- Academic Editor
†These authors contributed equally.
Background: Cycloheximide (CXM), an antifungal antibiotic, causes
impaired memory consolidation as a side effect partially by disturbing the
activities of the central catecholaminergic and cholinergic system. Some reports
indicated that puerarin prevented memory impairment in various models in rodents.
However, the protective effects of puerarin on the side effects of cycloheximide
for memory consolidation impairment have not yet been investigated.
Methods: The protective effects of puerarin on CXM-induced
memory-consolidation impairment, and memory impairment produced by central
administration of AF64A neurotoxin, were investigated using a passive avoidance
task in rats. A combination of transmitter receptor agonists and antagonists was
used to explore the effects of puerarin on nervous system function. The activity
of antioxidant defense systems and neurotransmitter systems in the prefrontal
cortex and hippocampus were assayed. Results: Systemic (25 and 50 mg/kg,
i.p.) or central (5 and 10 µg/brain, i.c.v.) administration of puerarin
attenuated CXM-induced memory-consolidation impairment produced by 1.5 mg/kg CXM
(s.c.) in rats. The improvements produced by 50 mg/kg puerarin were blocked by
cholinergic antagonists, a 5-HT
Memory loss, including anterograde and retrograde amnesia, is the first and major symptom in Alzheimer’s disease (AD). Anterograde amnesia is characterized by an inability to learn new things and happens in the early stages of memory loss. Retrograde amnesia is characterized by loss of memory for information acquired before the onset of amnesia and almost always occurs in association with anterograde amnesia. Retrograde amnesia is closely connected to the loss of memory consolidation. It is currently well established that memory consolidation and long-lasting synaptic plasticity require the synthesis of new proteins [1]. Some studies [2, 3] have pointed out that antibiotics such as anisomycin, puromycin and cycloheximide (CXM) have the side effect of causing memory loss by blockading protein synthesis. CXM has been shown to produce impairment of memory consolidation and to produce retrograde amnesia in various behavioral paradigms in rodents. Furthermore, memory consolidation is also associated with the activation of receptor-linked enzymes through neurotransmitters such as acetylcholine, dopamine and serotonin, which are responsible for the synthesis of intracellular and intercellular proteins [4, 5]. Nabeshima et al. [6, 7, 8] indicated that CXM treatment caused impairment of memory consolidation partially by reducing cholinergic and catecholaminergic activities, and by increasing the serotonergic activity, in experimental animals. Hence, CXM-induced impairment of memory consolidation serves as a useful model for evaluating the development and mechanism of anti-amnestic drugs [9, 10, 11].
Puerarin (PUR), one of the major isoflavonoid bioactive ingredients of
Pueraria lobata (Willd.) Ohwi, is available in common nutritional
supplements to treat cerebrovascular diseases, diabetes, and neurodegenerative
disorders such as AD (in animal disease models) [12]. Earlier reports suggested
that total isoflavonoids of Pueraria lobata improved memory deficits
caused by muscarinic receptor blocker scopolamine (SCOP), D-galactose or cerebral
artery obstruction in rodents [13, 14]. In a purified form, puerarin also
attenuated memory deficits caused by D-galactose or cerebral artery obstruction
in rodents [15, 16]. Furthermore, puerarin improved memory impairment caused by
central injection with amyloid
Puerarin (purity
Male Sprague-Dawley rats (200–250 g), obtained from BioLASCO Taiwan Co., Ltd.
(Taipei, Taiwan), were housed in a temperature- (23
The procedure was as described in our previous reports [21]. The test was conducted in two days, with the first day being the training trial and the second day being the retention trial. During the training trial, each rat was placed in the light compartment with its back to the guillotine door. The step-through latency (STL) before the rat entered the dark compartment was recorded. When the rat entered the dark compartment, the door was closed and an inescapable footshock (0.8 mA for 2 s) was delivered through an MCU-101 Controller (Muromachi Kikai Co., Tokyo, Japan). After the footshock, the rat was put back into the home cage. The next day (24 h later), the rat was again placed in the light compartment and the STL of the retention trial was recorded. The cut-off time was 300 sec. The experiments were performed between 09:00 and 17:00 hours.
