†These authors contributed equally.
Academic Editor: Yan Topilsky
Background: The best anticoagulation choice
for patients undergoing transcatheter aortic valve replacement (TAVR) with
indications of oral anticoagulation (OAC) remains uncertain. We carried out a
comprehensive analysis adopting updated evidence that investigated the efficacy
and safety of direct oral anticoagulants (DOACs) versus vitamin K antagonists
(VKAs) in this population. Methods: A systematic search has
been conducted through PubMed, Embase, and Cochrane Library to collect randomized
controlled trials (RCTs) and real-world studies comparing the therapy outcomes of
DOACs with VKAs in patients undergoing TAVR with indications of OAC up to Dec
2021. Included studies reported all-cause mortality, bleeding, stroke, or
composite endpoint. A random-effects model was used and followed
a sensitivity analysis based on the heterogeneity. In addition, five scenario
analyses were performed to robust our findings. Results: Our
analysis included 11 articles enrolling a total of 8934 patients undergone TAVR
with indications of OAC (DOACs group = 3890, VKAs group = 5044). Pooled analysis
revealed no significant different risk of all-cause mortality (aHR: 0.95, 95% CI: 0.65–1.39, I
Transcatheter aortic valve replacement (TAVR) is an increasingly used procedure for patients with severe aortic stenosis (AS), which is considered the preferred strategy in inoperable or high-risk patients [1, 2]. The updated European guidelines recommend that single antiplatelet therapy should be treated for life after TAVR if there is no evidence of anticoagulation. However, lifelong oral anticoagulation (OAC) is recommended for TAVR patients with anticoagulation indications [3, 4]. Direct oral anticoagulants (DOACs) have proven identical efficacy and safety to vitamin K antagonists (VKAs) and provided new anticoagulant strategies for patients with atrial fibrillation (AF) [5]. Nevertheless, DOACs, or VKAs, the optimal anticoagulation strategy for TAVR patients needing OAC, remained elucidated. One meta-analysis revealed DOACs are non-inferior to VKAs in patients undergoing TAVR, which were limited by only including retrospective cohort studies and reporting unadjusted pooled odds ratios [6]. On the other hand, a recent meta-analysis showed VKAs are more protective in disabling or non-disabling stroke for post-TAVR patients requiring anticoagulation than DOACs [7]. However, the randomized controlled trial (RCT) included was not eligible for inclusion criteria due to applying antiplatelet therapy as control [8], possibly leading to untenable results. Meanwhile, two pivotal randomized controlled trials, ENVISAGE-TAVI and ATLANTIS [9, 10], concerning this issue have been completed, and an updated study is warranted. Therefore, we conducted a pooled analysis by summarizing all available evidence from RCTs and real-world studies comparing DOACs and VKAs in post-TAVI patients requiring anticoagulation therapy.
The present pooled analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [11]. We searched for relevant publications on the PubMed, Cochrane Library, and Embase databases from inception to Dec 29, 2021. A flowchart of the search strategy is presented in Supplementary Table 1. Moreover, the data not reported in articles were searched through the ClinicalTrials. Reference lists of identified articles were also reviewed to find potential studies that met inclusion criteria. Two reviewers (J.W. and F.Z.) retrieved the eligible documents independently from databases and resolved the differences through consulting with a third author (Z.G.).
Inclusion criteria were as follows: (I) the study design was a retrospective,
prospective cohort study, or RCT; (Ⅱ) studies with patients who underwent TAVR
and in need of OAC; (III) enrolled participants were distributed to intervention
group (using at least one of DOACs) and control group (using at least one of
VKAs); (IV) the reported outcomes included either all-cause mortality, any
stroke, any bleeding, or composite endpoint. Moreover, trials absenting control
or interventional groups, case-control or cross-sectional studies, and studies
lacking baseline data or insufficient efficacy and safety outcomes were excluded.
