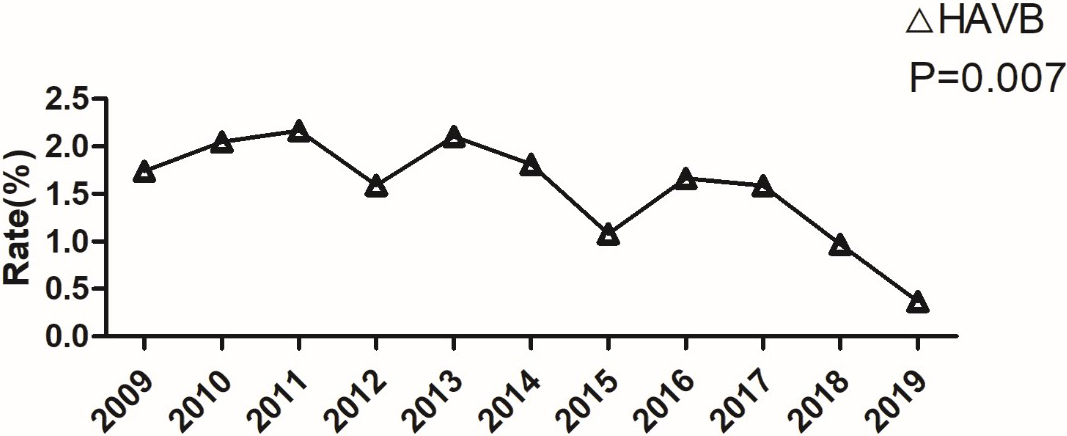 Fig. 2.
Fig. 2.Incidence of acute myocardial infarction complicated with high-degree. HAVB, high-degree atrioventricular block.
 Fig. 3.
Fig. 3.Incidence of acute myocardial infarction complicated with ventricular arrythmia storm, temporary pacemaker implantation and in-hospital mortality in the last 10 years. tPM, temporary pacemaker.
The baseline characteristics of 552 patients are summarized in Table 1. Among
552 patients with AMI with arrythmia, 139 (25.2%) of them received tPM
implantation. There were no significant differences in hypertension, diabetes,
peripheral vascular disease, stroke, myocardial infarction, percutaneous coronary
intervention, coronary artery bypass graft, heart failure, adenosine diphosphate (ADP) receptor blocker,
| With temporary pacemaker (n = 139) | Without temporary pacemaker (n = 413) | p-value | |||
|---|---|---|---|---|---|
| Age | 66.5 |
63.8 |
0.022 | ||
| Male (%) | 92 (66.2) | 348 (84.3) | 0.001 | ||
| Medical history | |||||
| Hypertension | 90 (64.7) | 270 (65.4) | 0.812 | ||
| Diabetes | 57 (41.0) | 166 (40.2) | 0.866 | ||
| Dyslipidaemia | 91 (65.5) | 312 (75.5) | 0.021 | ||
| Peripheral vascular disease | 11 (7.9) | 42 (10.2) | 0.435 | ||
| Stroke/TIA | 25 (18.0) | 76 (18.4) | 0.913 | ||
| Myocardial infarction | 12 (8.6) | 63 (15.3) | 0.050 | ||
| Percutaneous coronary intervention | 7 (5.0) | 52 (12.6) | 0.913 | ||
| Coronary artery bypass graft surgery | 5 (3.6) | 23 (5.6) | 0.203 | ||
| Heart failure | 10 (7.2) | 49 (11.9) | 0.123 | ||
| Prehospital medication | |||||
| Aspirin | 62 (0.45) | 290 (0.7) | 0.001 | ||
| ADP receptor blocker | 58 (0.42) | 188 (0.46) | 0.436 | ||
| 27 (0.19) | 107 (0.26) | 0.123 | |||