The schematic diagram of experimental designs and stages is shown in Fig. 1. For evaluating the mechanism of the attenuating action of puerarin on memory-consolidation impairment, we designed two series of experiments. The first, to evaluate the memory-improving effects of systemic treatment with puerarin, was performed using an injection of puerarin (10, 25, 50 mg/kg, i.p.) on CXM-induced impairment of memory-consolidation [21]. The second series, to evaluate the memory-improving effects of central treatment with puerarin, was performed using an intracerebroventricular (i.c.v.) injection of puerarin (1, 5, or 10 µg/brain) on rats with CXM-induced or AF64A-induced memory impairment. The detailed experimental performance was described as follows.
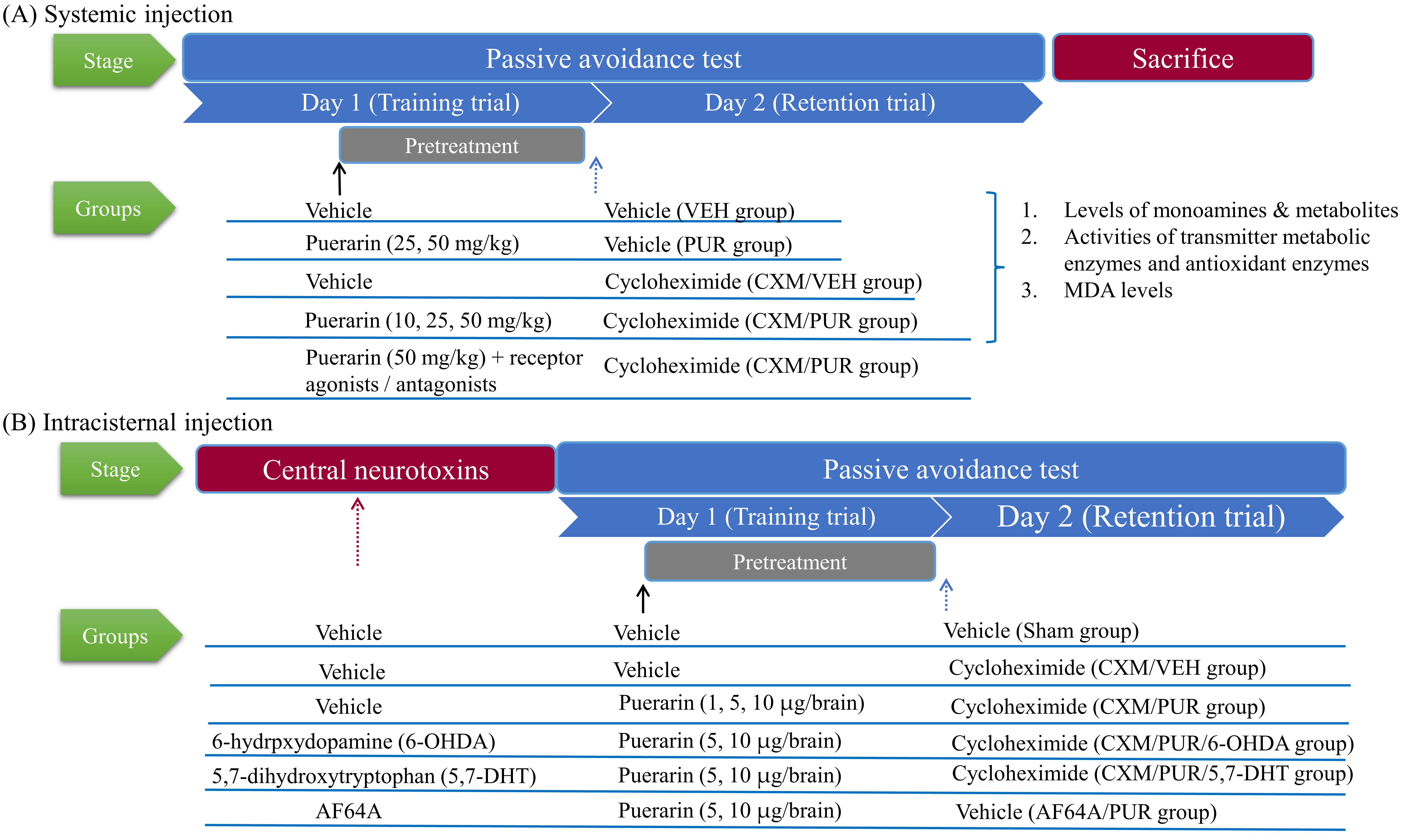 Fig. 1.