The included observational studies have eliminated the patients with absolute
contraindication on using DOACs, including mechanical valves, estimated
glomerular filtration rates
Two investigators (J.W. and F.Z.) extracted data independently. Detailed data
obtained from the retrieved studies includes the following sections: (I) study
characteristics; (Ⅱ) patient demographics; (III) clinical characteristics; (IV)
data of recorded outcomes. The Cochrane Risk of Bias Tool was used for quality
assessment of included RCTs, containing the following domains: random sequence
generation; allocation concealment; blinding of participants and personnel;
blinding of outcome assessors; incomplete outcome data; selective reporting; and
other forms of bias [12, 13]. The Newcastle-Ottawa scale (NOS) system was applied
to evaluate each included real-world study. According to the NOS scoring
criterion, 9 points were the maximum score,
Results for primary analysis were treated as dichotomous data. Adjusted hazard
ratios (aHRs) and 95% confidence intervals (CIs) were calculated using
random-effects models. Heterogeneity was tested adopting the
I
Our analysis included 1 RCT and 10 real-world studies that involved 8934 TAVR
participants with anticoagulant indications, divided into the DOACs (n = 3890) or
VKAs groups (n = 5044) [10]. The details of the article selection are presented
in Fig. 1. The characteristics of the eligible studies are listed in Tables 1,2. For RCT [10], 713 patients received edoxaban, and 713 received VKAs. The
follow-up was 2 years. Among the 10 real-world studies [19, 20, 21, 22, 23, 24, 25, 26, 27, 28], 3177 patients
took DOACs, and 4331 patients took VKAs. Among them, 3 studies applied
propensity-score matching (PSM) to adjust for
the difference in baseline, 2 used inverse probability of treatment weighting
(IPTW), 1 used Cox regression model, and the rest 4 did not adopt any adjustment
