1 Department of Clinical and Experimental Medicine, Cardiology Section, Azienda Ospedaliera Universitaria Policlinico “Gaetano Martino", University of Messina, 98124 Messina, Italy
2 Division of Cardiology, Department of Internal Medicine, St. Marianna University School of Medicine, 216-8511 Kawasaki, Japan
Academic Editors: Sophie Mavrogeni and Rajesh Katare
Abstract
TakoTsubo Syndrome (TTS) is a stress-induced cardiac disease characterized by temporary and segmental left ventricle dysfunction, typically involving the apex. Post-menopause women are more frequently affected. ECG and clinical features at presentation may be similar to those observed in acute coronary syndrome (ACS). However underlying pathomechanisms are completely different and, for what concerns TTS, extremely debated and not yet completely understood. Some hypotheses have been proposed during years, mostly regarding catecholamine-induced cardiotoxicity and microvascular dysfunction, usually following a trigger event which may be either “emotional” (primary TTS) or “physical” (secondary TTS). Additional modulators like neuroendocrine disorders (particularly hypothalamic-pituitary-adrenal axis dysfunction and estrogen drop in menopause) may play a crucial role in TTS onset. Despite being originally considered more benign than ACS, several studies have enlightened that TTS and STEMI are burdened by the same in-hospital mortality and complications. However, TTS and ACS complications somehow differ for what concerns incidence, the underlying mechanisms, and both long- and short-term outcomes. Full recovery in TTS requires weeks to months and cases of recurrences have been described, but no single clinical feature seems to predict subsequent episodes so far. By now, apart from inhibitors of the Renin-Angiotensin-Aldosterone System (RAASi), no drug has proved to be effective either in the acute or chronic phase in reducing mortality, improving outcome, or preventing recurrences.
Keywords
- TakoTsubo Syndrome
- acute coronary syndrome
- stress cardiomyopathy
- catecholamine
- heart failure
- left ventricular dysfunction
TakoTsubo cardiomyopathy, more recently preferred as a syndrome (TakoTsubo Syndrome, TTS), is a cardiac disease characterized by temporary hypokinesis, dyskinesis, or akinesis in the left ventricle (LV) wall segments with (more frequent) or without apical involvement, exceeding a single coronary vessel blood flow distribution. Women are more susceptible to TTS, and a stress elicitation (personal or social occasion, as well as acute disease) is the most common trigger [1, 2, 3, 4, 5].
Angiography usually shows no significant coronary artery disease (CAD), but a coexistence of bystander CAD and TTS is possible. Relevant abnormalities are commonly observed on the electrocardiogram (ECG) such as ST-segment elevation and/or T-wave inversion, as well as prolonged QT interval, mimicking a typical acute coronary syndrome (ACS) [1, 3, 4, 6].
The precise pathomechanism of TTS is still uncertain and probably multifactorial. Many hypotheses have been linked to the occurrence of cardiomyopathy. Both physical and emotional stress can trigger the onset of the syndrome. Either physical or emotional stress has been reported in 60–80% of patients. There is another 20–30% in whom no triggers were identified. The most common physical stressors included surgery, infections, and acute respiratory failure. Emotional triggers were the death of a loved one, relationship conflicts, fear, anger, and anxiety depicts the recent inter-TAK classification [1, 2, 3, 7].
The most accepted pathogenetic theories are catecholamine-induced cardiotoxicity and microvascular dysfunction, but additional modulators like neuroendocrine disorders, dysfunctional cognitive and emotional brain centers, especially of the hypothalamic-pituitary-adrenal axis, have been outlined [1, 3, 5, 8, 9, 10].
The plasma levels of epinephrine were critically elevated in many patients with emotional stress in a study by Wittstein [11]. Authors also found that serum catecholamine concentration was 2- to 3-fold higher in this clinical setting than in typical ACS patients.
