1 Department of Neurology, Affiliated Hospital of Nantong University, Medical School of Nantong University, 226001 Nantong, Jiangsu, China
2 Department of Neurology, The Affiliated Wuxi People's Hospital of Nanjing Medical University, 214023 Wuxi, Jiangsu, China
3 Center of Reproduction and Genetics, The Affiliated Suzhou Hospital of Nanjing Medical University, Suzhou Municipal Hospital, Gusu School, Nanjing Medical University, 215006 Suzhou, Jiangsu, China
4 Research Institute for Reproductive Health and Genetic Diseases, Wuxi Maternity and Child Health Care Hospital, Women's Hospital of Jiangnan University, Jiangnan University, 214002 Wuxi, Jiangsu, China
†These authors contributed equally.
Abstract
The utilization of assisted reproductive technologies (ART) is on the rise, resulting in a growing population of ART-conceived offspring. The health concerns of this unique population have attracted significant attention. During ART procedures, gametes and early-stage embryos are exposed to various non-physiological conditions, such as manipulation, culture media, and cryopreservation, which may disrupt embryonic development and potentially impact the health of offspring. Notably, the potential impact of ART on neurodevelopment and its association with an increased risk of neurodevelopmental disorders (NDD) later in life remains a subject of debate. This review aims to summarize the current research advancements concerning the effects of ART on neurodevelopment, specifically focusing on the evidence of the relationship between ART, epigenetic modifications, and NDD, including autism spectrum disorder, intellectual disability, attention deficit hyperactivity disorder, and cerebral palsy. Future studies should prioritize large sample sizes, rigorous adjustment for confounding factors, and the use of interdisciplinary approaches to effectively monitor the neurodevelopmental outcomes of ART-conceived children.
Keywords
- assisted reproductive technology
- epigenetics
- DNA methylation
- offspring neurodevelopment
- neurodevelopmental disorders
Approximately 10–15% of reproductive-aged couples worldwide experience fertility issues, leading to an increasing demand for assisted reproductive technologies (ART) to overcome these challenges [1]. The utilization of ART has led to a significant increase in the population born through these techniques, with an estimated 8 million children conceived by ART as of 2020 [2, 3]. ART procedures involve a series of non-physiological procedures, including manipulations of gametes such as ovulation stimulation, in vitro fertilization, intracytoplasmic sperm injection (ICSI), cryopreservation of sperm and oocytes, as well as manipulations of embryos such as in vitro culture, cryopreservation, and pre-implantation genetic diagnosis. These interventions take place during critical periods of gamete and embryo development, and may have potential adverse effects on gamete maturation, fertilization, and embryo development [4]. The health outcomes of individuals born through ART as a form of artificial environmental exposure have received widespread attention [3], particularly with regard to the potential effects of these procedures on neurodevelopment [5, 6, 7, 8].
The development of the nervous system in early embryonic stages is tightly regulated and highly susceptible to the influence of both endogenous and exogenous factors [9]. Abnormal development of the nervous system can lead to various neurodevelopmental disorders (NDD), including attention deficit hyperactivity disorder (ADHD), intellectual disability (ID), autism spectrum disorder (ASD), and cerebral palsy [10]. The etiology of these disorders is multifaceted and involves genetic, environmental, and immunologic factors [11]. Epigenetic processes have emerged as a critical mechanism underlying the pathogenesis of NDD [12]. Increasing evidence suggests that epigenetic dysregulation, including DNA methylation and histone modifications, disrupts the regulation of gene expression patterns essential for neural development and function, and plays a critical role in the pathophysiology of NDD [13, 14, 15].