methods. Publication periods were from 2018 to 2021, and the follow-up period
spanned from 0.25 years to 3 years. Furthermore, 7 studies included the
population with the OAC indications for AF; the other 4 studies without specified
OAC indications. Patient demographics of 11 studies are outlined in
Supplementary Table 3. The quality assessment of all studies
was identified as modest to high (Supplementary Tables 4 and 5). We
considered the quality of RCT moderate due to the open-labeled study design. For
real-world studies, the NOS score of each study
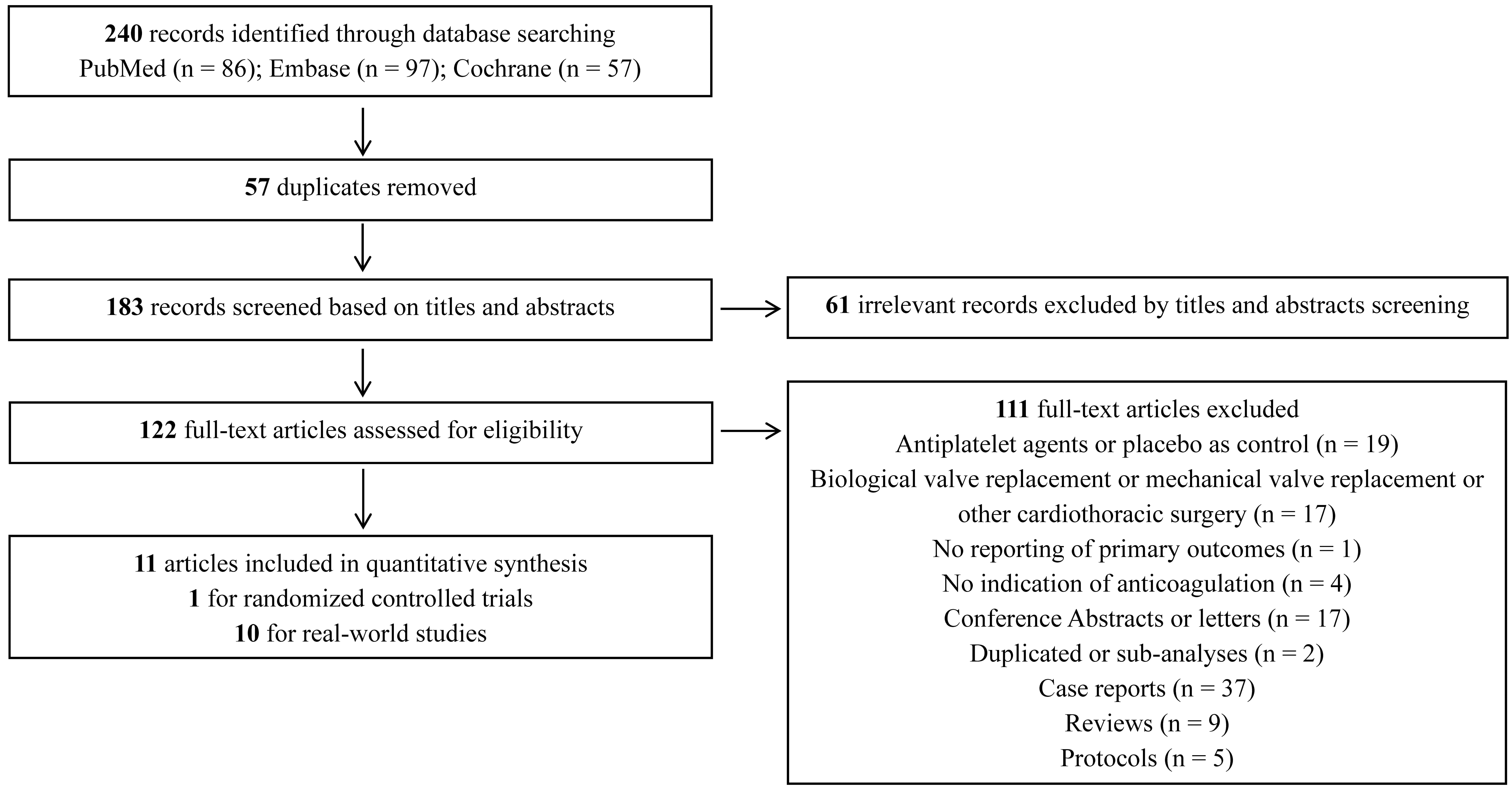 Fig. 1.
Fig. 1.Flow diagram for the selection of eligible studies.
| Study (year) | NCT number | Intervention with dosage | Patients (number) | Comparison | Patients (number) | Follow up (year) | Indication for OAC |
| ENVISAGE-TAVI 2021 | NCT02943785 | Edoxaban 60 mg once | 713 | Vitamin K Antagonist | 713 | 2.0 | Atrial fibrillation |
| OAC, oral anticoagulation; ENVISAGE-TAVI, Compare the efficacy and safety of edoxaban with vitamin K antagonists in patients with atrial fibrillation as the indication for oral anticoagulation after successful transcatheter aortic valve replacement. | |||||||
| Study (year) | Country or region | Data source | Inclusion period | Intervention/Numbers | Comparison/Numbers | Adjusted method | Adjusted variables | Follow up (year) | Indication for OAC | Numbers of each DOAC |
| Didier et al. 2021 [19] | France | Single-payer national health data system | 2010.1–2017.12 | DOACs/1378 | VKAs/1093 | PSM | (1) | 3.0 | Different indications | Api/724; Riva/488; Dabi/166 |
| Kawashima et al. 2020 [20] | Japan | Optimized transcatheter valvular intervention registry | 2013.10–2017.5 | DOACs/227 | VKAs/176 | IPTW | (2) | 2.0 | AF | NR |
| Mannacio et al. 2020 [21] | Italy | A retrospective, multicentre, cohort study | 2013.7–2019.12 | DOACs/340 | Warfarin/692 | PSM | (3) | 2.7 | AF | NR |