| Statin | 51 (0.37) | 221 (0.54) | 0.001 | ||
| ACEI | 29 (0.21) | 95 (0.23) | 0.512 | ||
| ARB | 28 (0.2) | 89 (0.22) | 0.726 | ||
| Clinical presentation | |||||
| Systolic blood pressure (mmHg) | 117.5 |
119.2 |
0.428 | ||
| Killip class | |||||
| I | 83 (0.6) | 284 (0.69) | 0.071 | ||
| II | 32 (0.23) | 70 (0.17) | 0.111 | ||
| III | 7 (0.05) | 18 (0.04) | 0.740 | ||
| IV | 17 (0.44) | 41 (0.1) | 0.444 | ||
| Arrythmia due to AMI | |||||
| High-degree AVB | 130 (0.94) | 366 (0.89) | 0.098 | ||
| Sinus arrest | 13 (0.09) | 32 (0.08) | 0.550 | ||
| Ventricular arrythmia storm | 8 (0.06) | 60 (0.15) | 0.006 | ||
| ST-segment elevation | 121 (0.87) | 341 (0.83) | 0.216 | ||
| ST-segment depression | 17 (0.12) | 65 (0.16) | 0.223 | ||
| Left bundle branch block | 5 (0.036) | 7 (0.017) | 0.276 | ||
| LVEDD (mm) | 48.5 |
50.4 |
0.005 | ||
| LVEF (%) | 51.7 |
53.9 |
0.021 | ||
ADP, adenosine diphosphate; AMI, acute myocardial infarction; TIA, transient ischaemic attack; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; AVB, atrioventricular block; LVEDD, left ventricular end diastolic diameter; LVEF, left ventricular ejection fraction.
However, there were significant differences in age, male sex, dyslipidemia, aspirin, statin use, ventricular arrythmia storm, left ventricular end diastolic diameter (LVEDD) (mm), and left ventricular ejection fraction (LVEF) (%). Older people, females tended to be more prevalent in the tPM group.
Specifically, for patients with tPM insertion during AMI, cardiogenic shock
(p = 0.044, 95% CI, HR = 4.384), acute kidney injury (p =
0.019, 95% CI, HR = 11.9), left main coronary artery as the culprit vessel
(p
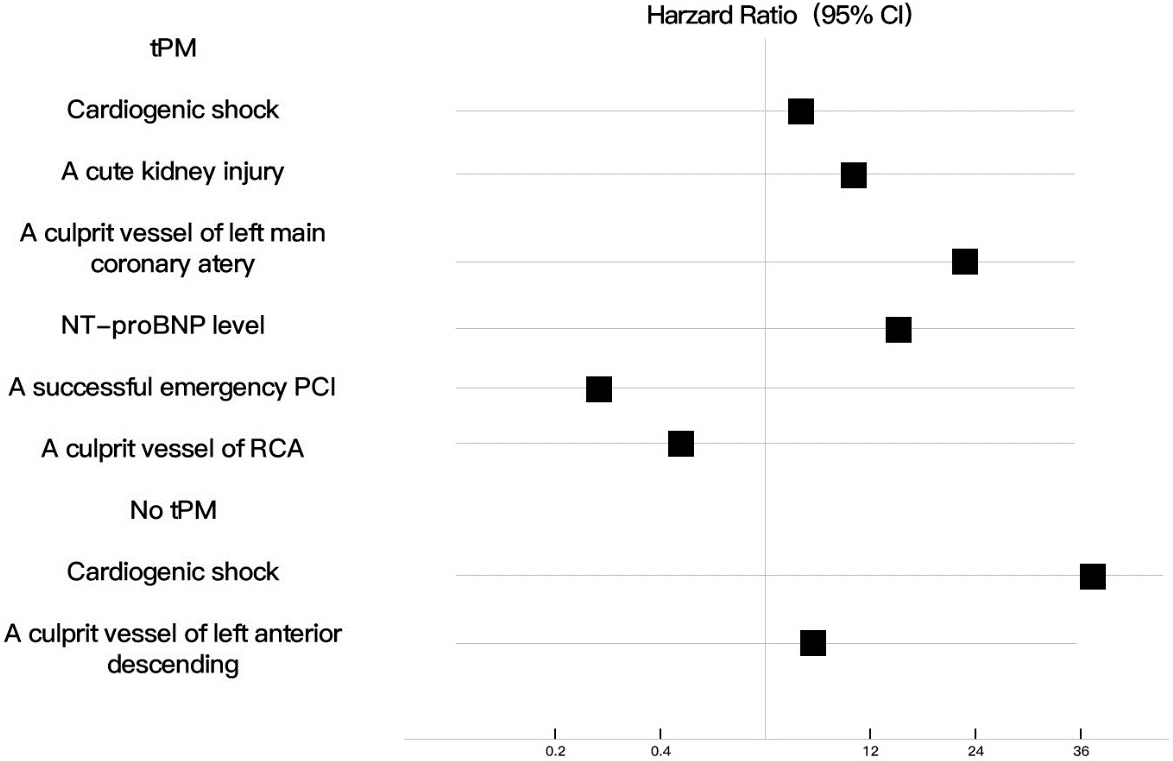 Fig. 4.
