Objective: To compare the efficacy and safety of antiviral agents currently studied for the treatment of the COVID-19 pandemic. Methods: A literature search was conducted on the PubMed, EMBASE, Web of Science, CNKI (Chinese Database), and MedRxiv for studies published from 1966 till May 10, 2020, and identified articles containing “COVID-19” and “antiviral agents”. Studies were reviewed and screened in the guidance of PRISMA. STATA 15.1 software was used to build a random-effects model. Heterogeneity was assessed using I2. The Cochrane Risk of Bias or Newcastle-Ottawa-Scale (NOS) was employed to evaluate the public bias. Results: We identified 916 papers and included 7 studies involving 878 patients. The network meta-analysis was centered on comparing the efficacy and safety of presently used antiviral drugs for COVID-19. Among the antiviral agents applied in the treatment of COVID-19 treatment, including lopinavir/ritonavir, remdesivir, favipiravir, arbidol (umefenovir) or placebo, favipiravir exhibited significantly better efficacy in nucleic acid conversion rate [RR 2.38, 95%CI (1.05, 5.41)] and CT improvements [RR 1.85, 95%CI (1.07, 3.2)] than arbidol as well as other included antivirals though no significant association were found. ARB had advantages in nucleic acid conversion rate and ADRs incidence. Besides, favipiravir was more superior in safety than other antiviral drugs assessed by SUCRA (Surface Under the Cumulative Ranking Curve) ranking. Conclusions: Favipiravir had better efficacy in clinical recovery as well as more acceptable safety, though further clinical research should be designed to confirm these results. With the limited data of remdesivir, we could not conclude a statistical advantages of using remdesivir.
At the end of 2019, a new type of pneumonia emerged in Wuhan, central China, and rapidly spread through the entire world. Lacking effective drugs for this disease significantly hindered the treatment at the very beginning. Not until Lu Roujian et al. unveiled the results of pathogen isolation and genomic characterization [1], a new human-infected beta-coronavirus was found and named as SARS-CoV-2 by ICTV (International Committee on Taxonomy of Viruses, ICTV) [2]. Currently, coronavirus infectious disease-19 (COVID-19) has been announced as a pandemic by the WHO and became the focus of the world as the number of patients infected with COVID-19 has increased up to over 4,400,000, with a mortality rate over 6% by May 15th, 2020. SARS-CoV-2 shares some similarities with MERS-CoV (with about 50% identity) and SARS-CoV (about 79% identity), and notably it shares a similar receptor-binding domain structure to that of SARS-CoV. Consequently, antiviral drugs that had proved to be effective in the previous SARS or MERS treatment have been tested in COVID-19 treatment, though SARS-CoV and MERS-CoV disappeared suddenly and no further antiviral studies were continued. To deal with the pandemic crisis, scientists have registered hundreds of clinical trials around world, with varied antiviral drugs, study designs, and endpoints et al.
Additionally, preliminary results showed that remdesivir (REM) had vigorous antiviral activity against SARS-CoV-2 in vitro [3]. Besides, due to lack of effective agents, some other antiviral agents, such as darunavir (DRV), was used to test the efficacy against SARS-CoV-2. However, in vitro study showed DRV had no antiviral activity against SARS-CoV-2 at clinically relevant concentrations (EC50 > 100 µM) [4]. Currently, the NIH COVID-19 treatment guidelines updated the antiviral section [5]: REM was recommended as the investigational antiviral agent as it has not yet been approved by the FDA and it was available through an FDA emergency use authorization for the treatment of COVID-19 in hospitalized patients with severe disease. Some latest results of clinical trials have been released, and favipiravir (FPV) and arbidol (ARB; umefenovir) showed potential antiviral efficacy against COVID-19. However, due to the varied clinical research design, it is crucial to conduct a systematic review of the published data, in order to provide rationale evidence for clinicians in choosing anti-viral drugs to combate COVID-19.