| Kalogeras et al. 2019 [22] | Athens, Tokyo, London, and Hammersmith | Athens–Tokyo–London-Aortic-Stenosis (ATLAS) registry | NR | DOACs/115 | Warfarin/102 | PSM | (4) | 2.0 | Different indications | NR |
| Jochheim et al. 2019 [23] | Europe | An investigatorinitiated multicenter observational registry study | 2007.6–2017.4 | DOACs/326 | VKAs/636 | IPTW | (5) | 1.0 | Different indications | Riva/175; Api/128; Dabi/23 |
| Kosmidou et al. 2019 [24] | The United States and Canada | Randomized PARTNER II (Placement of Aortic Transcatheter Valve II) trial and associated registries | NR | DOACs/155 | Warfarin/778 | NR | NR | 2.0 | AF | NR |
| Butt et al. 2019 [25] | Denmark | Danish healthcare system | 2012.1–2017.6 | DOACs/213 | VKAs/516 | COX | (6) | 1.0 | AF | NR |
| Mangner et al. 2018 [26] | Germany | A retrospective cohort study | 2011.1–2016.3 | DOACs/182 | VKAs/115 | NR | NR | 0.25 | AF | Riva/111; Api/41; Dabi/29; Edo/1 |
| Geis et al. 2018 [27] | Germany | A retrospective cohort study | 2008.7–2017.4 | DOACs/154 | VKAs/172 | NR | NR | 0.5 | Different indications | Riva/79; Api/54; Dabi/14; Edo/7 |
| Seeger et al. 2017 [28] | Germany | A prospective cohort study | NR | Api/81 | VKAs/50 | NR | NR | 1.0 | AF | Api/81 |
| DOACs, Direct Oral Anticoagulants; VKAs, Vitamin K Antagonist; COX, Cox proportional hazards models; AF, atrial fibrillation; OAC, oral anticoagulation; NR, not reported; IPTW, inverse probability of treatment weights of propensity scores; PSM, propensity score matching. (1): Adjusted variables including age, sex, body mass index, diabetes, New York Heart Association functional class III and IV, prior coronary artery bypass graft, prior percutaneous coronary intervention, prior stroke, peripheral artery disease, pacemaker, chronic renal failure, atrial fibrillation, ejection fraction, valve-in-valve procedure, aspirin, and year of inclusion in the registries; (2): Adjusted variables including body mass index, HAS-BLED score, Society