Fig. 4.Independent risk factors in patients with or without tPM insertion. tPM, temporary pacemaker; NT proBNP, N-terminal pro-brain natriuretic peptide; PCI; percutaneous coronary intervention; RCA, right coronary artery.
Table 2 shows the clinical characteristics of in-hospital events, coronary
lesions and culprit vessels in the two groups of patients with and without tPM
implantation. Coronary artery PCI; coronary artery bypass grafting; myocardial
reinfarction; heart failure; cardiogenic shock; persistent ventricular
tachycardia; stroke; permanent pacemaker implantation; implantable cardioverter-defibrillator (ICD); acute renal injury;
degree of coronary artery stenosis greater than 50% (left main trunk, anterior
descending branch, circumflex branch, or right coronary artery); culprit vessels
(left main, anterior descending branch, circumflex branch, or right coronary
artery); thrombolysis in myocardial infarction (TIMI) blood flow of culprit vessels before coronary PCI (grade 0, grade
1, or grade 2); number of coronary arteries with stenosis greater than 50% [1, 2]; and being discharged on aspirin, statin, and ARB or ACEI drugs in the two
groups of patients were not statistically different. In-hospital death in the tPM
implantation group was significantly higher than that in the non-tPM implantation
group (p = 0.017). The culprit vessels were right coronary artery