We searched MEDLINE, Embase, preprint database and the Cochrane Central Library for clinical trials and cohort studies published in English from 1966 till May 10, 2020. We also searched ClinicalTrials.gov and the chictr.org.cn of Controlled Trials to identify any clinical study that was not yet published, but potentially eligible for inclusion. Additionally, we also searched medRxiv, ChinaXiv.org for any clinical literature that meets our inclusion criteria.
We included studies of outcomes in the treatment of adults diagnosed with COVID-19. The diagnostic criteria were in accordance with Chinese clinical guidance for COVID-19 [6]. The treatment of the patients with antiviral agents, included Lopinavir/Ritonavir (LPV/RTV), arbidol (ARB; umefenovir), favipiravir (FPV), remdesivir (REM) or placebo (CON), with at least two-arm design comparison. The outcome indicator included: (ⅰ) SARS-CoV-2 nucleic acid positive-to-negative conversion rate 7 days after initiating antiviral treatment. One of the vital diagnosis criteria included SARS-CoV-2 nucleic acid positivity by real-time reverse-transcriptase polymerase-chain-reaction (RT-PCR) assay for nasal and/or pharyngeal swab specimens. (ⅱ) SARS-CoV-2 nucleic acid positive-to-negative conversion rate 14 days after initiating antiviral treatment. (ⅲ) 7 days computerized tomography (CT) improvements: the efficacy of antiviral agents was assessed by chest CT 7 days after initiating antivirals treatment; (ⅳ) 14 days CT improvements: chest CT assessment 14 days after initiating antivirals treatment; (ⅴ) Alleviation of cough: cough relief 7 days after antiviral treatment; (ⅵ) Incidence of adverse effects: any abnormality examined by clinical laboratory testing or daily vital signs in the follow-up period; All the clinical outcomes were important indicators of clinical recovery listed in the Chinese Clinical Guidance for COVID-19 [6, 7]; (ⅶ) All-cause mortality at day 7,14 or 28 after antiviral treatment initiating.
Two investigators (Peng Hongwei and Liu Lili) worked independently, searched and read all titles and abstracts and titles in duplicate to identify papers relevant to this study. When discrepancies occurred, a third coauthor (Chen Zhangren) made the final judgement. Peng and Liu assessed eligibility from full-text articles, with a similar process for potential disagreements as described above. Data extraction from included studies (i.e., published abstracts and manuscripts) was duplicated with a standardized extraction form. The characteristics of the research (study design, antiviral regimen, therapy duration, etc.), the outcomes of interest mentioned above, and biased risk assessment were extracted.
We assessed the quality of individual clinical research using the Cochrane Risk of Bias instrument evaluating seven key domains [8]. We employed the Grading of Recommendations Assessment, Development and Evaluation (GRADE) system for rating overall quality of evidence, using the four-step approach proposed to assess the quality of treatment effect estimates from network meta-analyses. For cohort study or case-control study, Newcastle-Ottawa-Scale (NOS) was employed [9]. We determined the quality of evidence for each primary outcome after considering each of these elements, and classified them as high, moderate, low, or very low quality [10].
We completed network meta-analysis using Stata 15.1 software. Heterogeneity was assessed by the χ2 test. In the cases of heterogeneity, sensitivity analysis was made and the heterogeneity sources were investigated. We analyzed two outcomes (CT improvements and nucleic acid conversion rate) at two timepoints: 7 days and 14 days after initiating treatment. Estimates of comparative efficacy and safety are represented as relative ratios (RR) with associated 95% confidence intervals (CI) for binary outcomes. We made a comparison of each intervention by surface under the cumulative ranking curve (SUCRA).
We have identified 916 publications through database searching and 2 additional records. Through titles and abstract reading, we finally included 7 articles for network meta-analysis. These articles included four randomized clinical trials and three cohort studies. The results of the literature search are depicted in Fig. 1. The evaluation of research bias was showed in Supplementary Fig. 1.