of Thoracic Surgeons score, New York Heart Association functional class III or IV symptoms, history of stroke and coronary artery disease, year of TAVR procedure, access site, implanted transcatheter aortic valve type, antiplatelet regimen at discharge, and anticoagulant choice per center; (3): Adjusted variables including sex, age, body mass index, hypertension, diabetes, renal disease, liver disease, history of stroke, bleeding or myocardial infarction, previous paroxysmal or persistent AF, CHA2DS2-VASc Score, HAS-BLED Score, EURO Score II, left atrium enlargemen, poor left ventricular ejection fraction, left ventricular hypertrophy, left ventricle dilatation, native aortic valve disease, aortic bioprosthesis size, prosthesis-patients mismatch, adherence to therapy and follow-up length; (4): Adjusted variables including age, gender, smoking status, previous cardiac surgery, left ventricular ejection fraction, logistic euroscore, post procedural aortic regurgitation, type of valve and use of dual antiplatelet therapy post procedure; (5): Adjusted variables including center, year of TAVR procedure, patient age at TAVR, gender, incidence of prior transcatheter aortic valve replacement, diagnosis of chronic kidney disease, Society of Thoracic Surgeons Predicted Risk of Mortality score, left ventricular function, and prosthesis type; (6) Adjusted variables for all-cause mortality including age, sex, a history of arterial thromboembolism, ischaemic heart disease, heart failure, hypertension, peripheral arterial disease, diabetes, chronic kidney disease, liver disease, and antiplatelet therapy, adjusted variables for bleeding including the components of the modified HAS-BLED score and sex; Riva, rivaroxaban; Api, apixaban; Dabi, dabigatran; Edo, edoxaban. | ||||||||||
The pooled results indicated similar risk of all-cause
mortality (aHR: 0.95, 95% CI: 0.65–1.39, I
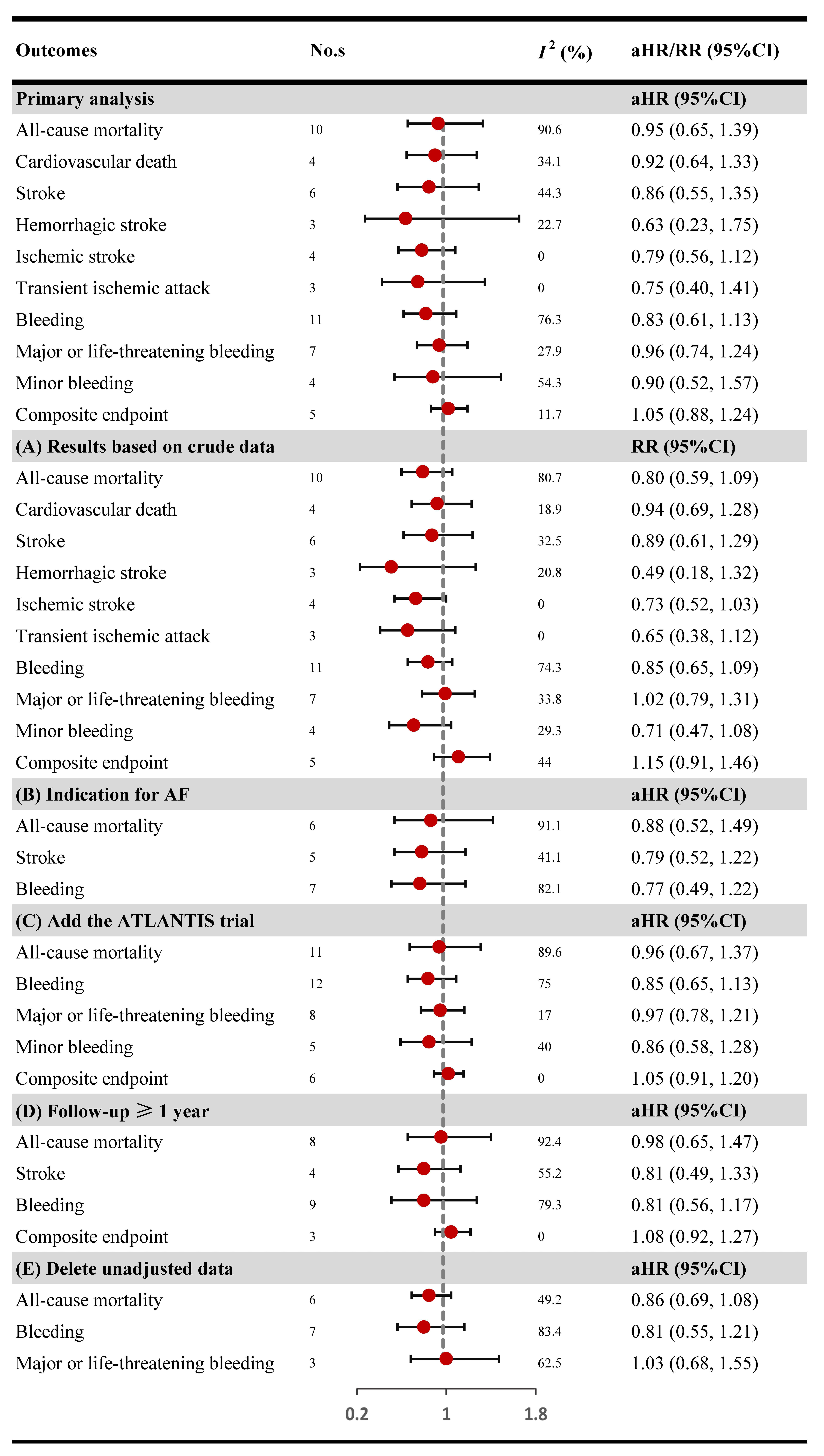 Fig. 2.