Fig. 1.Schematic diagram of experimental timeline and experimental designs. VEH,Vehicle; CXM, cycloheximide; PUR, Puerarin; MDA, malondialdehyde; 6-OHDA, 6-hydroxydopamine; 5,7-DHT, 5,7-dihydroxytryptamine.
The first series of experiments was designed to document the ameliorating
effects of systemic treatment of puerarin on the impairment of memory
consolidation that is produced by CXM treatment. CXM (1.5 mg/kg, s.c.) was
administered immediately after the training trial of the passive avoidance task
in order to impair memory consolidation [28]. Puerarin (10, 25, or 50 mg/kg,
i.p.) was administered to CXM-treated rats 30 min before the training trial, in
accordance with our previous report [21] and as per the pharmacokinetic report of
Kong et al. [23] showing that T
We investigated whether central treatment with puerarin improved memory impairment in the second series of experiments. For central injection of puerarin, rats were anesthetized with sodium pentobarbital (45 mg/kg, i.p.) and mounted in a stereotaxic frame (Stoelting, Wood Dale, IL, USA). A hole was drilled in a rat’s skull (anteroposterior (AP): –0.8 mm, mediolateral (ML): –1.5 mm from bregma) and a cannula (12 mm, 23 ga) was inserted to a depth of 3.6 mm below dura (Paxinos and Watson [29]). Post-operative care was provided for the first 2 h after surgery, including a single sterile saline injection, a single cefamandole injection and a heating pad under the cage. After 14 days of recovery, vehicle or puerarin (20 µL, 1, 5, or 10 µg/brain) was injected into the lateral ventricle with a 30-gauge injection needle attached to a 25-µL Hamilton syringe (Model 702RN, Reno, NV, USA), with a microinfusion pump (KDS310 syringe pump, KD Scientific Inc., Holliston, MA, USA), at a rate of 1 µL/min. After injection, the needle was left in place in the cannula for 2 min to allow puerarin to diffuse into the surrounding tissues of the injection area.
These cannula-implanted rats were divided into nine groups: vehicle (Sham), CXM
injection (CXM/VEH), pretreatment with puerarin in CXM injection (CXM/PUR),
pretreatment with 6-OHDA in CXM injection (6-OHDA), pretreatment with puerarin
and 6-OHDA in CXM injection (CXM/PUR/6-OHDA), pretreatment with 5,7-DHT in CXM
injection (5,7-DHT), pretreatment with puerarin and 5,7-DHT in CXM injection
(CXM/PUR/5,7-DHT), AF64A entral injection (AF64A), and pretreatment with puerarin
in AF64A central injection (AF64A/PUR). The AF64A (3 nmol/brain) was
administrated 14 days before the passive avoidance test [27]. The 5,7-DHT (10
µg/2 µL) was delivered to the dorsal raphe nuceli, bilaterally (AP:
–7.8 mm, ML:
The day after the retention trial, each rat was euthanized and the brain was separated into the prefrontal cortex and hippocampus on ice, 30 min after injection with vehicle or puerarin (25, 50 mg/kg, i.p.). These tissues were homogenized with ice-cold phosphate-buffered saline. Homogenates were centrifuged at 12,000 rpm for 15 min at 4 °C and the supernatants were collected. The supernatants were stored at –80 °C for subsequent neurotransmitter function and oxidative stress analysis.
