†These authors contributed equally.
Academic Editor: Yan Topilsky
Background: The best anticoagulation choice
for patients undergoing transcatheter aortic valve replacement (TAVR) with
indications of oral anticoagulation (OAC) remains uncertain. We carried out a
comprehensive analysis adopting updated evidence that investigated the efficacy
and safety of direct oral anticoagulants (DOACs) versus vitamin K antagonists
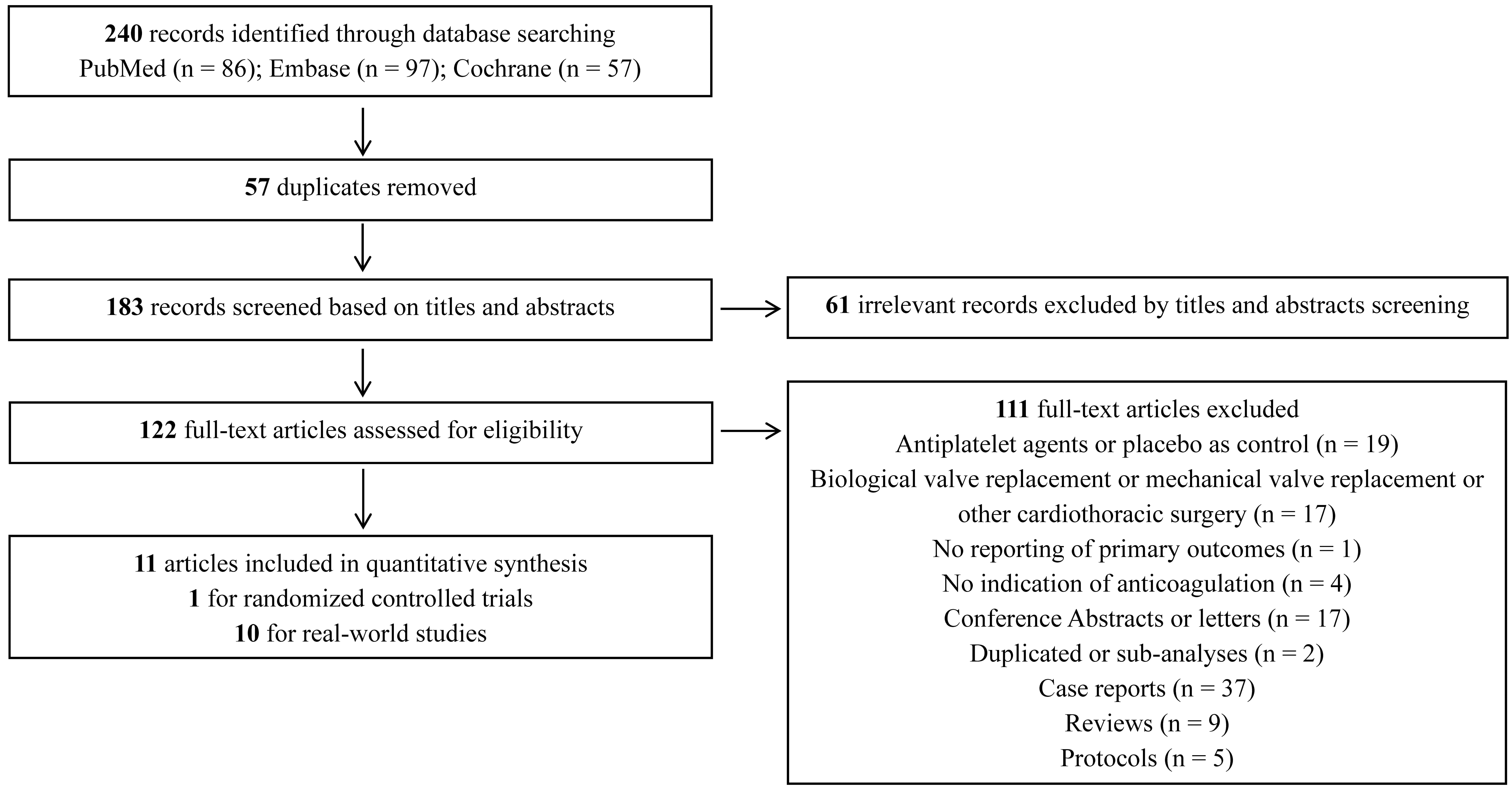
(VKAs) in this population. Methods: A systematic search has
been conducted through PubMed, Embase, and Cochrane Library to collect randomized
controlled trials (RCTs) and real-world studies comparing the therapy outcomes of
DOACs with VKAs in patients undergoing TAVR with indications of OAC up to Dec
2021. Included studies reported all-cause mortality, bleeding, stroke, or
composite endpoint. A random-effects model was used and followed
a sensitivity analysis based on the heterogeneity. In addition, five scenario
analyses were performed to robust our findings. Results: Our
analysis included 11 articles enrolling a total of 8934 patients undergone TAVR
with indications of OAC (DOACs group = 3890, VKAs group = 5044). Pooled analysis
revealed no significant different risk of all-cause mortality (aHR: 0.95, 95% CI: 0.65–1.39, I