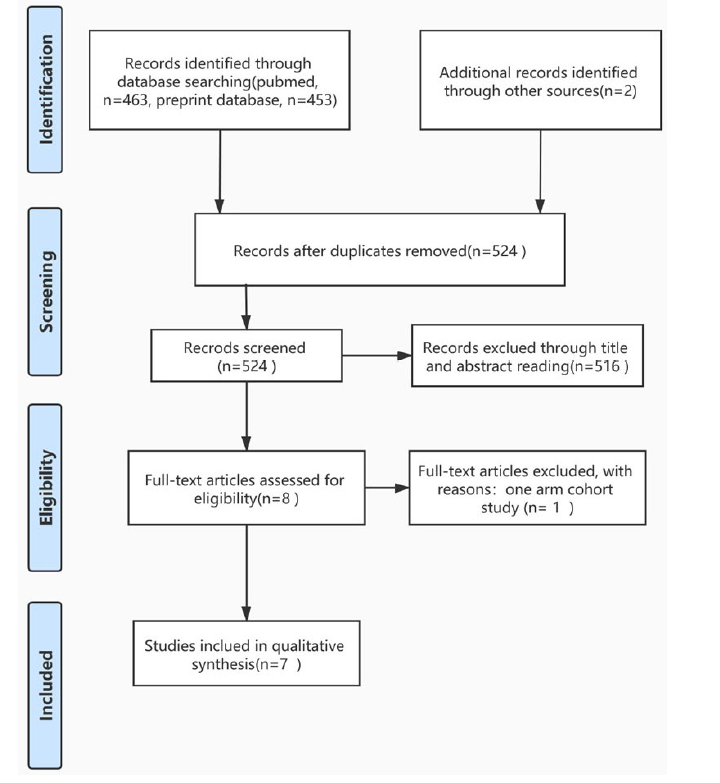 Figure 1.
Figure 1.The PRISMA flow of literature searching and screening.
The characteristics of the research included are shown in Table 1. The network relationships of the outcome indicators were shown in Fig. 2. We have analyzed 6 outcome indicators mentioned above and the heterogeneity test revealed no significant differences, so we therefore chose a random-effect model for further analysis.
| Author | Year | Design | Country | Antiviral therapy | Number of participants | Outcomes |
| Zhu Zhen [11] | 2020 | cohort study | China | LPV/RTV versus ARB | 34/16 | (ⅰ) (ⅱ) (ⅵ) |
| Deng Lisi [12] | 2020 | retrospective cohort study | China | ARB plus LPV/RTV versus LPV/RTV | 16/17 | (ⅰ) (ⅱ) (ⅲ) |
| Li Yueping [13] | 2020 | Randomized, controlled study | China | LPV/RTV versus ARB versus basic therapy | 21/16/7 | (ⅰ) (ⅱ) (ⅲ) |
| Chen Chang [14] | 2020 | randomized trial | China | FPV versus ARB | 116/120 | (ⅴ) (ⅵ) |
| Cai Qingxian [15] | 2020 | open-label control study | China | FPV versus LPV/RTV | 35/45 | (ⅰ) (ⅱ) (ⅲ) |
| Wang Yeming [16] | 2020 | Randomized, double-blind, placebo-controlled, multicenter | China | REV versus placebo | 158/78 | (ⅰ) (ⅱ) (ⅵ) (ⅶ) |
| Cao Bin [17] | 2020 | Randomized, double-blind, placebo-controlled, multicenter | China | LPV/RTV versus basic therapy | 99/100 | (ⅰ) (ⅲ) (ⅵ) (ⅶ) |
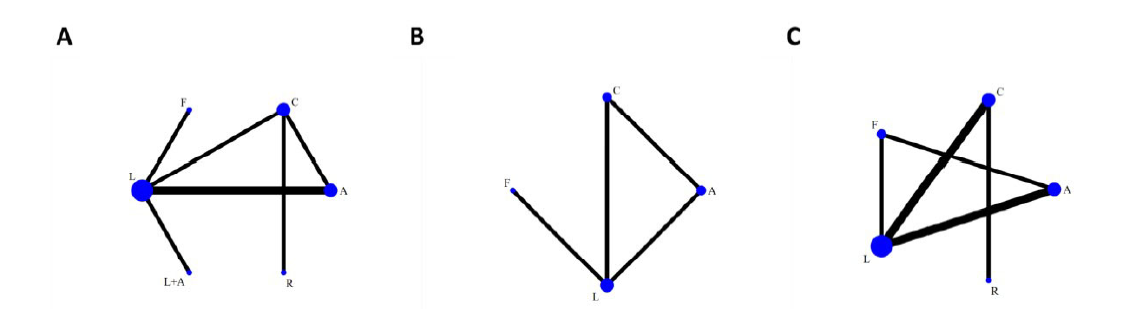 Figure 2.