Neurotransmitter function in the prefrontal cortex and hippocampus was assessed by measuring monoamines (NE, DA, 5-HT) and their metabolite (MHPG, DOPAC, HVA, 5-HIAA) concentrations, and AChE and monoamine oxidases (MAO-A and MAO-B) activity. Biogenic amines and their metabolite concentrations in the prefrontal cortex and hippocampus were measured using HPLC/ECD. Briefly, the above supernatants were again centrifuged with 0.22 µM Ultrafree MC centrifugal filter units (Millipore, Merck KGaA, Darmstadt, Germany) at 14,000 rpm for 10 min at 4 °C. The collected samples were assayed with an HPLC Model PM80 (Bioanalytic system Inc., West Lafayette, IN, USA), a Data Model M746 (Waters Co., Taipei, Taiwan), a Model LC-4C electrochemical detector (Bioanalytic system Inc.) and a Bioanalytic system MF-6026 column. AChE, MAO-A and MAO-B activity in the prefrontal cortex and hippocampus were measured using our previous method [30]. The supernatant or the AChE standard solution was reacted with DTNB for 10 min at 25 °C, and then ACtCh was added as a substrate for color development. The production of 5-thio-2-nitrobenzoic acid was measured at 412 nm with a microplate reader (BioTek Inc., Winooski, VT, USA). AChE activity was expressed as U AChE /mg protein. MAO-A and MAO-B activity was determined by the color produced by the reaction products of rat brain homogenate, horseradish peroxidase, and amplex red, and the substrate (5 mM serotonin for MAO-A or 5 mM benzylamine for MAO-B) for 60 min at 25 °C. The enzyme activity was expressed as a percentage of Sham-group values. The protein content was measured with a Bio-Rad protein assay kit (Bio-Rad Laboratories Inc., Taipei, Taiwan).
Antioxidant enzyme activity (including superoxidase dismutase [SOD], glutathione peroxidase [GPx], glutathione reductase [GR] and catalase) and glutathione (GSH) and malondialdehyde (MDA) levels in the prefrontal cortex and hippocampus were measured. Antioxidant enzyme activity was analyzed with a spectrophotometric microplate reader as specified in our previous report [31]. SOD activity was assayed by the trend of absorbance at 560 nm for the production of nitroblue tetrazolium. Catalase activity was assayed by the decrease in the absorbance at 560 nm for amplex red. GPx and GR activity was measured using a Cayman assay kit (Cayman Chemical Co., Ann Arbor, MI, USA). GPx, SOD and catalase activity was expressed as U/mg of protein. GR activity was expressed as mU/mg of protein. GSH and MDA levels were also determined as described previously [30]. Briefly, the supernatant or GSH standard solution was reacted with the reaction solution including DTNB, NADPH (nicotinamide adenine dinucleotide phosphate), and GR. Then the absorbance was recorded at 405 nm for 5 min with a microplate reader. GSH levels were expressed as nmol/g of protein. The TBARS (thiobarbituric acid reactive substances) assay was used to measure MDA levels in the prefrontal cortex and hippocampus. Briefly, a thiobarbituric acid (TBA) test was performed with the supernatant or MDA standard solution and the absorbance was determined at 532 nm. MDA levels were expressed as nmol/mg of protein.