(p = 0.015), TIMI blood flow of culprit vessels before coronary PCI was
grade 3 (p = 0.011), the number of vessels with coronary stenosis
greater than 50% was 3 (p = 0.023), and the number of patients
discharged on a
| With tPM (n = 139) | Without tPM (n = 413) | p-value | |||
|---|---|---|---|---|---|
| In-hospital procedure (%) | |||||
| Cardiac catheterization | 95 (68.3) | 346 (83.8) | 0.001 | ||
| Percutaneous coronary intervention | 90 (64.7) | 316 (76.5) | 0.210 | ||
| Percutaneous coronary intervention |
88 (64.0) | 280 (67.8) | 0.332 | ||
| Coronary artery bypass graft surgery | 3 (2.2) | 6 (1.5) | 0.549 | ||
| Thrombolytic therapy | 1 (0.7) | 3 (0.7) | 1.000 | ||
| In-hospital events (%) | |||||
| Myocardial re-infarction | 1 (0.7) | 2 (0.5) | 0.744 | ||
| Congestive heart failure | 13 (9.3) | 31 (7.5) | 0.314 | ||
| Cardiogenic shock | 6 (4.3) | 12 (2.9) | 0.418 | ||
| Sustained ventricular tachycardia | 5 (3.6) | 22 (5.3) | 0.413 | ||
| Stroke/TIA | 1 (0.7) | 1 (0.2) | 0.441 | ||
| Permanent pacemaker | 13 (9.4) | 36 (9.2) | 0.820 | ||
| ICD | 2 (1.4) | 8 (1.9) | 0.703 | ||
| Death | 18 (13.0) | 26 (6.3) | 0.017 | ||
| Acute kidney injury (n, %) | 2 (1.4) | 3 (0.7) | 0.443 | ||
| Coronary-artery stenosis |
|||||
| Left main | 12 (8.6) | 40 (9.7) | 0.774 | ||
| Left anterior descending | 82 (59.0) | 270 (65.4) | 0.075 | ||
