- Academic Editor
†These authors contributed equally.
Background: Systematic evaluation of the effects of vitamin D supplementation in heart failure (HF) patients. Methods: Searches were conducted on National Library of Medicine, Web of Science, Cochrane Library, Google Scholar, China National Knowledge Infrastructure, and WANFANG databases. We analyzed data by using Review Manager 5.4 software. All are from the earliest records to March 2023. Outcome indicators analyzed the left ventricular ejection fraction (LVEF), the left ventricular end-diastolic internal diameter (LVEDD), the B-type brain natriuretic peptide (BNP) level and the 25-hydroxy vitamin D (25(OH)D) level. Results: Ten studies with 1099 patients were included. LVEF (mean difference (MD) = 0.74, 95% CI: –0.29 to 1.76, p = 0.41), LVEDD (MD = –0.59, 95% CI: –1.83 to 0.66, p = 0.25), BNP (MD = –0.08, 95% CI: –0.24 to 0.08, p = 0.34), 25(OH)D (MD = 0.41, 95% CI: –0.28 to 1.11, p = 0.25) are not statistically significant. And there is no heterogeneity in the results of LVEF, LVEDD and BNP indicators. Conclusions: Vitamin D supplementation may not be helpful in the clinical management of patients with HF.
Heart failure (HF) is a potential outcome of end stage of heart disease. It impairs the cardiac circulation due to a systolic and/or diastolic function damage of the heart [1, 2]. HF occurs mainly due to remodeling of heart muscle cells [3]. Vitamin D deficiency triggers excessive activation of the renin-angiotensin-aldosterone system (RAAS) which damages the endothelial function, accelerates the ventricular remodeling, and thus may lead to HF [4]. More than 1 billion people worldwide are deficient in vitamin D leading to the World Health Organization defining it as a public health problem [5]. Vitamin D deficiency is associated with lifestyle risk factors, living conditions, and diseases which usually reduce vitamin D intake, absorption, or synthesis [6]. Low levels of vitamin D may exacerbate chronic HF [7]. However, it has been reported that vitamin D supplementation does not produce long-term benefits in HF patients [8]. Furthermore, a meta-analysis has indicated that vitamin D supplementation does not reduce mortality or improve the left ventricular function [9].
Vitamin D is an essential fat-soluble steroid hormone [10]. Under the ultraviolet
B radiation from sunshine, 7-dehydrocholesterol inside skin is converted to
vitamin D under the non-enzymatic photolysis [11]. After being released into
blood circulation, it is metabolized into 25-hydroxy vitamin D (25(OH)D) by the
25-hydroxylase in the liver [12]. Then 25(OH)D is converted to the active
calcitriol by the enzyme 1a-hydroxylase in the kidney [13]. The best method of
assessing vitamin D levels in human body is through 25(OH)D [14].
Decreased levels of 1
Vitamin D plays an important regulatory role in calcium and phosphorus metabolism [16]. It is anti-inflammatory, immunomodulatory, and affects vascular remodeling, blood glucose regulation, reduction of renin, angiotensin, and aldosterone activity in addition to playing a variety of other biological roles [17]. When calcitriol binds to the vitamin D receptor (VDR), its physiological effects are exerted [18]. Because VDR can express in vascular tissues, it may affect calcium in-flow, muscle relaxation, and diastolic function of vascular tissues [19]. As a result, the vitamin D has potential inhibitory effects on cardiac hypertrophy and anti-heart failure [20]. Vitamin D on cardiac function in patients with HF has controversial findings. This study intends to clarify the role of vitamin D in patients with HF.
Searches were conducted on National Library of Medicine, Web of Science, Cochrane Library, Google Scholar, China National Knowledge Infrastructure, and WANFANG databases. All are from the earliest records to March 2023. Searching terms included “heart failure”, “vitamin D”, “Cardiac failure”, “Randomized Controlled Trials”, “Vitamin D3” and “cardiac function”. The languages of the literature were mainly English and Chinese. The relevant literature was traced in the references of the retrieved clinical trial report papers or reviews. Protocol registration prior to initiating the meta-analysis was not possible due to missing a selective time point in the design period.