All results were expressed as the mean
Our previous report pointed out that systemic administration of puerarin improved learning disabilities induced by MECA, PCA, and MK-801 in a passive avoidance task [21], therefore this study was conducted to evaluate the improving effect of systemic administration of puerarin on CXM-induced impairment of memory consolidation on a passive avoidance task. CXM, a protein inhibitor, impaired memory consolidation in rodents when subcutaneously injected immediately after behavioral performance [7]. Our present study confirmed that CXM administration immediately after the training trial impaired memory consolidation in rats, and showed that systemic administration of puerarin (25 or 50 mg/kg, i.p.) before the training trial significantly attenuated the impairment of memory consolidation caused by CXM (Fig. 2A).
 Fig. 2.
Fig. 2.Effects of puerarin (PUR, 10, 25, 50 mg/kg, i.p.) on
cycloheximide (CXM, 1.5 mg/kg, s.c.)-induced passive avoidance response
impairment in rats. (A) Absence of transmitter receptor agonists and
antagonists. (B) Presence of transmitter receptor agonists and antagonists. Data
are expressed as mean
Furthermore, the underlying neurochemical mechanisms of the improving effect of systemic administration with puerarin against CXM-induced memory consolidation impairment were clarified by combining the puerarin treatment with cholinergic receptor antagonists such as SCOP and MECA, serotonergic receptor agonists such as DPAT and DOI, and adrenergic receptor blockers such as PHEN and PROP, which, by themselves did not cause memory impairment [8, 28]. The present data showed that SCOP, MECA, DOI, and PROP blocked the improving effects of puerarin (50 mg/kg) on the impairment of memory consolidation produced by CXM treatment in rats (Fig. 2B).
Several researchers have shown that puerarin could cross the BBB and become distributed in various areas of the brain [20, 23]. We further evaluated the effects of i.c.v. injection of puerarin on the CXM-induced impairment of memory consolidation in rats. Administration of puerarin (5 or 10 µg/brain; i.c.v.) before the training trial significantly attenuated the CXM-induced impairment of memory consolidation (Fig. 3A). Furthermore, we investigated the role of monoaminergic systems in the ameliorating effects of puerarin by destroying central monoaminergic systems with central parenchymal injection of monoaminergic neurotoxins 5,-7-DHT or 6-OHDA. The results (Fig. 3A) showed that bilateral injection with 6-OHDA into the locus coeruleus did not affect CXM-induced impairment of a passive avoidance response, but blocked the puerarin from ameliorating the deficit caused by CXM treatment. However, bilaterally injected 5,7-DHT into the dorsal raphe nuclei did not affect CXM-induced impairment of passive avoidance response or the ameliorating effect of puerarin on the CXM-induced impairment (Fig. 3A).
 Fig. 3.
Fig. 3.Effects of puerarin (PUR, 5, 10 µg/brain, i.c.v.) on
cycloheximide (CXM, 1.5 mg/kg, s.c.)-induced or AF64A-induced (B) passive
avoidance response impairment in rats. (A) Absence or presence of
6-hydroxydopamine (6-OHDA) or 5,7-dihydroxytryptamine (5,7-DHT). (B) AF64A. Data
are expressed as mean
The basal forebrain cholinergic system (especially the prefrontal cortex and hippocampus) plays an important role in memory. Therefore, we further evaluated the ameliorating effect of puerarin on AF64A-induced memory impairment in rats. Administration of puerarin (10 µg/brain, i.c.v.) before the training trial significantly attenuated AF64A-induced memory impairment (Fig. 3B).