Figure 2.Network relationships of different outcomes. A. positive-to-negative conversion of SARS-CoV-2 nucleic acid; B. CT improvements; C. incidence of ADRs. A = Arbidol; C = Placebo or basic therapy; F = Favipiravir; L = Lopinavir/Ritonavir; L + A = Lopinavir/Ritonavir + Arbidol.
There were 7 studies reported the SARS-CoV-2 nucleic acid conversion rate.
3.4.1 Nucleic acid positive-to-negative conversion rate
There were 5 papers reporting the COVID-19 nucleic acid positive-to-negative conversion rates after different interventions. As shown in Fig. 2A, there were 4 direct comparison concerning LPV/RTV (LPV/RTV vs. FPV, LPV/RTV vs. LPV/RTV + ARB, LPV/RTV vs. CON, LPV/RTV vs. ARB), two studies compared the efficacy of LPV/RTV vs. ARB. The three interventions, LPV/RTV, CON, ARB formed a closed loop, indicating direct and indirect comparison could be made among these three interventions.
Fix effect network analysis showed that the nucleic acid conversion rate 7 days after initiating treatment in the LPV/RTV + ARB arm [RR 2.31, 95%CI (1.05, 4.29)] was better than LPV/RTV monotherapy. Besides, the LPV/RTV treatment was inferior to the FPV [RR 0.25, 95%CI (0.13, 0.48)] or ARB monotherapy [RR 0.59, 95%CI (0.36, 0.96)]. Additionally, FPV displayed better activity than ARB [RR 2.38, 95%CI (1.05, 5.41)]. There was no significant difference when comparing between other interventions (Fig. 3A). When it comes to SUCRA ranking (7 days nucleic acid positive-to-negative conversion rate), the FPV arm obtained the highest SUCRA score, followed by LPV/RTV + ARB, REM, CON, ARB and LPV/RTV. In the outcome of 14 days’ nucleic acid conversion rate, no statistical difference was found and the ranking was FPV > LPV/RTV + ARB > ARB > CON > REM > LPV/RTV (Table 2).