| Left circumflex | 72 (51.8) | 218 (52.8) | 0.840 | ||
| Right coronary artery | 95 (68.3) | 329 (79.7) | 0.409 | ||
| Culprit vessel (%) | |||||
| Left main | 1 (0.7) | 3 (0.7) | 1.000 | ||
| Left anterior descending | 10 (7) | 27 (6.5) | 0.789 | ||
| Circumflex | 10 (7) | 18 (6.5) | 0.188 | ||
| Right coronary artery | 81 (58.3) | 289 (70) | 0.015 | ||
| Saphenous graft bypass | 1 (0.7) | 4 (1) | 0.789 | ||
| TIMI grade of culprit vessel before PCI | |||||
| 0 | 79 (56.8) | 214 (51.8) | 0.305 | ||
| 1 | 7 (5) | 42 (9.9) | 0.066 | ||
| 2 | 2 (1.4) | 13 (3.1) | 0.284 | ||
| 3 | 9 (6.5) | 61 (14.8) | 0.011 | ||
| Number of coronary vessels with |
|||||
| 1 | 11 (7.9) | 57 (13.8) | 0.067 | ||
| 2 | 29 (20.9) | 106 (25.7) | 0.137 | ||
| 3 or more | 46 (33.1) | 182 (44.1) | 0.023 | ||
| Discharge medications | |||||
| Aspirin | 121 (87) | 387 (93.7) | 0.898 | ||
| 7 (5.0) | 78 (18.9) | 0.001 | |||
| Statin | 112 (80.6) | 358 (86.7) | 0.319 | ||
| ACEI | 88 (84.2) | 293 (70.9) | 0.864 | ||
| ARB | 33 (23.7) | 97 (23.5) | 0.599 | ||
| Discharge Permanent pacemaker | 7 (0.05) | 6 (0.01) | 0.024 | ||
TIA, transient ischaemic attack; ICD, implantable cardioverter-defibrillator; TIMI, thrombolysis in myocardial infarction; PCI, percutaneous coronary intervention; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker.
However, for in-hospital mortality, 13.0% of patients with tPM implantationdied