We include randomized controlled trials (RCT) of vitamin D in patients with HF.
The definition of HF is based on the New York Heart Association (NYHA)
classification
The followings are excluded: (1) trials reported in abstract only, (2) low-quality literature, (3) cohort studies, (4) retrospective case-control studies, (5) conference literature, (6) repetitive articles, (7) and nonclinical trials.
Evaluators first independently completed the initial screening of the included literature by reading the title and abstract. The methodological criteria of quality assessment are based on Cochrane and meet the inclusion criteria. By reading some full texts, the following evaluation criteria were used: (1) randomization, (2) concealment of allocation, (3) subject and intervention blinding, (4) blinding on outcome assessments, (5) data integrity, (6) selective outcome reporting, and (7) other biases. “Uncertain risk”, “low risk”, and “high risk” evaluations were used for the assessment of bias. Two independent researchers evaluated data quality. One third party was solicited to advice when discussion could not resolve the inconsistent opinion of a particular study’s inclusion.
The data extracted includes the following: (1) general information, such as title, author, year of publication and trial quality score, (2) comparability of data and interventions across patient data groups, and (3) outcome data including 25(OH)D, LVEF, left ventricular end-diastolic internal diameter (LVEDD), and B-type brain natriuretic peptide (BNP).
Statistical analysis was performed using Review Manager 5.4 software
(International Cochrane Collaboration Network, TX, USA), with a test level of
The basic characteristics of the included studies are shown in Table 1 (Ref. [23, 24, 25, 26, 27, 28, 29, 30, 31, 32]). In all included studies, 25(OH)D is an outcome indicator, and the most timeframes are from 3 months to 4 months. The study’s quality evaluation is indicated in Table 2 (Ref. [23, 24, 25, 26, 27, 28, 29, 30, 31, 32]). Most studies have low risk for all items, so the included studies are quality.
| Author | Experimental design | Vitamin D supplementation dose | Periodicity | Test population | Key outcome indicators |
| Qu et al. [23], 2015 | Forward looking | 1000 U/d | 3 months | Ischemic heart failure; NYHA classification III–IV | 25(OH)D; BNP; LVEF |
| Li et al. [24], 2015 | Forward looking | 1000 U/d | 3 months | Children with chronic heart failure; 3 years |
25(OH)D; NYHA classification; Cardiac efficacy |
| Wu et al. [25], 2011 | Forward looking | 1600 U/d | 10 weeks | Chronic heart failure; NYHA classification |
25(OH)D; BNP; NYHA classification; 6-minute walking distance (6MWD) |
| Nicolas [26], 2013 | Forward looking | 2000 U/d | 6 weeks | Chronic heart failure; age |
25(OH)D; NYHA classification; 6MWD |
| Zittermann et al. [27], 2019 | Forward looking | 4000 U/d | 12 months | Advanced heart failure; 25(OH)D |
25(OH)D; LVEF |
| Soad et al. [28], 2012 | Forward looking | 1000 U/d | 3 months | Infants with ischaemic heart failure; EF |
25(OH)D; LVEF; RAS cytokines |
| Klaus et al. [29], 2016 | Forward looking | 4000 U/d | 12 months | Chronic heart failure; LVEF |
25(OH)D; LVEF; 6MWD |
| Rebecca et al. [30], 2014 | Forward looking | 50,000 U/w | 6 months | Heart failure; age |
25(OH)D; PTH |
| Woo et al. [31], 2022 | Forward looking | 4000 U/d | 4 months | Chronic heart failure; 25(OH)D |
25(OH)D; LVEF; NYHA classification; 6MWD |
| Heidi [32], 2017 | Forward looking | 10,000 U/d | 6 months | Heart failure; NYHA classification II–III; age |
25(OH)D; BNP; QOL; CPX; PTH |
Note: 25(OH)D, 25-hydroxy vitamin D; BNP, B-type brain natriuretic peptide; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; 6MWD, 6-minute walking distance; RAS, renin-angiotensin; PTH, parathyroid hormone; QOL, quality of life; CPX, complete physical examination.