The early-life exposure to adverse environmental factors, such as stress, drug exposure, and malnutrition, can have a significant impact on epigenetic regulation during critical periods of life [16]. The utilization of diverse ART procedures during critical embryonic developmental periods may have implications for the fetal epigenome, posing concerns regarding potential disruptions in development and long-term health effects [16]. Some investigations have reported a higher frequency of alterations in DNA methylation patterns at imprinting control centers in children conceived through ART compared to those conceived naturally [17, 18]. A recent study found significant differences in DNA methylation and genes related to neurodevelopment between ART and naturally conceived newborns [18]. However, a Nordic register study indicated minimal variations in neurodevelopmental outcomes between ART and non-ART singletons, despite the fact that singletons in the ART cohort exhibited higher adjusted risks of learning and motor functioning disorders (hazard ratio [HR], 1.17 [1.11–1.24]), a tendency towards a higher risk of ASD (HR, 1.07 [0.98–1.16]), and ADHD (HR, 1.17 [0.99–1.12]) [6]. The strength of the evidence for these associations remains limited and inconsistent, with controversy surrounding their validity.
This review aims to summarize the current evidence regarding the potential association between ART and NDD in offspring. We will explore different types of ART and their impact on NDD, focusing on potential mechanisms that contribute to these risks through epigenetic regulation (Fig. 1). Additionally, we will provide insights into future directions for refining ART protocols to ensure optimal health outcomes for offspring.
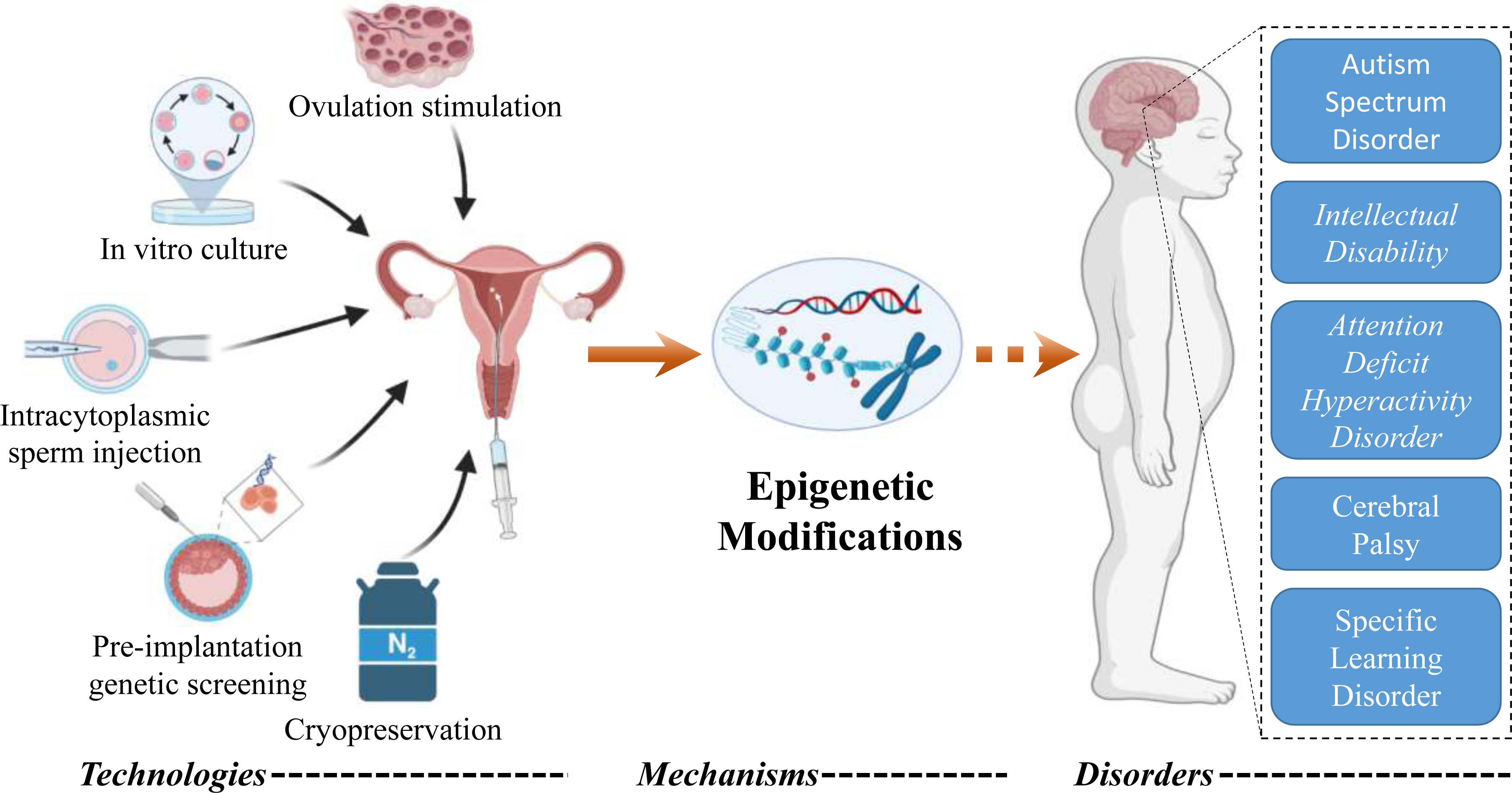 Fig. 1.