prior to hospital discharge compared with 6.3% without tPM insertion (p
= 0.012, Fig. 5). After adjusting for three-vessel disease and successful
percutaneous revascularization, this association also had statistically
significant, with an odds ratio (OR) of in-hospital death of 4.0 (95% CI, 3.5–4.6;
p
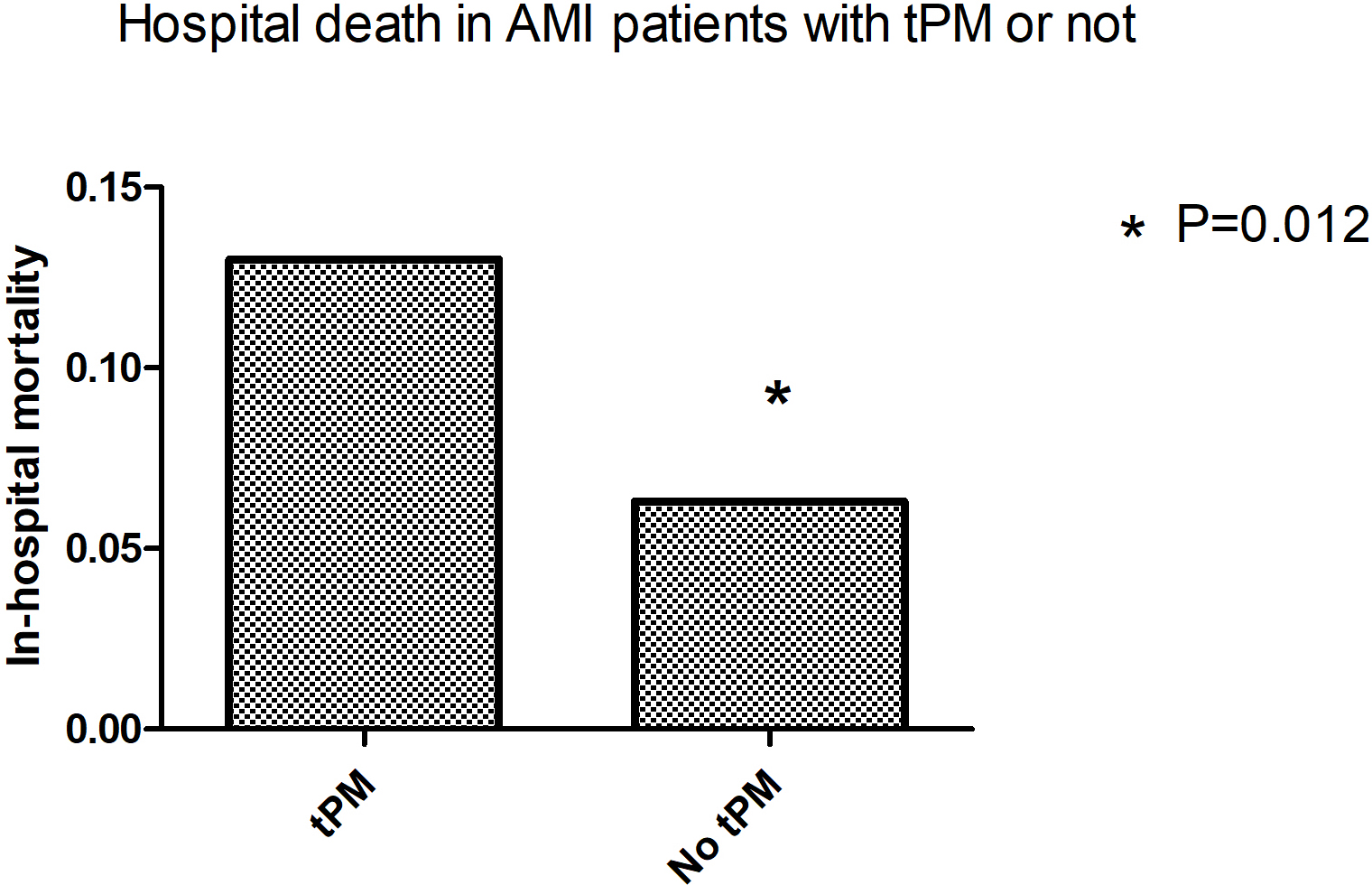 Fig. 5.
Fig. 5.In-hospital mortality in patients with AMI with or without tPM insertion. tPM, temporary pacemaker; AMI, acute myocardial infarction.
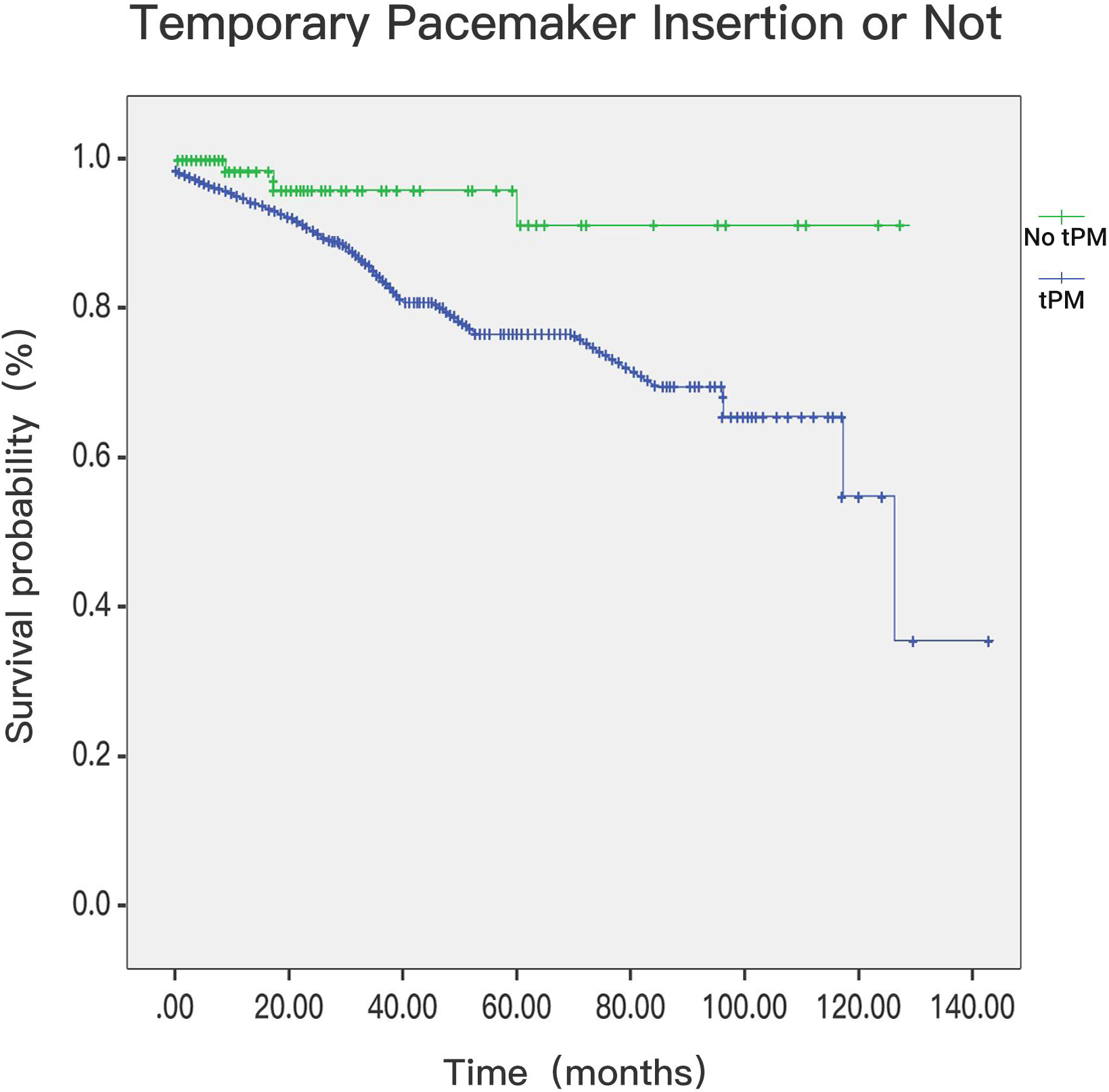 Fig. 6.
Fig. 6.In-hospital mortality in patients with tPM or not (p
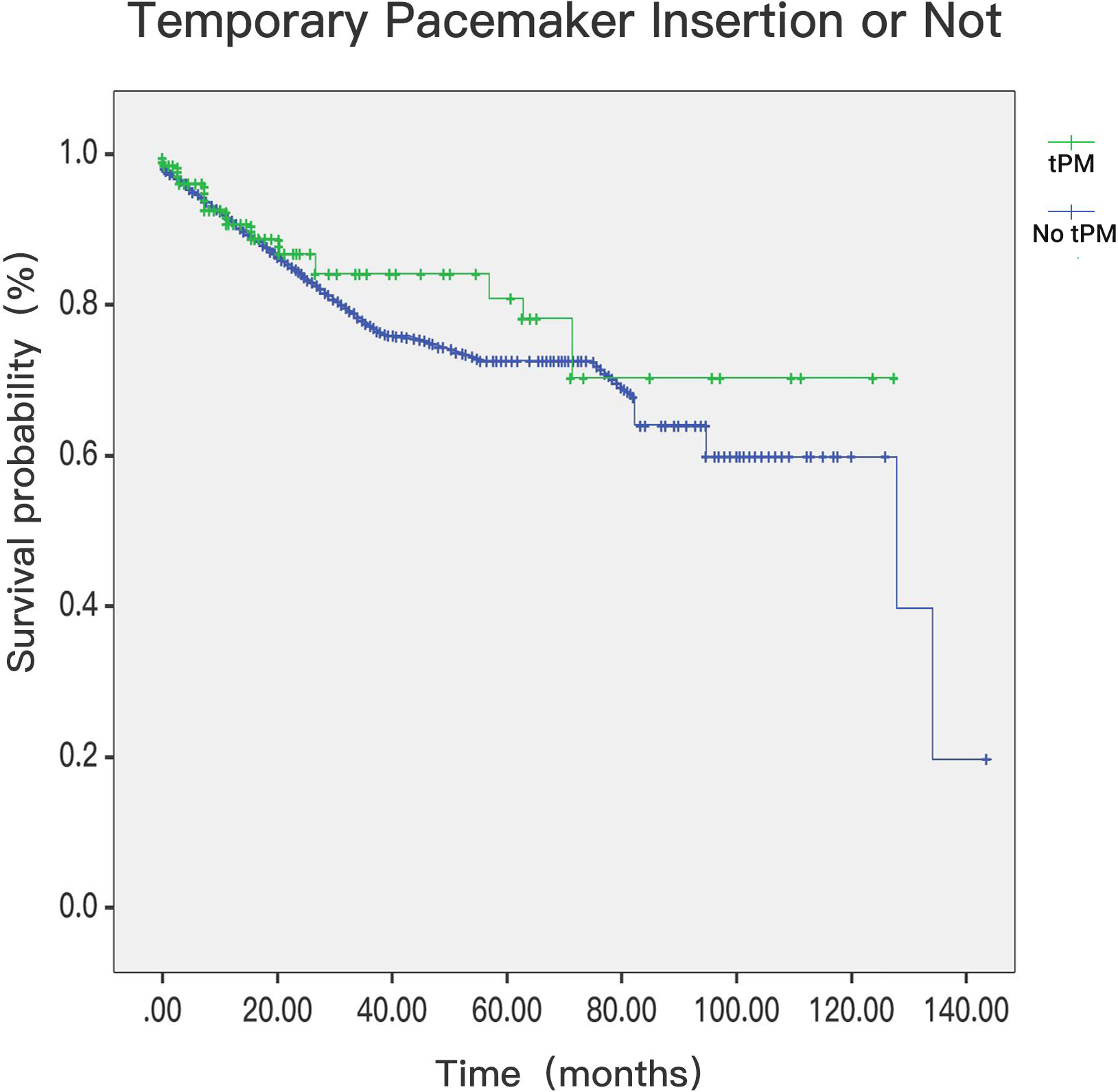 Fig. 7.
Fig. 7.Postdischarge mortality in patients with tPM or not (p = 0.006). tPM, temporary pacemaker.
This article describes the clinical characteristics and prognosis of patients with AMI complicated with arrhythmia for the first time. A total of 36,294 hospitalized patients with AMI in four medical centres over 10 years were analysed. Early studies showed that, before the era of intravenous thrombolysis and emergency PCI, AMI complicated with arrhythmia had a high in-hospital mortality rate. In 1970, Narva [2] found that the in-hospital mortality of patients with AMI complicated with high-degree AVB was 33%, and the proportion of tPM implantation in such patients was as high as 60%, which was much higher than that in the era of intravenous thrombolysis and emergency PCI. Our study found that, in the current era of vascular reconstruction, the in-hospital mortality of patients with AMI complicated with high-degree AVB was 8%, and the proportion of tPM implantation in patients with AMI complicated with arrhythmia (including sinus arrest, high-degree AVB and ventricular tachycardia storm) was 0.39%. In 1971, Rokseth [3] and others found that patients with AMI complicated with arrhythmia accounted for approximately 1.92% of patients, which was 1.37% compared with our study, indicating that the basic situation of AMI complicated with arrhythmia was no different from that in previous years.
In the last 10 years, the number of patients with high-degree AVB, tPM insertion, ventricular arrythmia storm, and in-hospital mortality has decreased year by year in the era of coronary artery revascularization. Our study confirms that, in the era of thrombolysis or emergency PCI for coronary artery revascularization, patients with AMI with arrhythmia had reductions in the critical rate of arrhythmia, decreases in the proportion of tPM insertion and reductions of in-hospital mortality due to coronary artery revascularization. These patients benefit from coronary revascularization, and this conclusion is consistent with the research of Hwang [4].