A catecholaminergic mechanism was hypothesized in past studies and confirmed by reviews and consensus statements [1, 2, 4, 5, 12]. This theory subtends similar features observed in exogenous catecholamine administration and pheochromocytoma, which, however, TTS has been established to be discerned from, though there is a lack of agreement on this aspect in the scientific community [9, 10, 11, 13, 14, 15, 16, 17, 18].
The abnormal distribution of catecholamine receptors in the myocardium was
highlighted by Lyon et al. [19] in 2008. The elevated concentration of
norepinephrine, released by the sympathetic system, showed high affinity to
More recently, G protein-coupled kinase 2 and
About 90% of patients with TTS are (postmenopausal) women, but the syndrome also affects men, especially in Japan [21]. For that reason, the pathogenetic role of estrogens has also been widely studied in this clinical setting [22, 23, 24].
The risk of experiencing TTS was higher in patients carrying anomalous T-allele of the gene encoding estrogen receptor 1, but confirmatory studies are still lacking [25]. In fact, though prior evidence, hormone replacement therapy was not protective against the development of TTS, leading researchers to conclude that estrogenic deficiency or anomalous receptors are possible drives of the syndrome [21, 22, 23, 25].
Small coronary artery and microvascular dysfunction might also play a role in precipitating the syndrome. Studies have revealed the incidence of TTS in women resembling that of migraine headache and the Raynaud phenomenon [26]. Then, vascular dysfunction may be a common feature in such patients. Accordingly, a catecholamine-based microvascular spasm was also thought to cause a transient drop in coronary blood flow reserve and ensuing apical ballooning phenomenon [27].
The same functional impairment may occur during histaminergic distress caused by an anaphylactic response to drugs, foods, or contrast agents [28]. In some patients, a combination of TTS and type-1 Kounis syndrome was hypothesized [29, 30, 31], as demonstrated by Desay et al. [32] from the US National Inpatient Sample in a 7-year period (2007–2014). These authors identified such a combination in African and Asian elderly females the most, also suffering from hypertension in 100% and dyslipidemia in 62% of cases.
Interestingly, studies have demonstrated a seasonal variability in the occurrence of TTS. More cases were observed in summer (30%) than in winter (18%) in 260 consecutive patients (95% women) from a New Zealand study, compared to the highest occurrence of ACS in winter [33]. A recent multicenter study in Japan also confirmed such a trend that demonstrated seasonal variation only in the female group [34]. The authors reported more events from July to December, especially in the afternoon. These results also suggest that the pathogenesis and clinical features of TTS might therefore differ according to climate.
Despite being originally considered less malign than ACS, several studies have
enlightened that TTS is burdened by the same in-hospital mortality rate as in
ST-elevation myocardial infarction (STEMI), with overall occurrence as by 4–5%
of patients admitted for chest pain [1, 4, 35]. It is not surprising that these
two conditions share some predisposing factors (smoking habit and endothelial
dysfunction appeared to be important aspects in both diseases) and complications
[36]. However, patients with ACS and TTS are substantially different for what
concerns sex, age, CV risk factors, and comorbidities, as the latter are more
likely to be women, have younger age, and have fewer comorbidities). It is
interesting to note that, despite indicators of poor outcome (male sex, age
Furthermore, it is crucial to differentiate primary from secondary TTS. Most primary TTS affect postmenopausal women without coronary artery disease (CAD); in this case, moderate LV dysfunction is probably caused by microvascular dysfunction during strong emotional stress (Table 1, Ref. [41]). In secondary TTS, on the other hand, severe LV dysfunction is triggered by physical stress. Previous studies reported that most in-hospital TTS are secondary TTS, which are strongly comorbidities-related and burdened by higher in-hospital mortality compared with out-of-hospital TTS (more likely to be primary TTS) [42]. There is a discordance between European and Japanese registries for what concerns in-hospital outcome: in-hospital mortality appears to be higher among Japanese patients, however, ethnicity itself is not likely to play a role in increasing mortality [43].