Fig. 1.Schematic illustration of the association between assisted reproductive technologies (ART) and neurodevelopmental disorders (NDD). Various ART procedures may increase the risk of NDD, and epigenetic modifications are believed to be one of the potential mechanisms underlying this increased risk.
Advances in reproductive technology have revolutionized the ability to conceive for many couples facing fertility issues. However, these technologies have raised concerns about potential health impacts on the offspring. During critical periods of development, the nervous system exhibits a heightened sensitivity to environmental factors, and epigenetic modifications play a crucial role in regulating gene expression within this context.
ART encompasses a variety of medical interventions aimed at helping couples overcome infertility and related reproductive problems. These interventions include artificial insemination (AI), in vitro fertilization (IVF), ICSI, and embryo transfer, as well as related techniques such as ovarian stimulation, gamete and embryo cryopreservation, pre-implantation genetic screening (PGS), and assisted hatching (AH). Ovarian stimulation involves the use of medication to encourage egg development and release, aimed at increasing the chances of conception in cases of infertility [19]. ICSI is a specialized form of IVF where a single sperm is directly injected into an oocyte to increase the likelihood of fertilization, and is primarily used for cases of severe male infertility [20]. Gamete and embryo cryopreservation involves the freezing and storage of oocytes, sperm, or embryos for later use in assisted reproductive technologies [21]. PGS is a genetic screening test that can be used to detect genetic abnormalities in embryos prior to implantation [22]. AH is a technique used to assist the hatching of embryos in order to facilitate implantation [23].
These techniques involve invasive interventions to manipulate gametes and embryos in vitro and in vivo, creating a pathway for successful conception and providing an optimal environment for gamete fusion. However, the use of hormones, the extraction and storage of gametes, gamete maturation, in vitro embryo culture, cryopreservation, and microsurgery all involve non-physiologic interventions. All of these may interfere with gamete and embryo development, and the overall pregnancy environment, and pose risks to the safety of offspring, leading to short-term and long-term complications [3, 24]. Short-term complications can include a higher risk of preterm birth, low birth weight, and neonatal complications. Furthermore, there is ongoing research exploring the potential long-term complications, which may involve effects on various systems such as the cardiovascular, metabolic, and nervous systems. With the increasing number of children born through ART reaching an estimated 8 million, there is a rising concern regarding the possibility of birth defects and developmental outcomes, particularly in relation to neurodevelopment outcomes.
Multiple pregnancies following ART treatments have been reported to have an incidence rate ranging from 17% to 29% [25]. This upward trend is primarily attributed to the transfer of multiple embryos, aiming to maximize the likelihood of successful implantation and pregnancy. However, it is important to note that multiple pregnancies are associated with an elevated risk of complications, including preterm birth, low birth weight, and neonatal complications, which can have detrimental implications for the health outcomes of both the mothers and infants [25, 26].