The results indicated that the ameliorating effect of puerarin on CXM-induced impairment of memory consolidation are related to the catecholaminergic system of the brain, we further measured the monoamine levels (including their metabolites) and transmitter enzyme activity (including AChE, MAO-A and MAO-B) in the prefrontal cortex and hippocampus to clarify the role of neurotransmitter function in the ameliorating effects of puerarin. CXM treatment decreased DA and NE levels in the prefrontal cortex, and also decreased monoamine levels (including DA, NE and 5-HT) and their metabolite levels (including MHPG, DOPAC, HVA and 5-HIAA) in the hippocampus (Table 1). Monoamine turnover rates, which were calculated from the above data (monoamine levels vs their metabolite levels), showed that CXM treatment produced increased both NE and DA turnover rates in the prefrontal cortex; but only increased the NE turnover rate in the hippocampus (Fig. 4). Puerarin treatment restored the decreased NE levels in the prefrontal cortex and the decreased monoamine levels in the hippocampus that had been produced by CXM treatment, but only at the 50 mg/kg puerarin dose (Table 1). Puerarin administration at 25 and 50 mg/kg decreased DOPAC and HVA levels in the prefrontal cortex that had been increased by CXM, but only at the 50-mg/kg dose did puerarin increase the lowered levels of MHPG and 5-HIAA in the hippocampus (Table 1). Puerarin increased the DA levels in the hippocampus of vehicle-treated rats only at the 50-mg/kg dose (Table 1). Puerarin decreased the higher NE and DA turnover rates in the prefrontal cortex and hippocampus only at 50 mg/kg (Fig. 4). When puerarin was administered to vehicle-treated rats, it decreased the DA turnover rates in the prefrontal cortex and hippocampus, but only at the 50 mg/kg dose (Fig. 4).
| MHPG | NE | DOPAC | HVA | DA | 5-HIAA | 5-HT | |
| Monoamines and metabolites in prefrontal cortex (ng/mg) | |||||||
| VEH | 7503.6 |
844.7 |
548.6 |
236.6 |
1308.4 |
615.9 |
603.5 |
| Puerarin 25 mg/kg | 7159.5 |
830.5 |
465.7 |
250.5 |
1155.3 |
598.5 |
488.4 |
| Puerarin 50 mg/kg | 7464.9 |
918.0 |
390.9 |
219.9 |
1265.9 |
613.4 |
624.5 |
| CXM 1.5 mg/kg | 7261.2 |
650.5 |
573.5 |
348.7 |
1094.7 |
750.6 |
598.48 |
| CXM + puerarin 25 mg/kg | 6974.5 |
658.1 |
422.7 |
243.1 |
1079.3 |
655.7 |
577.6 |
| CXM + puerarin 50 mg/kg | 6395.2 |
804.5 |
403.8 |
239.6 |
1200.2 |
645.8 |
646.8 |
| Monoamines and metabolites in hippocampus (ng/mg) | |||||||
| VEH | 996.1 |
354.7 |
61.7 |
111.2 |
30.7 |
294.0 |
189.5 |
| Puerarin 25 mg/kg | 1104.9 |
333.3 |
57.3 |
89.3 |
30.5 |
296.6 |
180.9 |
| Puerarin 50 mg/kg | 1005.6 |
426.4 |
50.4 |
101.4 |
57.2 |
356.4 |
237.2 |
| CXM 1.5 mg/kg | 674.9 |
158.1 |
32.0 |
62.1 |
15.9 |
200.8 |
134.2 |
| CXM + puerarin 25 mg/kg | 723.6 |
211.7 |
33.5 |
70.0 |
17.9 |
233.4 |
125.7 |
| CXM + puerarin 50 mg/kg | 874.3 |
334.6 |
33.9 |
79.9 |
30.7 |
260.9 |
198.3 |
Data were expressed as mean
 Fig. 4.
Fig. 4.Effects of puerarin (PUR, 25, 50 mg/kg, i.p.) on monoamine
turnover rates in the prefrontal cortex and hippocampus of vehicle (VEH)- and
cycloheximide (CXM, 1.5 mg/kg, s.c.)-treated rats. (A) MHPG/NE. (B)
(DOPAC+HVA)/DA. (C) 5-HIAA/5-HT. Data are expressed as mean
CXM increased MAO-A activity in the prefrontal cortex and AChE, MAO-A and MAO-B activity in the hippocampus (Fig. 5). At 25 and 50 mg/kg, puerarin inhibited MAO-A and MAO-B activity in the prefrontal cortex of CXM-treated rats, but it inhibited the CXM-elevated levels of AChE and MAO-B activity in the hippocampus only at 50 mg/kg (Fig. 5). When puerarin was administered to vehicle-treated rats, it decreased AChE and MAO-B activity in the prefrontal cortex and hippocampus only at 50 mg/kg. However, puerarin decreased only hippocampal MAO-A activity in vehicle-treated rats at 50 mg/kg (Fig. 5).