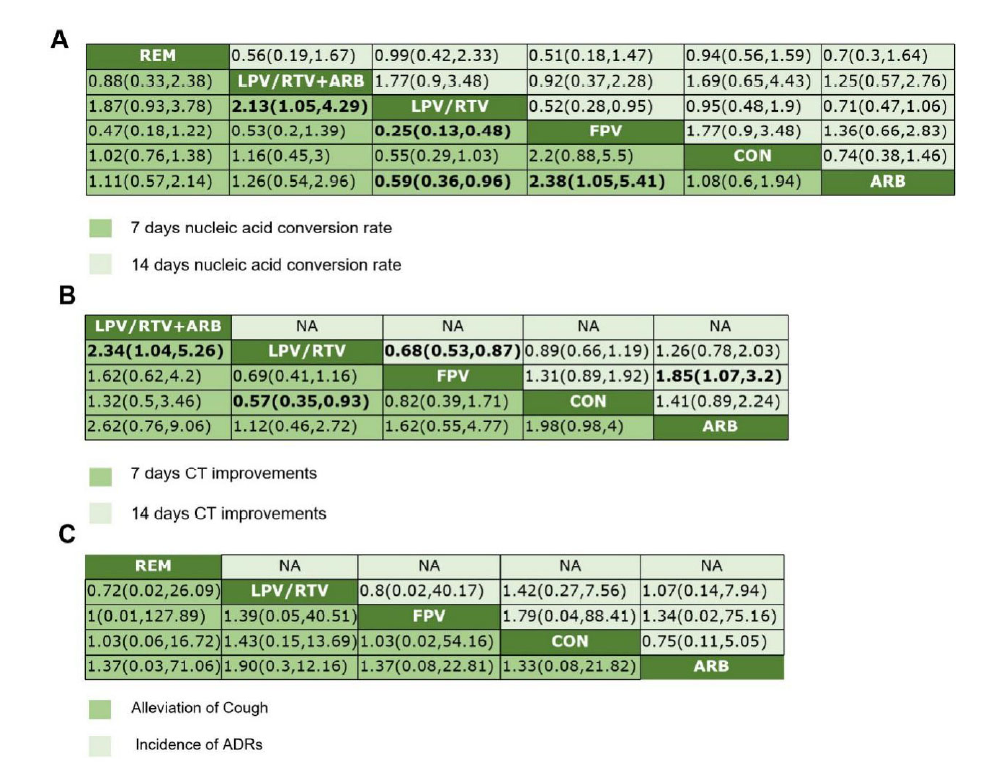 Figure 3.
Figure 3.Random-effects network meta-analysis of different clinical outcomes. A. network meta-analysis of nucleic acid conversion rate; B. network meta-analysis of CT improvements; C. network meta-analysis of cough relief and ADRs incidence. Data are mean difference (95% CI) of the row treatment relative to the column treatment. Bold values indicate comparisons that are statistically significant. CI = credible interval. REM = Remdesivir, LPV/RTV = Lopinavir/Ritonavir, ARB = Arbidol, FPV = Favipiravir, CON = Placebo or basic therapy.
3.4.2 CT improvements
There were 3 articles reporting the CT improvements upon antiviral treatments for 7 or 14 days. Closed comparison loop was formed among CON, ARB and LPV/RTV, another direct comparison was made between LPV/RTV and FPV (Fig. 2B). According to the network meta-analysis, the outcome of CT improvements 7 days after antiviral treatment were as follows: LPV/RTV + ARB [RR 2.34, 95%CI (1.04, 5.26)] had better efficacy than LPV/RTV monotherapy; LPV/RTV [RR 0.57, 95%CI (0.35, 0.93)] was inferior to CON. For the clinical outcome of CT improvements 14 days after antiviral treatment, LPV/RTV [RR 2.34, 95%CI (1.04, 5.26)] was inferior to FPV, and FPV [RR 1.85, 95%CI (1.07, 3.2)] was better than ARB (Fig. 3B). The SUCRA ranking (7 days CT improvements) showed that the LPV + ARB treatment achieved the highest SUCRA score, followed by CON, FPV, LPV/RTV, and ARB; Regarding the indicator of 14 days CT improvements, the ranking was FPV > CON > LPV > ARB (Table 2).