Neurodevelopment refers to the complex biological processes that shape the development of the nervous system, including the formation of neurons, synapses, and neural circuits, as well as the establishment of neuronal communication pathways [27]. These processes begin in utero and continue throughout childhood and adolescence, ultimately shaping the structure and function of the brain. Environmental factors, such as maternal stress, infection, and drug exposure, can also influence neurodevelopment [28, 29].
Neurodevelopmental disorders (NDD) are a group of conditions that arise from disruptions in the normal processes of neurodevelopment, leading to alterations in the structure and function of the brain [10]. These disorders can have a range of effects on cognitive, emotional, and behavioral functioning, and can manifest in a variety of ways, including difficulties with social interaction, communication, and motor skills [30]. Examples of NDD include autism spectrum disorder (ASD), intellectual disability (ID), attention deficit hyperactivity disorder (ADHD), neurodevelopmental motor disorders, and specific learning disorders [31].
The etiology of NDD is complex and multifactorial, involving a combination of genetic, environmental, and epigenetic factors [11, 32]. Environmental factors, including maternal stress, infection, and drug exposure, can also influence neurodevelopment. It has been reported that maternal exposure to alcohol during pregnancy results in fetal alcohol spectrum disorders, which can lead to various cognitive and behavioral impairments [33]. Recent research has suggested that ART may also contribute to the development of these disorders, possibly through the use of fertility drugs, alterations in early embryonic development, or epigenetic modifications [3, 34]. While the exact mechanisms of these effects are still being investigated, the potential risks associated with ART highlight the need for ongoing monitoring and research in this area.
Epigenetics is an emerging field that investigates stable and heritable alterations in gene expression, independent of changes in the DNA sequence. The term epigenetics encompasses a range of molecular modifications to DNA and associated proteins, including DNA methylation, histone modification, genomic imprinting, chromatin remodeling, and non-coding RNA-mediated regulation [35]. DNA methylation is one of the extensively studied epigenetic mechanisms, involving the addition of a methyl group to a DNA molecule, potentially altering its expression [36]. In DNA methylation, cytosines in CpG dinucleotides are targeted for methylation, with a methyl group added to the carbon 5 position of the cytosine ring. This modification plays a critical role in gene expression regulation, impacting processes like development, imprinting, and disease susceptibility. Methylation of CpG sites can silence genes by hindering the binding of transcription factors and other regulatory proteins to the DNA sequence. Conversely, hypomethylation of CpG sites can activate genes, leading to increased transcriptional activity. Abnormal DNA methylation patterns, such as global hypomethylation or regional hypermethylation, are associated with diseases such as cancer, neurodevelopmental disorders, and infertility [37].
Emerging evidence suggests that epigenetic regulation, through mechanisms such as DNA methylation, non-coding RNAs, and histone modifications, plays a crucial role in neurodevelopment [38, 39, 40]. Aberrant epigenetic modifications have been linked to various NDD, such as ASD [41] and ID [42]. Of note, ART procedures occur during the critical period of epigenetic reprogramming in gametes and early embryonic development [43]. These procedures may interfere with epigenetic gene regulation in gametes or early embryos, leading to abnormal neurodevelopment and subsequent neurodevelopmental disorders [5].
Overall, understanding the relationships between ART, NDD, and epigenetics is crucial for identifying potential health risks and developing strategies to minimize any negative impacts on offspring.
As the use of ART continues to increase, there is a growing body of research aimed at evaluating the health outcomes of individuals born through this technology, including neurodevelopmental outcomes. In the following sections, we have summarized the association between ART and ADHD, ASD, ID, and other NDD (Table 1, Ref. [44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70]), to gain a better understanding of the complex relationship between ART and NDD.