 Fig. 5.
Fig. 5.Effects of puerarin (PUR, 25, 50 mg/kg, i.p.) on the activities
of neurotransmitters degrading enzymes in the prefrontal cortex and hippocampus
of vehicle (VEH)- and cycloheximide (CXM, 1.5 mg/kg, s.c.)-treated rats. (A)
AChE. (B) MAO-A. (C) MAO-B. Data are expressed as mean
Due to fact that puerarin has radical scavenging and antioxidant properties [32, 33], we also measured the antioxidant enzyme activity and GSH levels in the prefrontal cortex and hippocampus to clarify the role of oxidative stress on the attenuating effects of puerarin on CXM-induced impairment of memory consolidation. CXM treatment decreased GSH-recycle system activity including GSH, GPx, and GR in the prefrontal cortex and hippocampus (Fig. 6). We further found that CXM decreased SOD and catalase activity, and increased MDA levels, in the prefrontal cortex and hippocampus (Fig. 7). Puerarin, at 50 mg/kg, restored GSH-recycle system activity and antioxidant enzyme activity in the prefrontal cortex and hippocampus that were decreased by CXM treaatment, and it also decreased the higher MDA levels that had been produced by CXM treatment (Figs. 6,7). In Sham rats, puerarin did not affect GSH-recycle system activity or antioxidant enzyme activity in the prefrontal cortex or hippocampus at any dose (Figs. 6,7).
 Fig. 6.
Fig. 6.Effects of puerarin (PUR, 25, 50 mg/kg, i.p.) on glutathione
(GSH) recycle system activities in the prefrontal cortex and hippocampus of
vehicle (VEH)- and cycloheximide (CXM, 1.5 mg/kg, s.c.)-treated rats. (A) GSH
levels. (B) glutathione peroxidase (GPx) activities. (C) glutathione
reductase (GR) activities. Data are expressed as mean
 Fig. 7.
Fig. 7.Effects of puerarin (PUR, 25, 50 mg/kg, i.p.) on antioxidant
enzyme activities and malondialdehyde (MDA) levels in the prefrontal cortex and
hippocampus of vehicle (VEH)- and cycloheximide (CXM, 1.5 mg/kg, s.c.)-treated
rats. (A) Superoxidase dismutase (SOD) activities. (B) Catalase activities. (C)
MDA levels. Data are expressed as mean
CXM, an antifungal antibiotic, interferes with the translocation step in protein
synthesis of eukaryotic cells to block the synthesis of new proteins needed in
memory formation, and thereby causes retrograde amnesia. Based on the neural
basis of memory consolidation, the cholinergic, serotonergic, and
catecholaminergic systems in the brain play an important role [34, 35, 36]. Nabeshima
et al. [7, 8] demonstrated that CXM treatment produced a
memory-consolidation deficits partially by decreasing cholinergic and
catecholaminergic activity and by increasing serotonergic activity in
experimental animals. Our previous report indicated that puerarin can attenuate
drug-induced learning acquisition by increasing cholinergic activity and by
decreasing serotonergic activity [21]. Other researchers have also suggested that
puerarin ameliorate the behavioral deficits in chronic stress and
ischemia/reperfusion by normalizing serotonergic activity and activating
nicotinic acetylcholinergic activity [22, 37]. This present study found that
puerarin, after systemic administration, attenuated CXM-induced impairment of
memory consolidation, and that cholinergic antagonists, 5-HT
Hippocampal-prefrontal cortex circuity plays an important role in cognitive
function and memory consolidation [24]. This present investigation showed that
CXM treatment mainly decreased all monoamines and their metabolite levels in the
hippocampus, but only decreased NE and DA levels in the prefrontal cortex. CXM
treatment mainly increased AChE, MAO-A and MAO-B activity in hippocampus, but
only increased MAO-A activity in prefrontal cortex. Early reports indicated that
CXM treatment inhibited monoamine synthesis and decreased choline