Fig. 2.Primary analysis and scenario analyses. CI, confidence interval;
aHR, adjusted hazard ratios; RR, relative risk. ATLANTIS: a multicenter,
randomized, phase IIIb, prospective, open-label, superiority study comparing
standard of care versus an apixaban-based strategy after successful TAVI. Part A:
scenario analysis by calculating RR based on crude data. Part B: scenario
analysis by limiting patients with anticoagulant indication as AF. Part C:
scenario analysis by adding the ATLANTIS trial. Part D:
scenario analysis by excluding studies with follow-up
The results of scenarios analyses are presented as follows (Fig. 2). (I): the
pooled RRs and associated 95% CI s of all-cause mortality (RR: 0.80, 95% CI:
0.59–1.09, I
We next examined the potential confounding factors that may impact the all-cause
mortality, stroke, bleeding, and composite endpoint outcome. Factors including
mean age, percentage of females, body-mass index (BMI), hypertension, diabetes
mellitus, chronic kidney disease, New York Heart Association (NYHA) class
We performed a comprehensive pooled analysis that simultaneously involves RCTs and real-world studies. The main findings can be summarized that the risk of all-cause mortality, stroke, bleeding, and composite endpoint was comparable between the DOACs and VKAs groups for post-TAVR patients requiring OAC therapy. Meanwhile, the rate of cardiovascular death, hemorrhagic stroke, ischemic stroke, transient ischemic attack, major or life-threatening, and minor bleeding was consistent between the two groups.
Compared to traditional anticoagulant VKAs, DOACs, including dabigatran, rivaroxaban, apixaban, and edoxaban, have provided an alternative therapy for venous thromboembolism and non-valvular atrial fibrillation due to their ease of use and proven efficacy and safety [29, 30, 31, 32]. Would a patient taking DOACs concurrent with valve heart disease (VHD) requires a return to VKAs is the choice we are facing? Regrettably, the results of RE-ALIGN trial were terrible, revealing excessive thromboembolism and bleeding events in patients treated with dabigatran; therefore, patients with mechanical heart valves (MHV) are contraindicated to take DOACs after the trial [33]. However, the mechanisms causing thromboembolic complications differed in MHV and bioprosthetic valves (BHV), and the latter considered less contact phase activation and device-related thrombosis [34]. A novel BHV technology, TAVR has gained popularity in patients with severe symptomatic AS [1]. The risk of thromboembolic events was increased when TAVR concomitant indications for anticoagulation. Therefore, an increasing interest in investigating the safety and efficacy of DOACs in post-TAVR patients in need of OAC therapy is arising. According to 2021 ESC/EACTS guidelines for managing valvular heart disease and 2021 ESC management of antithrombotic therapy in patients undergoing TAVR, lifelong OAC for TAVR patients who have other indications for anticoagulation were recommended intensively [3, 4]. However, robust evidence of the preferred oral anticoagulant agent for this population has not yet been established.
The initial study explored the safety and efficacy of the DOAC (apixaban) in patients with AF after TAVR was published in 2016 [28]. After that, cohort studies concerning this hot issue were conducted consecutively [26, 27, 28]. A large multicenter French TAVR registries demonstrated lower long-term mortality and major bleeding at 3 years with DOACs than VKAs at discharge [19]. An Athens–Tokyo–London-Aortic-Stenosis (ATLAS) registry showed DOACs use in patients who underwent TAVR with indication for OAC has a comparable risk of all-cause mortality and bleeding with VKAs [22]. However, all of the above studies had the drawbacks of an open-label registry treatment and controversial conclusions. Meanwhile, high-quality meta-analyses are currently lacking. The earliest meta-analysis, which combined 5 articles of 2569 patients, indicated a similar all-cause mortality, major and/or life-threatening bleeding, and stroke risk of DOACs compared with VKAs [6] (odds ratio [OR]: 1.07, 95% CI: 0.73–1.57; OR: 0.85, 95% CI: 0.64–1.12; OR: 1.52, 95% CI: 0.93–2.48, respectively). However, this study had 2 important limitations: first, only retrospective observational studies were considered, and the pooled ORs were unadjusted. Therefore, the findings should be elucidated cautiously due to the possible confounders. Second, the different indications for anticoagulation, especially AF, have not been analyzed independently. Another meta-analysis of 7 studies reported that VKAs have priority against DOACs in anti-thromboembolism (RR: 1.44, 95% CI: 1.05–1.99) but not in mortality or bleeding events [7]. Although this study was the first meta-analysis that included RCT, a severe error can not be neglected: the GALILEO trial, which compared the DOACs with antiplatelet-based therapy, did not meet the inclusion eligibility criteria [8]. Beyond that, it is noteworthy that two pivotal RCTs, ATLANTIS (NCT02664649) and ENVISAGE-TAVI (NCT02943785) [9, 10], have been reported recently. The issue could be interpreted by obtaining more valuable information on large populations. Therefore, we updated a comprehensive analysis to unite current proof from RCTs and real-world studies to judge the safety and efficacy of DOACs in the population undergoing TAVR and requiring OAC therapy. The final results revealed that using DOACs might be noninferiority to using VKAs in this setting.