This study found that patients with AMI complicated with arrhythmia and tPM implantation had a high in-hospital mortality rate (p = 0.012). Temporary pacemaker implantation was possibly an independent risk factor for these patients. The results of recent studies [5, 6, 7, 8, 9] suggest that the in-hospital mortality rate of the tPM group is 2–5 times higher than that of the non-tPM group. Our study is consistent with these findings. Murphy [10] pointed out that the common complications of tPM implantation are ventricular fibrillation, myocardial perforation and sepsis. Our study found that there were significant differences in three-vessel disease, heart failure, cardiogenic shock, ejection fraction and ventricular tachycardia storm in the tPM group compared with those without tPMs, suggesting that the increased in-hospital mortality may be due to three-vessel disease, heart failure, cardiogenic shock and ventricular tachycardia storm. It is mainly the severity of myocardial infarction in patients with AMI complicated with arrhythmia, resulting in multiple vessels or larger infarct areas. The research results are consistent with those of Singh [1].
Our results showed for the first time that the postdischarge mortality of
patients with AMI complicated with arrhythmia was lower in the tPM group. This
finding is very interesting. It may be that patients with tPMs have ameliorated
their arrhythmia in the hospital, and because of the severe condition of AMI, if
emergency tPMs are inserted, the proportion of emergency revascularization is
higher, resulting in a decrease in out-of-hospital mortality. This research
result needs to be further researched because of the benefit of tPM implantation.
Or does the increased proportion of revascularization in these patients reduce
out-of-hospital mortality? Our results suggest that the left ventricular end
diastolic diameter (50.4
This study found for the first time that, in the tPM group, if the culprit vessel was the left main artery, whether revascularized or not, it was an independent risk factor. In the tPM group, Cardiogenic shock, acute renal injury and high BNP levels were independent risk factors for patients with AMI complicated with arrhythmia. In patients without tPMs, cardiogenic shock and anterior wall infarction are independent risk factors for AMI complicated with arrhythmia. These results are consistent with previous studies [11, 12] showing that acute renal injury and high BNP levels are independent risk factors for AMI.
In myocardial infarction combined with high-degree AVB or sinus arrest, the main mechanism is acute left ventricular inferior wall or posterior wall myocardial infarction, is caused by ischemia of the atrioventricular node artery and sinoatrial node artery, which occurs in the early stage of myocardial infarction, reflecting myocardial reperfusion, and activating parasympathetic nerves [13, 14, 15, 16]. In the subgroup analysis of our study, there was no significant difference in the proportion of tPM implantation in the group with myocardial infarction complicated with high-degree AVB or sinus arrest, indicating that this kind of arrhythmia may be more common in the early stage of myocardial infarction or it may be easily ameliorated by timely drug treatment or revascularization. Another subgroup analysis found that the proportion of patients with AMI complicated with ventricular tachycardia storm with tPM insertion was less than that without tPM insertion, indicating that revascularization is more effective and provides long-term benefit for patients with such arrhythmias in the era of emergency thrombolysis and PCI.
In the era of emergency thrombolysis or PCI, coronary revascularization can ameliorate the prognosis of patients with AMI complicated with various arrhythmias. Temporary pacemaker insertion in patients with AMI complicated with arrhythmia can reduce the postdischarge mortality of these patients.
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
YJY designed the research study. PW, XLX, SDW, ZML, LS, BX, KFD, YJW, SBQ, RLG, GZ, MH, XMH, and HW performed the research. ZML, LS, BX, KFD, YJW, SBQ, RLG and GZ provided their selected cases and advice on the study. PW and XLX analyzed the data. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript.
This trial has been supported by the Medical Ethics Committee of University-Town Hospital of Chongqing Medical University (LL-202014). All participating patients signed informed consent forms.
Not applicable.
This work was supported by the Science and Technology Research Program of Chongqing Municipal Education Commission (Grant No. KJQN201800406). This work was supported by Chongqing Science and health joint project (Grant No. 2020GDRC020).
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