| NDD | ART type | Study design | N (case) | N (comparison) | Confounders adjusted | Risk | Reference |
| ADHD | IVF | Cohort | 28,158 | 2,417,886 | Maternal age, education, smoking, BMI, parity, and birth year, | Modestly increased | [44] |
| IVF/ICSI | Cohort | 14,991 | 555,828 | Maternal age, education, smoking, parity, psychiatric history, birth year, child’s sex, and multiplicity, mode of conception | No correlation | [45] | |
| OI/IUI | Cohort | 18,148 | 555,828 | Modestly increased | [45] | ||
| ART | Cohort | 46,868 | 1,450,254 | Parental characteristics and health history | Decreased | [46] | |
| ART | Cohort | 94,206 | 805,748 | Maternal age, smoking, obesity, parity, drug or alcohol use, income quintile, rurality, immigration status, history of illness and child’s sex, mode of conception | No correlation | [47] | |
| ICSI | Cohort | 3825 | 1,568,257 | Maternal age, paternal age, gestation status, and child’s sex | Increased | [48] | |
| ART | Cohort | 2057 | 6050 | Parental mood disorders, mode of conception | No correlation | [49] | |
| IVF | Case–Control | 139 | 143 | Maternal education, gestational age, parity, and birth weight | No correlation | [50, 51] | |
| ART | Cohort | 71 | 1577 | Maternal age, education, smoking, alcohol, parity, intelligence, BMI, child’s gender and age | No correlation | [52] | |
| ASD | ART | Case–Control | 473 | 473 | Maternal age, parity, multiplicity, country of origin, child’s birth weight, gender and age | Decreased | [53] |
| IVF or OI | Cohort | 33,139 | 555,828 | Maternal age, education, parity, smoking, child’s birth weight and multiplicity, mode of conception | No correlation | [54] | |
| IVF | Case–Control | 4164 | 16,353 | Maternal socioeconomic status and parity | No correlation | [55] | |
| IVF/ICSI | Cohort | 30,959 | 2,579,916 | Parental age and psychiatric history | No correlation | [56] | |
| ART | Cohort | 48,865 | 5,877,386 | Maternal age, parity, race, education, birth year, child’s gender and place of birth | Modestly increased | [57] | |
| ART | Cohort | 124,269 | 2,288,452 | Parental age at birth and history of mental disorder, birth year, child’s birth order, sex | Increased | [58] | |
| IVF | Cohort | 975 | 107,573 | Parental age at birth, birth year, district of residence, immigration status, smoking, clinical histories and infertility factors | Increased | [59] | |
| ART | Cohort | 10,147 | 441,898 | Maternal demographics, smoking, insurance, parity, gender, method of delivery, chronic and pregnancy hypertension, gestational and chronic diabetes, breech | No correlation | [60] | |
| ART | Case–Control | 100 | 200 | Maternal age at birth and the risk of ASD, child’s sex, delivery mode, history of preterm delivery, history of using ART | No correlation | [61] | |
| ID | ICSI | Case–Control | 97 | 110 | - | No correlation | [62] |
| IVF/ICSI | Cohort | 14,991 | 555,828 | Maternal age, parity, education, smoking, maternal psychiatric history, birth year, multiplicity, and child’s sex, mode of conception | No correlation | [45] | |
| OI/IUI | Cohort | 18,148 | 555,828 | No correlation | [45] | ||
| ICSI | Cohort | 11,514 | 2,510,166 | Parental age and psychiatric history | Modestly increased | [56] | |
| PGD | Case–Control | 47 | 48 | Maternal age at birth | No correlation | [63] | |
| ART | Cohort | 3107 | 208,800 | Maternal age, parity, delivery mode, private health insurance, marital status, and child’s sex | Increased | [64] | |
| CP | IVF | Cohort | 31,614 | 2,623,514 | Maternal age, birth year, parity, and smoking | No correlation | [65] |
| IVF | Cohort | 9255 | 394,713 | Maternal age, gender, parity, small-for-gestational age status, education, multiplicity or preterm delivery | Increased | [66] | |
| IVF or OI | Cohort | 33,139 | 588,967 | Maternal age, education, smoking, parity, and child’s sex, mode of conception | Increased | [67] | |
| ART | Cohort | 2893 | 208,571 | Maternal age, birth year, and parity | Increased | [68] | |
| SLD | ICSI | Case–Control | 40 | 40 | - | No correlation | [69] |
| ICSI | Cohort | 276 | 273 | Parental age, social class, and child’s age, sex | No correlation | [70] |
NDD, Neurodevelopmental disorders; ASD, Autism Spectrum Disorder; ADHD, Attention Deficit Hyperactivity Disorder; ID, Intellectual Disability; CP, Cerebral Palsy; SLD, Specific Learning Disorders; ART, assisted reproductive technologies; OI, ovulation induction; IUI, intrauterine insemination; IVF, in vitro fertilization; PGS, preimplantation genetic screening; ICSI, intracytoplasmic sperm injection; BMI, body mass index.