| Included studies | Radom allocation | Allocation concealment | Double blind method | Evaluation of blindness | Data integrity | Selective report | Others |
| Qu et al. [23], 2015 | Unclear | Low risk | Low risk | Unclear | Low risk | Low risk | Low risk |
| Li et al. [24], 2015 | Unclear | Low risk | High risk | Unclear | Low risk | Unclear | Unclear |
| Wu et al. [25], 2011 | Unclear | Low risk | Low risk | Unclear | Low risk | Unclear | Unclear |
| Nicolas [26], 2013 | Low risk | Unclear | Low risk | Low risk | Low risk | Low risk | Low risk |
| Zittermann et al. [27], 2019 | Unclear | Unclear | Unclear | Unclear | Low risk | Unclear | Low risk |
| Soad et al. [28], 2012 | Unclear | Unclear | Low risk | Low risk | Low risk | Low risk | Unclear |
| Klaus et al. [29], 2016 | Low risk | Unclear | Unclear | Low risk | Low risk | Unclear | Low risk |
| Rebecca et al. [30], 2014 | Low risk | Low risk | Low risk | Unclear | Low risk | Unclear | Low risk |
| Woo et al. [31], 2022 | Low risk | Unclear | Low risk | Unclear | Low risk | Unclear | Unclear |
| Heidi [32], 2017 | Low risk | Low risk | Low risk | Unclear | Low risk | Unclear | Low risk |
Unclear, Not specified in the article; Low risk, There are specific instructions in the article; High risk, Not mentioned in the article.
In total, 10 prior studies met the eligibility criteria and were included [23, 24, 25, 26, 27, 28, 29, 30, 31, 32]. The selection process is described in Fig. 1. There are a total of 1099 patients, with 548 in the vitamin D group and 551 in the control group. Overall, 5 studies [26, 29, 30, 31, 32] refer to the correct randomization method and 5 studies [23, 24, 25, 30, 32] adopt allocation concealment; 4 studies [26, 29, 31, 32] report LVEF, 4 studies [27, 28, 29, 31] report LVEDD, 4 studies [23, 25, 27, 32] report BNP, 7 studies [24, 27, 28, 29, 30, 31, 32] report 25(OH)D and 1 study [24] report the occurrence of adverse events during treatment . The bias of the study is analyzed in Fig. 2.