We noticed I
Although we first included the high-quality data and larger population from ENVISAGE-TAVI and ATLANTIS trials, the findings of this study should be guided for clinical applications within specific situations. The ENVISAGE-TAVI trial only involved a population of older adults undergoing OAC indication for AF. The results showed edoxaban was comparable to VKAs in terms of the composite primary outcome of adverse clinical events but presented a higher risk of major bleeding than VKAs; hence, these results may not be appropriate for younger patients with low surgical risk, patients with asymptomatic aortic stenosis, patients with high bleeding risk, and those committed to other OAC indications. On the other hand, the ATLANTIS trial found no significant differences in primary, secondary, or safety endpoints among apixaban and control groups in patients with indications for anticoagulant therapy, which was a post hoc analysis results without baseline characteristics and limited population. Therefore, more extensive RCTs are needed to confirm the efficacy and safety of dabigatran and rivaroxaban in post-TAVR patients with OAC needs.
Currently, several limitations should be considered in this research. First, the outcome evaluation of individual DOACs regimens was not performed. Second, the outcome for valve thrombosis, an important concern after TAVR, was not obtained. Besides, baseline data on critical clinical parameters, such as left ventricular ejection fraction, smoking habits, alcohol consumption, and data on procedural characteristics and complications concerning the TAVR procedure were not available, which might contribute to the mild to high heterogeneity between studies. Moreover, more detailed settings, including TAVR patients with OAC indications other than AF, prior AF before TAVR, and new-onset AF after TAVR, were not performed due to a lack of adequate data. Also, we can not provide results in patients concomitant with antiplatelet regimens, whether one antiplatelet drug or dual antiplatelet therapy (DAPT), between the DOACs and VKAs due to the paucity of available data. However, we noticed exploratory results on the post hoc analysis of the ENVISAGE-TAVI trial, which showed a higher bleeding rate of edoxaban in patients combined with antiplatelet therapy than VKAs. Large RCTs are warranted to establish the clinical outcomes of DOACs when compared with VKAs in combination with antiplatelets.
In TAVR patients with indication of OAC, the present study indicates that DOACs are as safe and effective as VKAs in term of all-cause mortality, bleeding, stroke, and composite endpoint. However, the ideal anticoagulation scheme should be chosen through a comprehensive evaluation of the patient’s condition and the physician’s discretion. Further precise randomized controlled trials are needed to explore more scenarios, such as single DOACs regimens and combination antiplatelet therapy.
ZCG, YB, JC, and HWL contributed to the study design and are guarantors of the entire manuscript. JW, FYZ, and LL contributed to the study conception, design, and critical revision of the manuscript for important intellectual content. MMP and CZ contributed to the data acquisition, analysis, and interpretation. All authors contributed to the study design, critically reviewed the first draft, approved the final version, and agreed to be accountable for the work.
Not applicable.
We would like to express our gratitude to all those who helped us during the writing of this manuscript. Thanks to all the peer reviewers for their opinions and suggestions.
This study was supported by the Research Project of Drug Clinical Comprehensive Evaluation and Drug Treatment Pathway (SHYXH-ZP-2021-001), Clinical Research Innovation and Cultivation Fund of Ren Ji hospital (RJPY-LX-008), Ren Ji Boost Project of National Natural Science Foundation of China (RJTJ-JX-001).
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