ADHD is a childhood-onset neurodevelopmental disorder characterized by inattention, hyperactivity, and impulsivity. It is a highly prevalent disorder, with estimates of a worldwide prevalence of around 5% [71]. Individuals with ADHD often experience challenges with executive functioning skills, including planning, organization, and time management. They may also struggle with emotional regulation and social skills. The causes of ADHD are multifactorial, with both genetic and non-inherited factors contributing to this disorder. While prenatal and perinatal factors have been identified as potential risk factors, the definitive causes of ADHD still remain unknown [72].
Here, we summarize several studies that investigated the potential effects of ART on the health and development of children, specifically with regard to ADHD. Wagenaar et al. [50] conducted a study evaluating the psychosocial well-being of IVF children and found normal behavior and socioemotional functioning, while their study [51] also indicated that children conceived through IVF exhibited normal cognitive abilities as assessed through neuropsychological tests. Similarly, Bay et al. [52] reported that parental subfertility and fertility treatment were not significantly associated with offspring intelligence, attention, or executive functions. However, Källén et al. [44] discovered a statistically significant but weak association between IVF and drug-treated ADHD, with an odds ratio of 1.18 (95% CI [confidence interval] 1.03–1.36), and the effect was observed to be stronger in girls (odds ratio [OR] = 1.40) compared to boys (OR = 1.11). Nevertheless, after adjusting for the length of involuntary childlessness, the association lost statistical significance. In a population-based cohort study by Fine et al. [47], the prevalence of ADHD was found to be 7.0% in the unassisted conception group, 7.5% in infertility without fertility treatment group, 6.8% in the OI/IUI (OI, ovulation induction; IUI, intrauterine insemination) group, and 6.3% in the IVF/ICSI group. These findings suggest that infertility itself may be associated with ADHD in offspring, while the use of fertility treatment did not amplify the risk.
The significant impact of various environmental factors on the development of ADHD in children is evident from the available evidence. These factors include maternal smoking, maternal alcohol and drug addiction during pregnancy, low birth weight and intrauterine growth restriction [73, 74, 75]. Thus, when exploring the possible correlation between ART and ADHD, it is crucial to consider these confounding factors. Although current evidence suggests that ART does not significantly affect cognitive function or psychosocial well-being, more research is necessary to fully comprehend the long-term effects of ART on ADHD.
ASD, a neurodevelopmental disorder with high heritability and heterogeneity, is characterized by difficulties in social communication and interaction, sensory abnormalities, repetitive behaviors, and varying levels of intellectual functioning. People with ASD often have co-occurring psychiatric or neurologic disorders such as hyperactivity and attention disorders, anxiety, depression, and epilepsy [76].