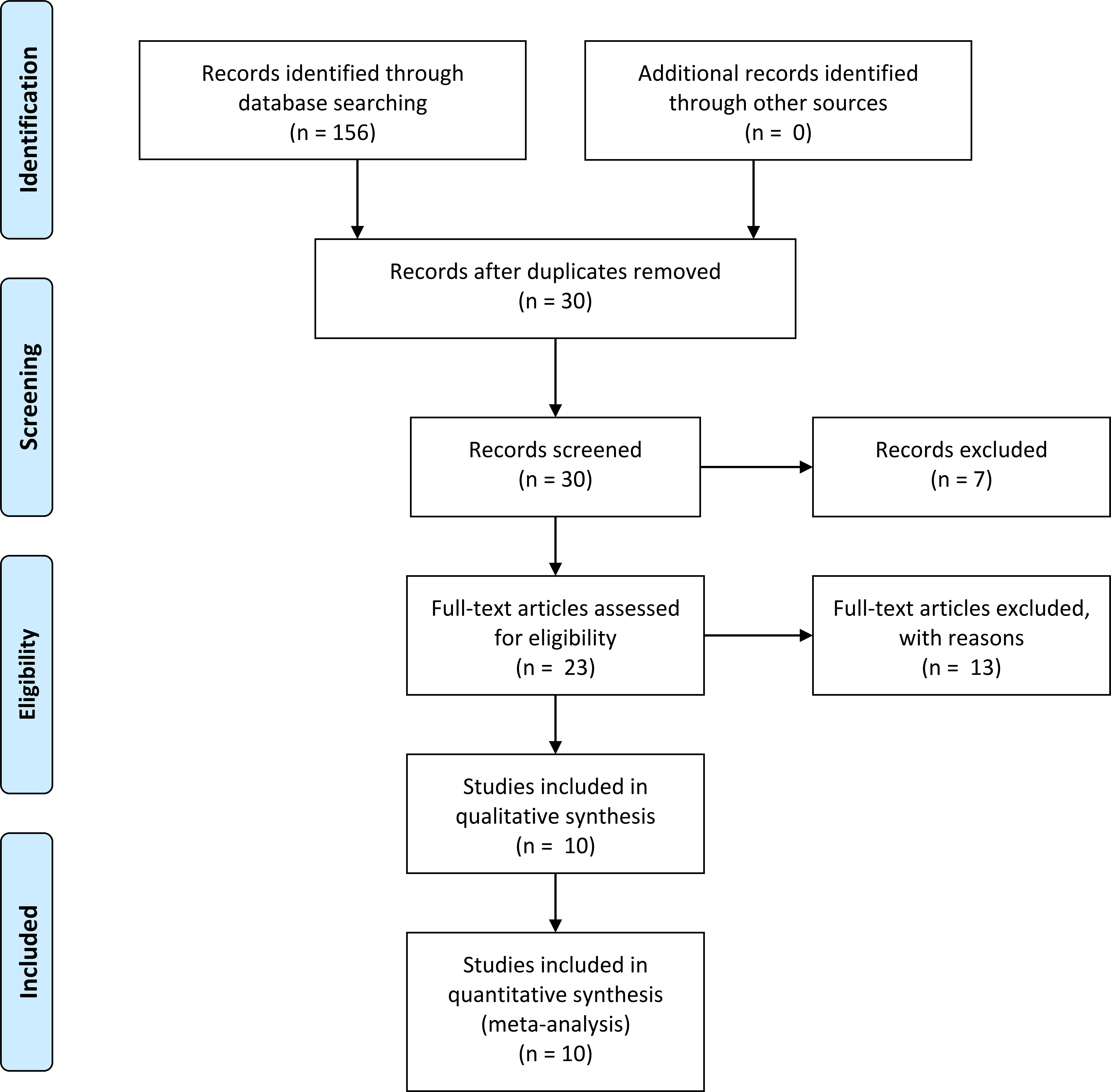 Fig. 1.
Fig. 1.PRISMA 2009 Flow Diagram.
 Fig. 2.
Fig. 2.Bias of studies.
Levels of LVEF were reported in four studies [26, 29, 31, 32]. There is no heterogeneity in the
results among studies (p = 0.41, I
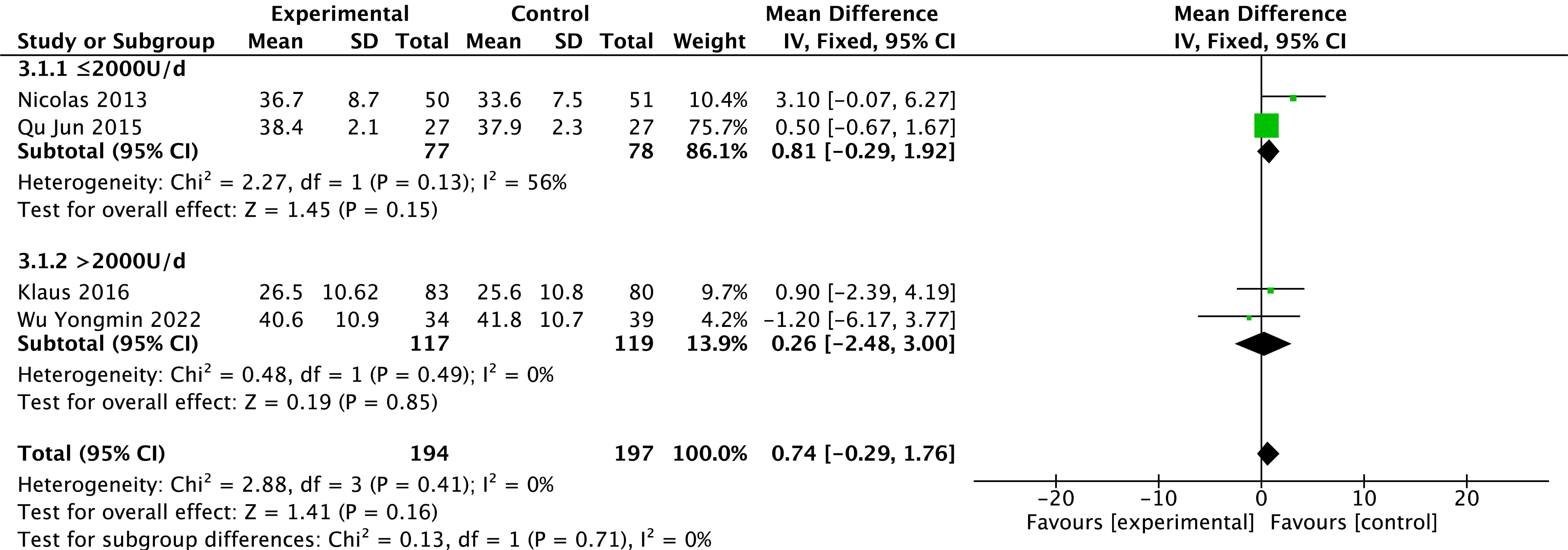 Fig. 3.
Fig. 3.Changes in LVEF after treatment in the vitamin D and control groups. LVEF, left ventricular ejection fraction.
Levels of LVEDD were reported in four studies [27, 28, 29, 31]. There is no heterogeneity in the
results among studies (p = 0.25, I
 Fig. 4.
Fig. 4.Changes in left ventricular end-diastolic internal diameter after treatment in the vitamin D and control groups.
Levels of BNP were reported in four studies [23, 25, 27, 32]. There is no heterogeneity in the
results among studies (p = 0.65, I
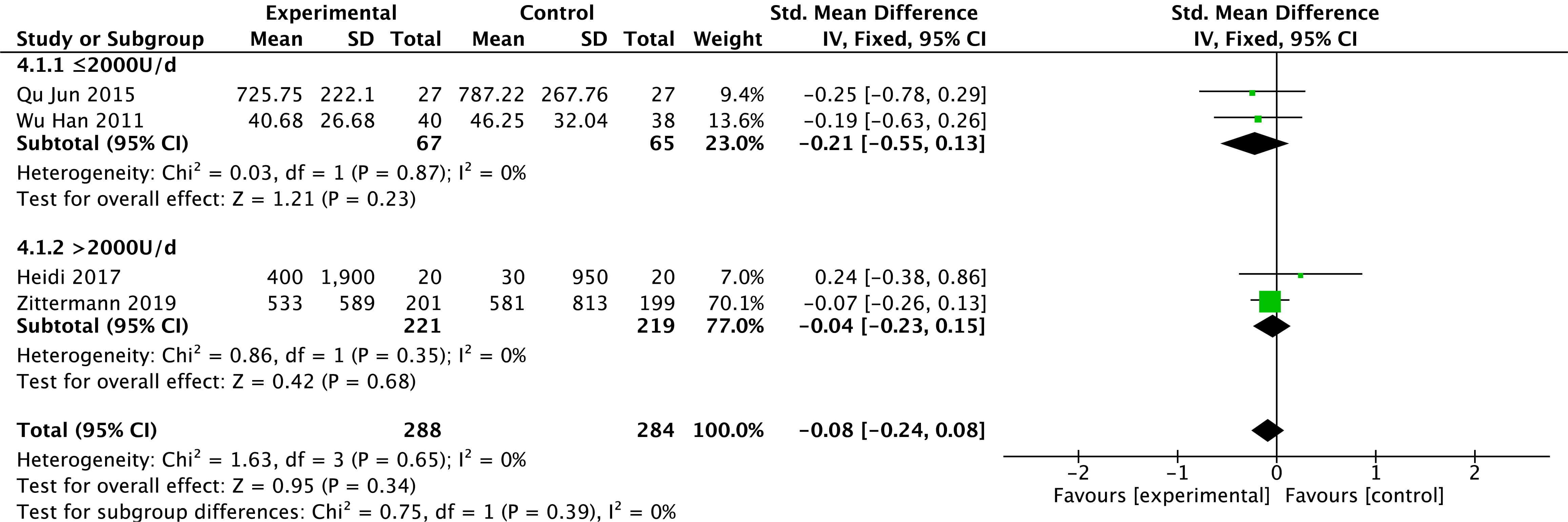 Fig. 5.
