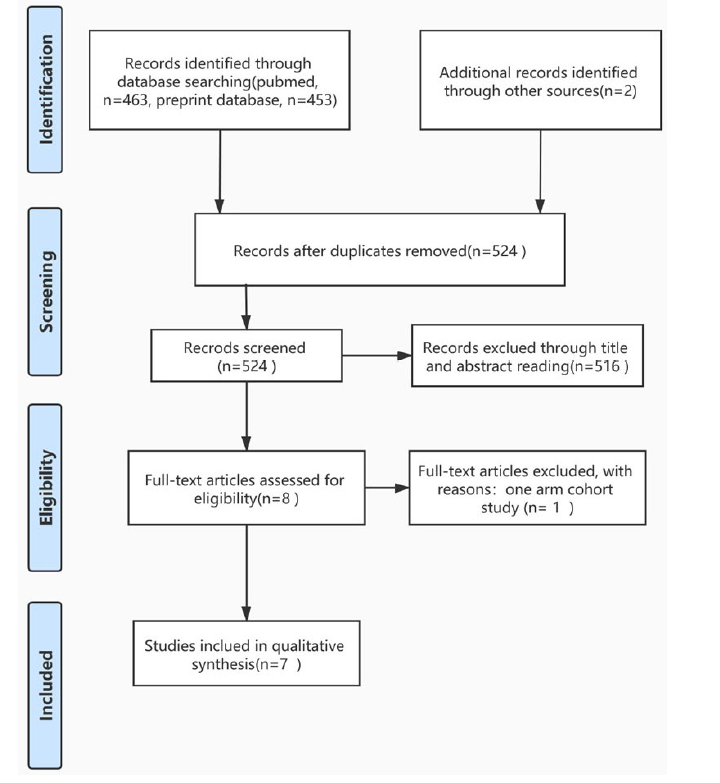
Objective: To compare the efficacy and safety of antiviral agents currently studied for the treatment of the COVID-19 pandemic. Methods: A literature search was conducted on the PubMed, EMBASE, Web of Science, CNKI (Chinese Database), and MedRxiv for studies published from 1966 till May 10, 2020, and identified articles containing “COVID-19” and “antiviral agents”. Studies were reviewed and screened in the guidance of PRISMA. STATA 15.1 software was used to build a random-effects model. Heterogeneity was assessed using I2. The Cochrane Risk of Bias or Newcastle-Ottawa-Scale (NOS) was employed to evaluate the public bias. Results: We identified 916 papers and included 7 studies involving 878 patients. The network meta-analysis was centered on comparing the efficacy and safety of presently used antiviral drugs for COVID-19. Among the antiviral agents applied in the treatment of COVID-19 treatment, including lopinavir/ritonavir, remdesivir, favipiravir, arbidol (umefenovir) or placebo, favipiravir exhibited significantly better efficacy in nucleic acid conversion rate [RR 2.38, 95%CI (1.05, 5.41)] and CT improvements [RR 1.85, 95%CI (1.07, 3.2)] than arbidol as well as other included antivirals though no significant association were found. ARB had advantages in nucleic acid conversion rate and ADRs incidence. Besides, favipiravir was more superior in safety than other antiviral drugs assessed by SUCRA (Surface Under the Cumulative Ranking Curve) ranking. Conclusions: Favipiravir had better efficacy in clinical recovery as well as more acceptable safety, though further clinical research should be designed to confirm these results. With the limited data of remdesivir, we could not conclude a statistical advantages of using remdesivir.