Numerous studies conducted globally have investigated the correlation between ART and ASD, with controversial results. Some studies have reported that ART is generally safe and does not increase the risk of ASD [54, 55, 56, 60, 61]. For instance, a population-based follow-up study conducted in Denmark, which included 588,967 children born between 1995 and 2003, reported no elevated risk of ASD in children born following assisted conception (adjusted hazard risk ratio 1.13, 95% confidence interval 0.97 to 1.31) [54]. Similarly, a case-control study conducted in Finland, involving 4164 individuals with ASD and 16,582 matched controls born between 1991 and 2005, revealed no significant association between IVF and ASD (adjusted odds ratio (OR): 0.9, 95% confidence interval (CI): 0.7–1.3), as well as its subtypes (OR: 0.8, 95% CI: 0.4–1.5). [55]. However, some studies have identified certain risk factors associated with ART and ASD [57, 58, 59]. For example, a study in the USA found a link between ASD and female infertility diagnoses, particularly conditions such as fallopian tube disorders and endometriosis [77]. Another study in Israel found that progesterone treatment was associated with a significantly higher risk of ASD (Relative Risk = 1.51, 95% CI 1.22–1.86) compared to the group without progesterone treatment [59]. Interestingly, a Danish population-based matched case-control study indicated that children conceived after assisted conception had a 59% lower risk of developing infantile autism compared to their matched controls [53]. This reduced risk could potentially be attributed to the better health status of the mothers and their improved access to healthcare during pregnancy.
Recently, a study was conducted through a search strategy that obtained 587 studies, among which 16 were included in the systematic review, and 15 were included in the meta-analysis [78]. Analysis of the subset of studies that examined all offspring and accounted for confounding factors revealed a higher risk of ASD associated with the use of ART, with a relative risk (RR) of 1.11 (95% CI 1.03–1.19). However, when focusing specifically on studies involving singletons, no statistically significant association between ART and ASD was observed, with an RR of 0.96 (95% CI 0.82–1.13). These findings should be interpreted cautiously, and additional large-scale, well-designed prospective studies with a larger sample size are necessary to gain a comprehensive understanding of the potential relationship between ART and ASD.
ID, also known as intellectual developmental disorder, is a neurodevelopmental condition characterized by significant limitations in intellectual functioning and adaptive behavior. The diagnosis of ID is based on standardized intelligence tests and assessments of adaptive behavior, which are used to identify limitations in communication, self-care, social skills, and other daily living activities. The severity of ID can be classified into four levels, ranging from mild to profound, based on Intelligence Quotient (IQ) score and adaptive behavior [79]. The etiology of ID is multifactorial and may involve genetic, environmental, and other factors such as chromosomal abnormalities, metabolic disorders, prenatal exposure to toxins, infections, and malnutrition [80].
A high-quality systematic review conducted by Rumbold et al. [81] aimed to investigate the impact of fertility treatments in school-aged children on cognitive outcomes, specifically intellectual functioning and adaptive behaviors. The review also explored potential differences in effects among specific fertility treatments. The findings suggested that cognitive outcomes were not significantly different between children conceived through IVF and those conceived naturally. However, ICSI may have a detrimental effect on cognitive development compared to conventional IVF (RR, 4.60 [95% CI, 2.14–9.88]; 135.7 vs 29.3 per 100,000 person-years) [56], with some studies reporting a higher risk of mental retardation and lower IQ scores among children conceived with ICSI (normal range: mean = 98.2, SD = 12.2; ICSI children: IQ = 94.1, SD = 13.8; IVF children: IQ = 102.0, SD = 9.1) [82]. In contrast, frozen embryo transfer and donor insemination did not seem to have a significant effect on cognitive development [56, 83]. The review highlighted the methodologic limitations of the available evidence, such as small sample sizes and retrospective designs, which call for further research with rigorous study designs, larger sample sizes, and more complete data to better understand the impact of fertility treatments on cognitive outcomes [81].
Thus, while the current evidence suggests that ART techniques may have some impact on children’s ID, further research is needed to fully understand the nature and magnitude of these effects.