acetyltransferase (ChAT) in mouse whole brains [43, 44]. The MAO inhibitors
pargyline and pheniprazine produced recovery from CXM-induced impairment of
memory consolidation [45]. We therefore suggest that CXM treatment impairs memory
consolidation partially by decreasing the activity of cholinergic and
monoaminergic systems by disrupting the synthesis and metabolism of acetylcholine
and monoamines (especially NE and DA) in the hippocampal-prefrontal cortex
circuity, especially in the hippocampus. However, some researchers have pointed
out that protein-synthesis inhibitors impair long-term potentiation through the
nitric oxide-dependent signaling pathway to inhibit neurotransmitter release and
cause impairment of memory consolidation [46, 47]. The inhibition of
neurotransmitter release by protein-synthesis inhibitors may be related to
S-nitrosylation enhancement and abnormal farnesylation, which were blocked by GSH
[46]. On the other hand, CXM is considered to be a neurotoxin because an early
report indicated that CXM (at
The findings from this study confirm that treatment with the antifungal antibiotic CXM impairs memory consolidation and causes retrograde amnesia, as indicated by a passive avoidance test. The impairment of memory consolidation caused by CXM treatment may be related to the inhibition of protein synthesis and the decrease in antioxidant-defense-system activation, and it may cause neurotransmitter dysfunction. Puerarin attenuates the toxicity of the antifungal antibiotic CXM, protecting the process for memory consolidation. Puerarin restored CXM-induced impairment of memory consolidation and the consequent retrograde amnesia. The protective mechanism of puerarin against CXM-induced impairment of memory consolidation may be related to decreased oxidative damage and normalization of neurotransmitter function in the prefrontal cortex-hippocampal circuit (Fig. 8).
 Fig. 8.
Fig. 8.Schematic representation of the protective mechanism of puerarin in cycloheximide-induced memory consolidation in passive avoidance test. GSH, glutathione; SOD, superoxidase dismutase; MDA, malondialdehyde.
5-HIAA, 5-hydroxyindoleacetic acid; 5-HT, 5-hydroxytryptamine; 5,7-DHT,
5,7-dihydroxytryptamine; 6-OHDA, 6-hydroxydopamine; AChE, acetylcholinesterase;
ActCH, acetylthiocholine iodide; AD, Alzheimer’s diseae; ChAT, choline
acetyltransferase; CXM, cycloheximide; DA, dopamine; DOI,
1-(2,5-dimethoxy-4-iodophenyl)-2 aminopropane; DOPAC, 3,4-dihydroxyphenyl acetic
acid; DPAT, 8-hydroxy-2-(di-n-propylamino) tetralin hydrobromide; DTNB,
5,5
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
CRW designed the research study. JCL and KJW performed the research. JCL and KJW analyzed the data. CRW wrote the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
All rats handling, manipulation and treatment were conducted according to the Guiding Principles for the Care and Use of Laboratory Animals, and approved by The Institutional Animal Care and Use Committee of China Medical University (CMUIACUC-2018-243).
We would like to express our gratitude to all peer reviewers for their opinions and suggestions.
This research was funded by the Ministry of Science and Technology, grant number 103-2320-B-039-028, 104-2320-B-039-027-MY2, 104-2622-B-039-005-CC2 and 105-2622-B-039-003-CC2.
The authors declare no conflict of interest. Chi-Rei Wu is serving as one of the Editorial Board members of this journal. We declare that Chi-Rei Wu had no involvement in the peer review of this article and has no access to information regarding its peer review. Full responsibility for the editorial process for this article was delegated to Gernot Riedel.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