| Primary | Secondary | |
| Gender | Women ( |
Women/Men |
| Stressor(s) | Emotional | Physical/Organic |
| CAD | Absent | Possible |
| Underlying pathomechanism | Microvascular/allergic dysfunction | Micro/macrovascular dysfunction |
| LV dysfunction | Moderate-to-severe | Often severe |
| LV complications | Uncommon | Frequent |
| LV functional recovery | Short term | Mid-late term |
| Prognosis | Variable (usually benign) | Variable (often poor) |
| Recurrences | Likely | More likely |
| Modified from Galiuto et al. [41]. CAD, Coronary Artery Disease; LV, Left Ventricle. | ||
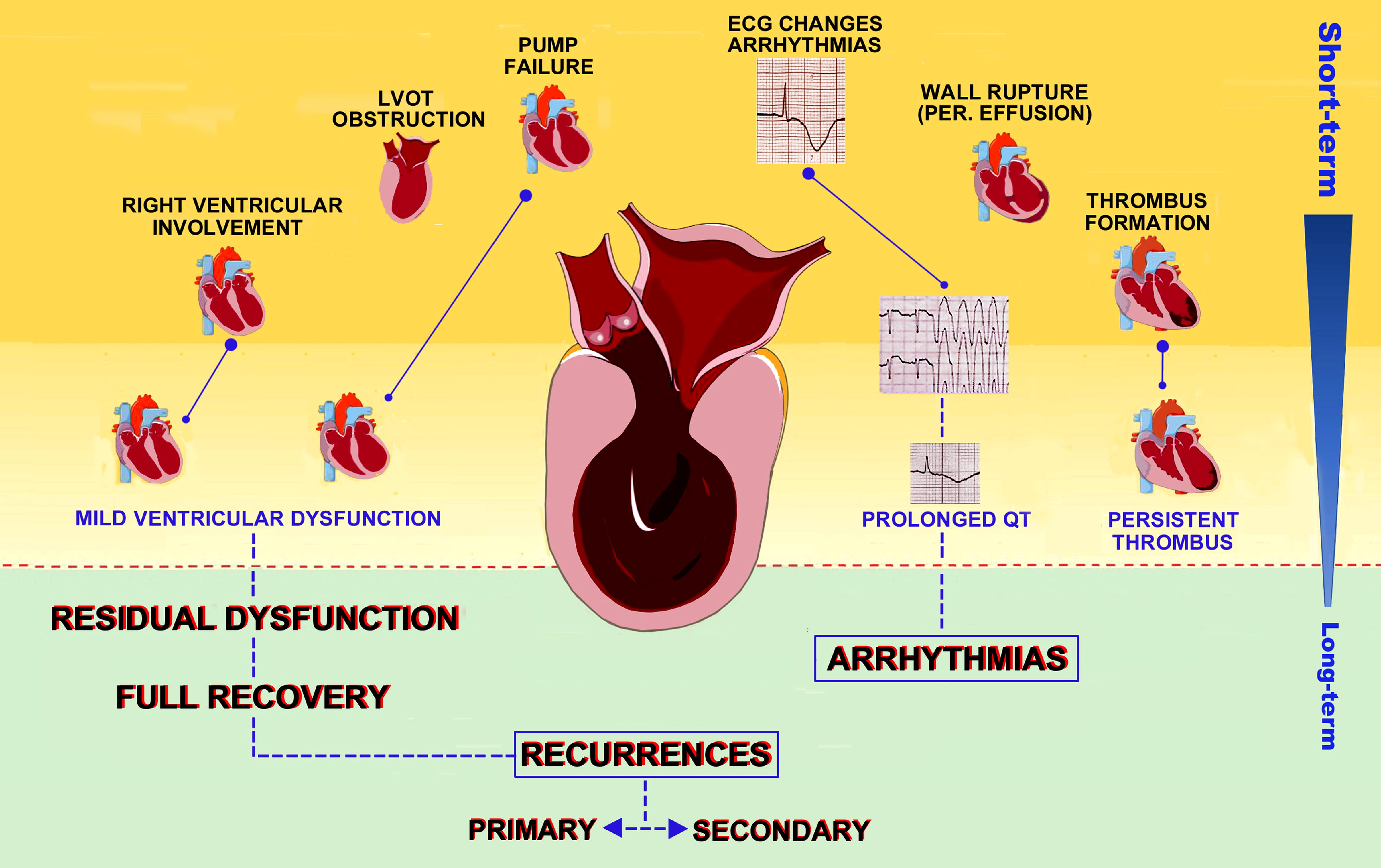
During hospitalization, 32.9% of TTS patients are estimated to have complications [44]. The most frequent adverse outcome in both conditions is left ventricular dysfunction, affecting approximately 20% (12–45%) of TTS patients and 13–32% of STEMI patients [45, 46, 47]. However, in acute myocardial infarction (AMI) LV failure is usually due to inotropism loss or acute mitral regurgitation, while in TTS (Fig. 1) up to 25% of heart failure (HF) are a consequence of left ventricular outflow tract obstruction (LVOTO) determined by hyperdynamic proximal LV chamber and systolic anterior motion (SAM) of the anterior mitral valve leaflet [36].