Fig. 5.Changes in B-type brain natriuretic peptide after treatment in the vitamin D and control groups.
Levels of 25(OH)D were reported in seven studies [24, 27, 28, 29, 30, 31, 32]. Because heterogeneity was
found in the study results (p
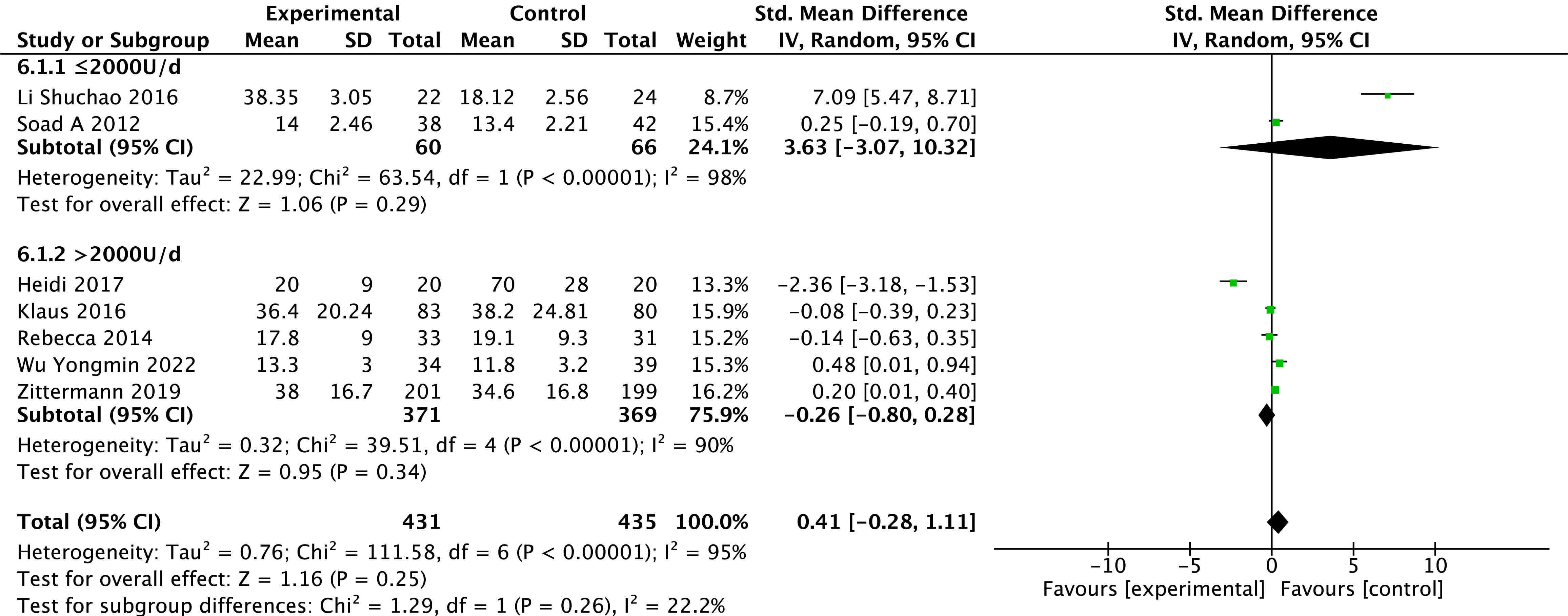 Fig. 6.