| Treatment | SUCRA | MeanRank | |
| 7 days nucleotide conversion rate | |||
| ARB | 40.9 | 4 | |
| CON | 48.3 | 3.6 | |
| FPV | 95.8 | 1.2 | |
| LPV/RTV | 2.5 | 5.9 | |
| LPV/RTV + ARB | 59.9 | 3 | |
| REM | 52.6 | 3.4 | |
| 14 days nucleotide conversion rate | |||
| ARB | 60.8 | 3 | |
| CON | 31 | 4.4 | |
| FPV | 83.1 | 1.8 | |
| LPV/RTV | 21.7 | 4.9 | |
| LPV/RTV + ARB | 76.5 | 2.2 | |
| REM | 26.8 | 4.7 | |
| 7 days CT improvements | |||
| ARB | 16.4 | 4.3 | |
| CON | 73.3 | 2.1 | |
| FPV | 54.7 | 2.8 | |
| LPV/RTV | 17.9 | 4.3 | |
| LPV/RTV + ARB | 87.7 | 1.5 | |
| 14 days CT improvements | |||
| ARB | 9 | 3.7 | |
| CON | 59.9 | 2.2 | |
| FPV | 96.7 | 1.1 | |
| LPV/RTV | 34.4 | 3 | |
| Incidence of ADRs | |||
| ARB | 53.7 | 2.9 | |
| CON | 47.1 | 3.1 | |
| FPV | 66.2 | 2.4 | |
| LPV/RTV | 33.4 | 3.7 | |
| REM | 49.6 | 3 | |
| Alleviation of Cough | |||
| ARB | 48.5 | 2.5 | |
| CON | 37.6 | 2.9 | |
| FPV | 58.3 | 2.3 | |
| LPV/RTV | 55.6 | 2.3 |
*Abbreviations: ARB = Arbidol, CON = placebo or basic therapy, FPV = favipiravir, LPV/RTV = lopinavir/ritonavir, REM = remdesivir.
3.4.3 Improvements in clinical symptoms and treatment safety
There were 2 articles comparing the cough alleviation and 6 articles reporting the frequencies of adverse effects. The net-work relationships of adverse effects among different interventions were shown in Fig. 2C. Closed comparison loop was formed among interventions of LPV/RTV, FPV and ARB, and other direct comparisons were made between CON vs. LPV/RTV and CON vs. REV, respectively. However, there were no significant difference between any interventions (Fig. 3C). Though there was no difference in the random-effect model, the SUCRA score suggested that the safety ranking was FPV > ARB > REM > CON > LPV/RTV and FPV also displayed the best ranking result in alleviation of cough, followed by LPV/RTV, ARB and CON (Table 2).
3.4.4 Efficacy in all-cause mortality
There were 2 studies reporting all-cause mortality. Wang Yeming et al. Compared motality of REM vs. Placebo at day 7, 14 and 28 , and no significant differences were found between two interventions [16]. Cao B. et al. compared motality at day 7 and day 14 after LPV/RTV vs. Standard care initiated in severe COVID-19 [17]. 199 patients underwent randomization and the total mortality rate of COVID-19 was 6% at day 7, with 5.1% in LPV/RTV cohort vs. 7% in standard care cohort. Although the motality rate climb up to 16.1% , with 15.2% in LPV/RTV cohort vs.1 7% in standard care cohort, no statistic significance was found between two cohorts.
Though the COVID-19 pandemic is currently under control in some countries, the WHO has announced COVID-19 as an international public health emergency. Thanks to the dedication of hard-working scientists and clinicians around the world, novel agents, or new activity of currently used drugs i.e. drug repurposing, such as chloroquine (CQ) and its analogue hydroxychloroquine (HCQ) were investigated for their anti-viral activity [3]. However, the specific pharmacokinetic characteristics (long time elimination, CYP3A4 inhibition that may induce various drug-to-drug interactions, etc.) and safety concerns (e.g. cardiac side effects) of CQ and HCQ limited their clinical application [18, 19]. REM was first developed as an antiviral drug against SARS-CoV and MERS-CoV [20, 21], both viruses of which suddenly disappeared in humans, thus markedly diminishing clinical research. LPV/RTV as well as ARB were proven to have activity either in in vitro studies as well as in animal models or for post-exposure prophylaxiss [22, 23], and they were being investigated in hundreds of registered clinical trials worldwide. According to our network meta-analysis, FPV was the best candidate antiviral drug in terms of both efficacy (i.e. CT improvements and nucleic acid positive-to-negative conversion rate) and safety.