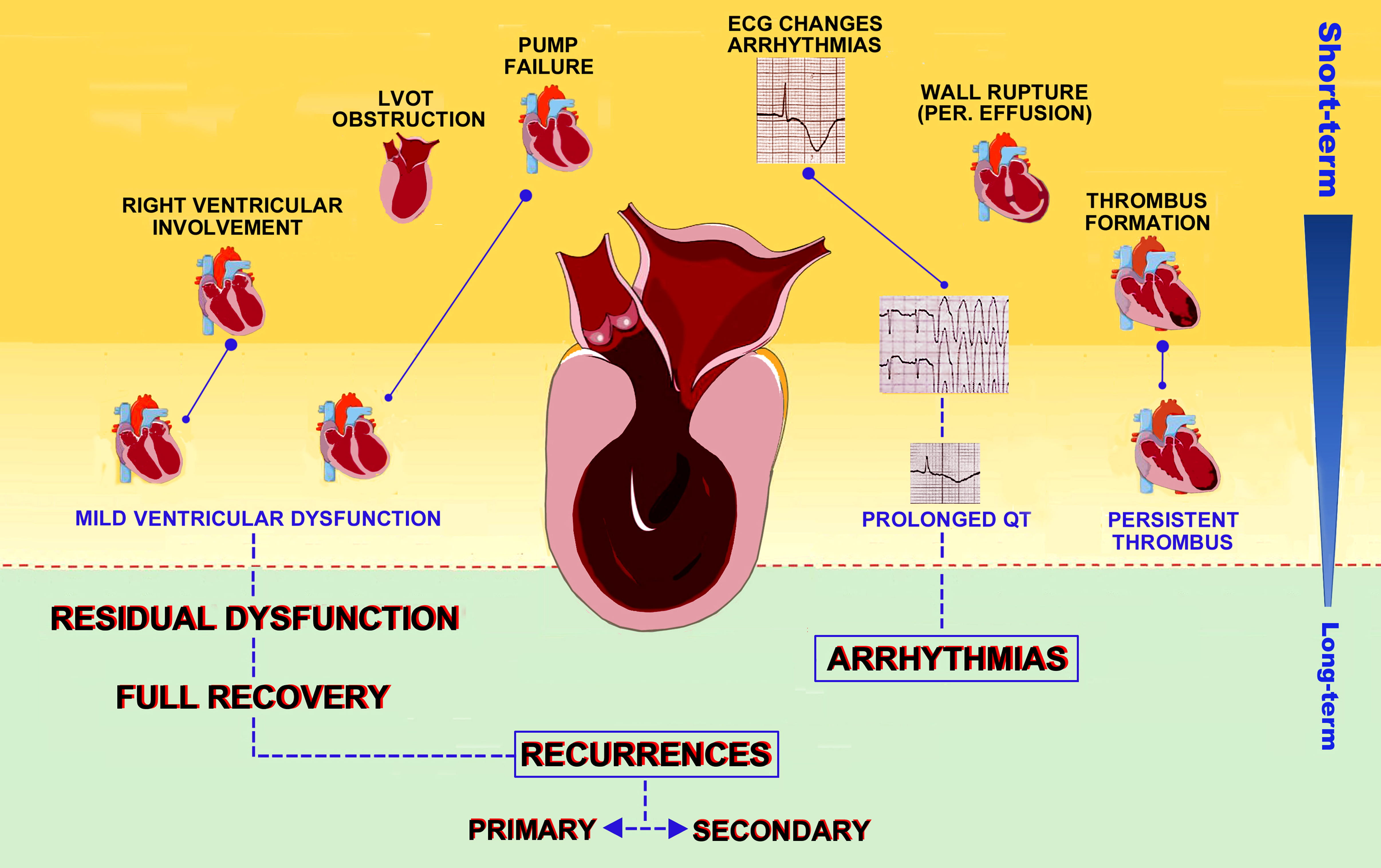 Fig. 1.