Fig. 6.Changes in 25-hydroxy vitamin D after treatment in the vitamin D and control groups.
Adverse events (AEs) were only reported in a single study [24]. The most frequent AEs include panic, nausea, dizziness, and fatigue. However, the incidence of adverse events between the two groups is not statistically significant.
Approximately ninety percent of people with chronic HF are vitamin D deficient and low levels of vitamin D are known to activate the RAAS system, triggering the inflammatory response, and leading to endothelial dysfunction [33]. This model predicts a correlation between deficiency of vitamin D and poor prognosis in chronic HF patients, suggesting that vitamin D supplementation may improve left ventricular remodeling and have a role in the recovery of cardiac function [34]. Vitamin D deficiency has been shown to result in cardiovascular complications, while a normal level may have protective effects in ventricular muscle [35]. It is notable that patients failing to complete the trial were excluded from the analysis, thus clinical events for this subgroup were not assessed. The results from this meta-analysis are in agreement with another study that found vitamin D supplementation resulted in no significant change to cardiac structure, systolic function or diastolic function, although the bioactive metabolite 25(OH)D, a nuclear hormone receptor ligand, has anti-hypertrophic activity [36].
The included studies did show an increase of 25(OH)D in the group of vitamin D
compared to the control group, and the increase of 25(OH)D was accompanied with
increased calcium concentrations in plasma. In one meta-analysis, it is shown
that increased calcium concentrations are the feature of HF [37]. HF patients
with vitamin D deficiency (25(OH)D
The results of this study show that LVEF, LVEDD, BNP, and 25(OH)D are not statistically significant, nor is there any significant effect on vitamin D supplementation in BNP. There was no heterogeneity in the results of LVEF, LVEDD and BNP indicators, suggesting that vitamin D supplementation is not significantly correlated with left ventricular remodeling. It suggests that vitamin D supplementation is not helpful to treat HF.
A RCT shows that moderately high doses of cholecalciferol adversely affected HF patients [39]. However, the recommended frequency and dose of vitamin D supplementation are not clear. Our subgroup analysis suggests that none of the measured doses of vitamin D supplementation improve the cardiac function of HF patients.
In recent years a number of RCTs have been conducted on the effects of vitamin D in HF patients, but different studies report controversial results. A meta-analysis shows that low vitamin D levels may associate with increased risks of all-cause mortality [40]. Another meta-analysis reported that supplementation of vitamin D did not improve LVEF or mortality in chronic HF [41]. While RCTs provide basis for clinical evidence, trials are often conducted in highly controlled settings with narrow inclusion and exclusion criteria, which can also reduce their generalizability and external validity [42].
This study has some limitations: (1) the included population number is small, (2) the presence of heterogeneity, particularly in blinding methods. It may also be related to differences in vitamin D doses and study populations, (3) the different dose cycles of vitamin D in different trials may also affect the results of the study, and (4) we did not include patients with preserved ejection fraction.
Since there is no advantage on the LVEF, LVEDD, BNP and 25(OH)D, Vitamin D supplementation may not be helpful in the clinical management of patients with HF.
HF, heart failure; RASS, renin-angiotensin system; 25(OH)D, 25-hydroxy vitamin D; VDR, vitamin D receptor; RCT, randomized controlled trial; LVEF, left ventricular ejection fraction; LVEDD, left ventricular end-diastolic internal diameter; BNP, B-type brain natriuretic peptide; MD, mean difference; CI, confidence intervals.
The data used to support the findings of this study are included within the article.
XMC, WLZ, YanZ, JCM, HEB and YeZ contributed to the design and concept. XMC and WLZ performed the literature searches and wrote the manuscript. YanZ and JCM critiqued the successive versions. HEB and YeZ approved the final manuscript. HEB and YeZ coordinated the effort and integrated the sections and comments. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
Not applicable.
The authors thank Dr. Qinglong Wang for assistance with data extraction.
This study was supported by the 2022 Key Discipline Development in Preventive Medicine of Traditional Chinese Medicine.
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