FPV was first approved in Japan and a recent research unveiled its activity against COVID-19 [14]. FPV, as a novel RNA-dependent RNA polymerase (RdRp) inhibitor, shows inhibitory effects on a wide range of RNA viruses, such as the influenza virus. Retrospective analysis of patients with Ebola virus disease (EVD) indicated that FPV treatment showed advantages in overall survival [24]. The genomic characteristics of SARS-CoV-2 is that it contains a single-stranded RNA beta-coronavirus genome harboring the RdRp gene, which shares great similarity with SARS-CoV as well as MERS-CoV [1]. Preclinical research demonstrated the efficacy of FPV against SARS-CoV-2 in vitro (EC50 = 61.9 µM determined using monkey African green kidney Vero E6 cells) [3]. Two clinical trials have investigated the comparative effectiveness and safety of FPV versus ritonavir-boosted lopinavir or ARB [14, 15], with randomized or controlled study, suggesting moderate-to-high level evidence. The most common adverse events of FPV were digestive tract reactions (nausea and diarrhea) and increased serum uric acid, side effects of which were irreversible. CFDA (China Food and Drug Administration, CFDA) approved FPV on the 16th Feb of 2020 as an antiviral agent for COVID-19. Consequently, the results of CT improvements, nucleic acid positive-to-negative rate, the efficacy and safety of FPV were apparently encouraging. These results provide clinicians with an apparently promising candidate for COVID-19 treatment.
REM was first developed as an adenosine analogue against EBOLA virus, which undergoes incorporation into nascent viral RNA chains and results in premature termination of viral replication. Actually, it is a prodrug of an adenosine analogue that has a broad antiviral spectrum, including all human and animal coronaviruses tested in vitro. As the first antiviral drug proved to be effective against SARS-CoV-2 in vitro [3], REM got high expectations especially after the first case in the United States recovered by REM treatment [25]. There are 8 registered clinical trials in the NIH, 3 of which have been completed and the results reported. However, the results of REM seem controversial: a multicenter, randomized, double-blind clinical trial launched in severe COVID-19 patients in China indicates REM is not associated with statistically significant clinical benefits, though it shortened the time to clinical improvements [16] (NCT04257656). The clinical trial of REM in adults with mild and moderate COVID-19 was suspended due to lack of patients. NIH clinical trials named “Adaptive COVID-19 Treatment Trial” (ACTT) launched in the United States showed that REM accelerated patient recovery from advanced COVID-19. Patients who received REM had a 31% faster time to recovery than those who received placebo. Besides, a favorable mortality rate was also observed in REM group (p = 0.059) (NCT04280705). The ACTT trial designed to be completed on April 1st, 2023, so we have only included Cao Bin's research in this manuscript due to lack of full data of another clinical study. In fact, the inconsistency of the REM results is related to the inconsistent criteria for efficacy evaluation. The clinical study launched by Cao Bin et al designed a six-point scale to evaluate the clinical improvements by at least two-point reduction in patients' admission status within 28 days after randomization. The primary clinical outcome of ACTT was the time to recovery within 29 days, which defined a three-point scale. Due to the circumstances of COVID-19 pandemic, Gilead Inc announced the latest news that the Japanese Ministry of Health, Labor and Welfare (MHLW) has approved REM as a treatment for severe patients COVID-19 patients. Generally, the activity of REM to COVID-19 patients in clinical improvements, mortality, as well as frequencies of adverse effects, needs to be confirmed in large-sample clinical study.