Fig. 1.Clinical outcomes in TTS patients. Possible complications of TTS are depicted according to timing of occurrence, from the inpatient phase to long term outcomes and recurrences.
Cardiogenic shock precipitates the acute disease in
approximately 20% of TTS and 6–10% of STEMI [4, 48]. There is no
unanimous consensus about the best strategy to treat cardiogenic shock in TTS,
but previous studies showed that TTS patients are more likely to require
mechanical respiratory support than ACS [49]. Notwithstanding, the mortality rate
is substantially higher among cardiogenic shock due to STEMI (
Left ventricular ejection fraction (LVEF) on admission is an important predictor of mortality in both TTS and ACS. In comparison to average ACS, TTS patients usually show significantly lower EF, but no differences were enlightened when compared with STEMI [45, 46, 47, 48, 50].
Supraventricular arrhythmias, in particular atrial fibrillation, are associated with a poor prognosis and a higher number of compliances in both TTS and STEMI [45, 51]. Atrial fibrillation onset is probably the result of high catecholamine release in addition to myocardium inflammatory state and atrial overload resulting from acute mitral insufficiency and LV dysfunction [45, 51].
Originally, ventricular arrhythmias have been described to
occur with the same incidence (8–9%) in patients with TTS and AMI [35, 47, 52].
However, it is now believed that ventricular arrhythmias are less frequent in TTS
than in AMI. Syed et al. [52] reported a prevalence of 3.4% of
ventricular tachycardia or fibrillation, while it is proposed that 6–8% of
patients with AMI develop malignant ventricular arrhythmias during the acute
phase. This may be due to
Mechanical complications, such as septal perforation, occur rarely, but consequences are extremely serious. Scant data is available due to the rarity of this complication, however, the mortality rate in TTS was assessed around 83%, reaching 90% during the first 8 days [54]. It has been suggested that high intraventricular pressure and high LV wall stress may be the major determinants [54]. It is interesting to observe how, despite the different etiology, in MI mechanical complications occur within the same time window (from 24 hours to a few weeks for septum and from 2 to 7 days for papillary muscles) and are burdened by comparable mortality (20–75%) [45]. Apical akinesia is a frequent issue in both conditions and is the main determinant of LV thrombosis. Intraventricular thrombosis occurs in approximately 5% of either TTS and STEMI patients of both TTS and ACS, leading to embolic stroke in 1–5% cases among TTS patients [4, 45, 47].
A Portuguese study assessed in-hospital stay lasting 5
Ventricular thrombus is a fearsome complication that might occur in all those clinical conditions causing ventricular dysfunction, such as dilated cardiomyopathy and AMI. TTS is of no exception in this respect, as it shows an increased likelihood of forming an LV thrombus with an estimated incidence of around 2–8% [56, 57, 58].
Patients who suffered from thromboembolic complications showed a significantly higher mortality rate (p = 0.02) over a mean follow-up of 3 years in a recent prospective study [59]. Thrombi generally occur 2–5 days after TTS onset and appear to be associated with both elevated C-reactive Protein and ST-segment elevation, despite the latter being present only in nearly half of patients with LV thrombosis [59]. Regional hypokinesia, endothelial activation, systemic hypercoagulability, and blood stasis due to myocardial stunning all combine to constitute a pro-thrombotic state, especially in patients presenting with apical type TTS, advanced age, and late hospital presentation [60, 61].
Cardiac alterations in TTS are both regional wall-motion and electrocardiographic abnormalities. Despite the former normalizes within a few weeks, the latter persist for several months [55]. As long as myocardial edema persists, T-wave inversion is evident, making such T-waves alteration an indirect index of myocardial edema [58]. However, ventricular arrhythmias usually occur only in the acute phase and so an implantable cardioverter-defibrillator is not indicated despite ECG abnormalities persisting in the subacute phase [55].
Usually, in TTS LV motion abnormalities recover up to normality (in both systolic and diastolic function) in 4 to 8 weeks [55]. Early signs of LV functional improvement can be recognized by analyzing the changes in global longitudinal strain and apical twisting/untwisting [55, 62, 63]. Improvement in global longitudinal strain (GLS) is associated with a reduction (up to a complete resolution) of LVOTO and mitral insufficiency [62]. It is curious to observe how improvement is not simultaneous for all myocardial segments, showing a different susceptibility to catecholamine insult and stunning [62]. This aspect should be further investigated, as it may reveal important implications to better understand the pathophysiology of disease [36, 55].
Up to 35% of TTS patients present with right ventricular dysfunction, often clinically silent [10]; however, biventricular involvement predicts a worse outcome [12, 61]. Biventricular involvement is typically identified on imaging studies such as echocardiography and MRI. In observational retrospective studies, the most frequently affected segments of the right ventricle were the apical-lateral, anterolateral, and inferior walls [21]. This involvement has been associated with a higher prevalence of in-hospital major adverse cardiovascular events, including heart failure, bilateral pleural effusion, cardiogenic shock, and in-hospital mortality, especially in older patients with low LV ejection fraction [21, 32].
Although TTS has long been considered a benign disease, recent observational registries seem not to confirm such a trend [62]. In a large retrospective study in 1750 patients, long term follow-up showed a patient-year risk of all-cause death of 5.6%, with a 9.9% per patient-year risk of major adverse cardiac, including death from any cause, recurrence of TTS, stroke or TIA, or AMI [21]. When compared to age- and sex-matched STEMI/NSTEMI patients, TTS showed similar long-term outcomes [62]. Among clinical determinants, male sex, diabetes mellitus, and Killip class III/IV at presentation were proven to significantly affect long-term prognosis [64]. Although sex-related differences in prognosis (poorer in men) have also been demonstrated,, information about the exact prognosis is still lacking, also because of ethnic disparities in both clinical characteristics and in-hospital outcomes [43].
The central role of cardiovascular imaging in the diagnosis of TTS is well
established. A growing body of evidence demonstrated that transthoracic
echocardiography, as well as being of great help to the clinician in the
differential diagnosis of TTS, provides independent and incremental long-term
prognostic value in addition to other clinical factors. Severely depressed LVEF
(
As in other cardiovascular diseases, conventional transthoracic echocardiography
may benefit from the use of advanced techniques such as speckle tracking to
better estimate ventricular function, providing incremental prognostic value. In
a recent study involving 650 TTS patients, Akashi and colleagues acquired LV GLS
at presentation in addition to LVEF [67]. At follow-up, long-term mortality
significantly differed among different quartiles depending both on the baseline
LVEF (p
A recent prospective study by Eitel and colleagues demonstrated that LV function, even if markedly reduced at presentation, fully recovered after days or months from the index event, as by echocardiography and/or MRI with a median time of 97 days (IQR, 36–123) [68].