As for LPV/RTV, the drug-to-drug interaction and side effects could not be ignored. LPV/RTV were first designed and developed as a peptidomimetic HIV protease inhibitors and tested against COVID-19 treatment due to its previous studies on SARS-CoV [19, 26] and its availability at the very beginning of the epidemic in Wuhan. The clinical trial completed by Cao Bin et al focused on severe COVID-19 patients, though LPV/RTV treatment could reduce the time to clinical improvement (15 days versus 16 days of standard care), LPV/RTV was not associated with statistically significant clinical benefits [17]. When compared with ARB, the latter was more superior to LPV/RTV in nucleic acid positive-to-negative conversion [11]. Additionally, a clinical trial launched by Guangzhou Eighth People's Hospital demonstrated that LPV/RTV, as well as ARB had little benefit in improving the clinical outcome of mild/moderate COVID-19, and LPV/RTV was associated with adverse reactions [13] , that was a major concern of LPV/RTV in clinical application. Ritonavir-boosted lopinavir could increase the bioavailability of lopinavir through inhibition of the CYP3A enzyme [27]. Lymphopenia was the most frequent and severe adverse effect of LPV/RTV during the treatment of COVID-19 [17]. The digestive tract side effects including diarrhea, vomiting, and nausea, were more common than that observed upon FPV treatment [25]. As the CYP3A enzyme was the principal enzyme of metabolism, LPV/RTV might induce various drug-to-drug interactions [28]. Some commonly applied agents in COVID-19 treatment including CQ, HCQ as well as moxifloxacin had strong interactions with LPV/RTV, leading to increased the serum concentration of those agents. That may also result in increasing side effects of LPV/RTV. Additionally, LPV/RTV monotherapy was inferior to other interventions concerning CT improvements, nucleic acid conversion rates, and adverse reactions et al.
ARB displayed better efficacy than LPV/RTV in nucleic acid conversion rate and fewer safety concerns. ARB is a non-nucleoside analogue designed and developed by Russian scientists. As a hemagglutinin inhibitor, it could block the entry of viruses and induce the secretion of interferon and display broad antiviral activity against influenza, human respiratory syncytial (HRSV), adenovirus, Coxsackie B5, parainfluenza, Ebola (EBOV), and hepatitis B and C viruses [29]. Previous studies showed its efficacy in inhibiting SARS-CoV as well as MERS-CoV [30, 31]. Based on this, professor Li Lanjuan et al., tested the antiviral activity of ARB and showed that it could significantly inhibit SARS-CoV-2 at the concentrations of ~10-30 µM in vitro. Due to the COVID-19 crisis, ARB was then recommended in the Chinese Clinical Guidance for the treatment COVID-19. According to our meta-analysis results, ARB was superior to LPV/RTV in the 7 days’ treatment in the nucleic acid conversion rate. The incidence of adverse effects of ARB was less than LPV/RTV, though no statistical significance was found.
The current literature review study has some limitations: (ⅰ) There is limited antiviral clinical research included due to lack of published data, and only three randomized clinical trials were included in our review, which may increase the risks of bias; (ⅱ) Only one REM clinical trial was included and due to varied assessment scale, there is limited data in 28-days mortality and direct comparison. The therapeutic efficacy and safety of REM need to be validated in future studies; (ⅲ) The majority of the sample size in most of the studies was small which may be due to the drug accessibility and the pandemic crisis.
Additionally, recent study have unveiled the fact that antioxidants and heme oxygenase contribute a lot in pathogenesis and progression of COVID-19 [32-34]. Clinical observations also reported the paradox of COVID-19 hospital admissions that fewer cigarette-smokers are admitted compared with non-smokers in general population, which could be explained by the fact that long-time smoke could induce the expression of HO-1, which plays a vital role in antioxidant and cytoprotective properties and could limit the infection’s damage [32]. Ahmed S. et al. found that alveoloar type Ⅱ cell SOD3 was the top-ranked gene that was most down-regulated in the elderly compared with young healthy donors through single cell RNA sequencing [33]. Taken together, the decreased ability in antioxidant may aggravate COVID-19 progression, especially in the elderly. Therapies with novel anti-oxidant agents as well as promisiong antiviral agents should be taken into consideration in order to prevent or treat COVID-19 disease.
In conclusion, FPV was superior to other antiviral agents in clinical improvement indicators (CT improvements, cough relief, nucleic acid conversion rate, and incidence of adverse reactions). ARB had advantages in nucleic acid conversion rate and the incidence of side effects. The activity of REM needs to be validated in more clinical research. Above all, the encouraging results of FPV may provide clinicians a rationale choice for COVID-19 treatment.
We would like to express our gratitude to all those who dedicated themselves in the COVID-19 pandemics and thanks to all the peer reviewers and editors for their opinions and suggestions.
The authors declare no conflict of interest.