TTS has long been considered a pro-arrhythmic condition, and cardiac arrhythmias
are certainly among the most feared long-term complications, due to their
severity and unpredictability, with an incidence that has been estimated from 7
to 14% [59, 69]. Among a cohort of 906 patients, El-Battrawy et al.
[70] recognized significantly higher 30-day mortality rates in those who had
arrhythmic disorders compared to non-arrhythmic patients (log-rank
Concerning mechanisms, corrected QT (QTc) prolongation has been reported in
different cohorts of patients, affecting up to one-half of the patients at
presentation, and a QTc
While myocardial fibrosis is a common finding in ischemic heart disease (IHD), with re-entrant arrhythmias stemming mostly from altered myocardial conduction patterns around ischemic scars and fibrotic areas, TTS does not seem to share such a pathological substrate. Indeed, in a large study involving 256 patients with TTS, Eitel and colleagues demonstrated minute focal or patchy nonischemic myocardial scarring at cardiac MRI in 9% of such cases, but using a much lower threshold of signal intensity and a smaller extent of late gadolinium enhancement when compared to IHD [64].
Differently from IHD, investigators found myocardial edema to be widely present in TTS patients on admission, with a prevalence of 162 out of 199 patients (81%), and a regional distribution consistent with the pattern of LV dysfunction, suggesting a role in the delayed and dispersed ventricular repolarization reflected on ECG by a prolonged QTc interval [68].
Anticoagulation in case of thrombus formation is the only therapy that proved to be effective and should be promptly started in the absence of high bleeding risk, and generally discontinued after 3 months or after echocardiographic demonstration of LV thrombus resolution and LVEF recovery at follow-up [5]. Despite the lack of evidence, prophylactic anticoagulation should be considered in patients with a severely reduced LVEF or apical akinesia on admission to hospital, with an individualized approach, as not all patients would benefit the same, depending on the risk-benefit profile [62].
However, long-term prognostic studies describing outcomes in TTS patients complicated by a ventricular thrombus, as well as those with cardioembolic events on admission, are lacking yet. In the acute phase, prothrombotic state was described as the result of platelet activation, peripheral vasoconstriction, and, of course, ventricular dysfunction, justifying a possible role of antiplatelet therapy in preventing major events in these patients.
Treatment with aspirin did not prove to reduce the risk of major adverse cardiovascular events in TTS patients in both short- and long-term follow-up [71].
Recurrent TTS after completely recovered LV dysfunction is still under
investigation [72]. Current literature suggests a recurrence rate of 8%, with a
variability ranging from 1 to
Recurrences were seen within the first 4 years in patients younger than 50 years at the first event. Their mortality rate was up to 2.7% at 5 years, strictly dependent on comorbidities and sex [12, 72, 73]. In a recent population-based cohort study on 519 US patients, recurrence of syndrome occurred in 7.5% of patients over a median of 5.2 years of follow-up. Authors found a higher proportion of elderly men with a 2.5-fold higher risk of death or recurrence [74].
Overall, no sure clinical features were recognized to predict subsequent episodes [72]. Like in AMI patients, the persistence of predisposing factors (diabetes and hypertension first) is the major determinant, but comorbidities are also reported to be potential triggers as well (Table 2, Ref. [73, 74, 75, 76]). Conversely, the impact of therapy is controversial and recurrence among TTS is substantially lower when compared to AMI, more likely to occur in primary TTS, which are subjected to psychological triggers. However, intercurrent diseases may be potential triggers, and this may suggest an overlapping between primary and secondary forms [62, 72, 73, 77].
| Clinical characteristic | |
| Female gender | |
| Timing range (days) | 30–3600 |
| Classical risk factors | Diabetes, Hypertension |
| P/N disorders | 35–45% |
| NC triggering diseases | Endocrine, Infective, Neurologic, Renal, Respiratory, Others |
| LVD pattern (vs index event) | Similar (60–70%), Different (30–40%) |
| Multiple recurrences | Rare |
| BB therapy (prior recurrence) | 60–80% |
| BB, Beta-Blocker; LVD, Left ventricle dysfunction; NC, Non-cardiac; P/N, Psychiatric/neurological. | |
Cases of multiple recurrences in the same patient have also been described, but the triggering event and the ballooning pattern may be different in each recurrence [36, 55, 62, 72]. Therefore, better identification of the stressors may help prevent further events, especially for what concerns emotional triggers [65, 72]. A targeted therapy, not only pharmacological but also stress-containing, anti-depressant, and for migraine, may help prevent future episodes [65].
Also, there is lacking unanimity on whether cardio-active therapy (particularly beta-blockers) may be useful to contrast the effects of catecholamine excess and then prevent subsequent events. Although beta-blockers appear to be the most intuitive prevention choice for recurrences, approximately 70% of recurrent-TTS patients were already on this therapy [55]. In the study of Lau et al. [74], beta-blocker exposure was associated with lower mortality and recurrences, while there was no association with ACE-inhibitors or ARBs. Conversely, ACE-inhibitors and ARBs have been demonstrated to be more effective against catecholaminergic damage, inflammation, and fibrosis [4, 5].
We are aware of the efforts to draw definite conclusions to avoid recurrences, but further evidence is needed first because most observational studies have been differently designed and managed, and important differences could also be related to the racial make-up of the study population.
On the basis that catecholaminergic tone may affect platelet activation, some authors advanced the hypothesis of a preventing role for Aspirin also in TTS patients, but recent studies did not prove its effectiveness in short- or long-term outcomes [5, 57, 71].
In recent years, important clinical registries and international trials enriched our knowledge on both the acute phase and long-term mortality related to TTS, raising awareness of this multifaceted and complex clinical disorder. Pathomechanism, clinical and prognostic features of TTS were summarized in the light of current literature.
Primary TTS seems to have a better prognosis, whereas secondary forms get worse outcomes, outpacing ACS. Recurrences are rare but still unpredictable, and blockade of the Renin-Angiotensin-Aldosterone system remains the primary therapeutic target for prevention.
Further research is encouraged to shed further light on the complex pathophysiology of TTS, define sure prognosticators and more effective treatments against recurrences.
ACE, Angiotensin-Converting Enzyme; ACS, Acute Coronary Syndrome; AMI, Acute Myocardial Infarction; ARB, Angiotensin Receptor Blockers; BNP, Brain Natriuretic Peptide; CAD, Coronary Artery Disease; ECG, electrocardiogram; EF, Ejection fraction; GLS, Global Longitudinal Strain; HF, Heart Failure; IHD, Ischemic Heart Disease; LV, Left Ventricle; LVEF, Left Ventricular Ejection Fraction; LVOTO, Left Ventricular Outflow Tract Obstruction; MACE, Major Adverse Cardiovascular Events; MI, Myocardial Infarction; MR, Mitral Regurgitation; MRI, Magnetic Resonance Imaging; NSTEMI, Non-ST-Elevation Myocardial Infarction; QTc, Corrected QT; RV, Right Ventricle; SAM, Systolic Anterior Motion; STEMI, ST-Elevation Myocardial Infarction; TIA, Transient Ischemic Attack; TIN, Takotsubo Italian Network; TT, TakoTsubo; TTS, TakoTsubo Syndrome; VT, Ventricular Tachycardia.
CdG and GA designed the research study. CdG, GA, OT performed the literature search. YJA provided an overview and advised on specific outcomes. CdG, GA, LP, MB analyzed the data. CdG, GA, LP, MB wrote the manuscript. CdG prepared Tables 1,2. CdG, LP, MB prepared Fig. 1. All authors read and approved the final manuscript.
Not applicable.
Not applicable.
This research received no external funding.
The authors declare no conflict of interest. Giuseppe Andò is serving as one of the Guest editors of this journal. We declare that Giuseppe Andò had no involvement in the peer review of this article and has no access to information regarding its peer review. Full responsibility for the editorial process for this article was delegated to Sophie Mavrogeni and Rajesh Katare.