In addition to ASD, ID, and ADHD, the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) recognizes several other NDD, including neurodevelopmental motor disorders and specific learning disorders. Neurodevelopmental motor disorders, such as cerebral palsy (CP), impact an individual’s motor coordination and posture control. In two recent studies, a cohort study conducted in Western Australia [68] and a meta-analysis of nine studies [84], it was found that children conceived by ART have a greater than two-fold increased risk of CP compared to children conceived naturally. The studies have identified preterm birth and multiple births as the primary factors responsible for the increased risk of CP in ART children. Specific learning disorders, also known as learning disabilities, are closely related to an individual’s cognitive development. A study conducted on mice born through ICSI exhibited deficits in discrimination, response acquisition and memory compared to naturally conceived control mice, with sexual dimorphism observed in some cognitive outcomes [85]. However, Liapi et al. [69] found that children through ICSI and spontaneous conception exhibit similar cognitive and socioemotional development, with comparable chances of experiencing learning disabilities.
In summary, the association between ART and NDD remains a topic of controversy. The evidence suggests that ART may increase the risk of CP, particularly in children born from multiple births and preterm deliveries. The impact of ART on ADHD, ASD, and ID is less clear, with conflicting results reported across multiple studies.
The etiology of NDD in ART-conceived offspring is multifactorial and complex, involving a convergence of genetic, epigenetic, environmental, and procedural factors. The background of couples undergoing ART, including genetic predispositions, pre-existing conditions, and lifestyle factors, can significantly influence the neurodevelopmental outcomes of their offspring. To study the association between ART procedures and NDD, it is crucial to consider and address these multifactorial influences.
The influence of maternal age and endocrinopathies on offspring disorders has been extensively investigated. Bergh et al. [86] emphasize that advanced maternal age is associated with elevated infertility rates, decreased pregnancy rates, and heightened risks of congenital malformations, chromosomopathies, metabolic diseases, and neurological disorders in offspring. Vanky et al. [87] review the detrimental effects of maternal polycystic ovary syndrome (PCOS) on infant and childhood growth, reproductive health, cardiometabolic health, and neurodevelopment in offspring. Nattero-Chavez et al. [88] investigated the consequences of maternal thyroid disorders and diabetes on offspring, highlighting connections with neurologic and behavioral conditions. Additionally, fetal exposure to hyperglycemia caused by maternal diabetes mellitus or gestational diabetes mellitus is associated with metabolic effects and an elevated risk of neurodevelopmental disorders and cardiovascular factors in adulthood [88].
Extensive research has demonstrated a negative correlation between male aging and various key factors related to fertility, including sperm parameters, reproductive hormone levels, and testicular function [89]. With advancing male age, one proposed mechanism suggests a gradual decline in the efficiency of the antioxidant defense system, leading to an elevation in the levels of reactive oxygen species (ROS) within sperm [90]. Consequently, this oxidative stress contributes to a decline in conventional semen parameters, such as semen volume, total sperm count, motility, and morphology, while also resulting in an increase in DNA fragmentation. Notably, this is just one of several theories regarding age-related events in male fertility. These age-related changes in sperm quality have a significant impact on male infertility and have adverse effects on the health of offspring. Moreover, advanced paternal age is intricately associated with notable genetic and epigenetic changes occurring within sperm [91]. These changes encompass sperm DNA damage, chromosomal abnormalities, heightened DNA fragmentation, and an increased incidence of single gene mutations. Notably, advanced paternal age is linked to an elevated susceptibility to neurodevelopment disorders, such as autism spectrum disorders and schizophrenia [92]. It is postulated that these associations arise from genetic defects and epigenetic modifications within sperm as the driving mechanisms.
Various lifestyle factors, including parental choices, have been demonstrated to
significantly influence reproductive outcomes and the long-term well-being of
offspring. Maternal lifestyle factors, such as diet, smoking, and alcohol
consumption, have a notable impact on epigenetic pathways, leading to lasting
effects on neurodevelopment in offspring [93, 94]. The benefits of incorporating
folic acid supplements during the periconception period to prevent neural tube
defects and promote normal brain development are well-established. However, it
has been observed that maternal consumption of folic acid supplement dosages
exceeding the Tolerable Upper Intake Level (
Given the complexity involved in infertility research and the use of ART, it is crucial to thoroughly consider and address potential confounding factors when studying the association between ART procedures and NDD in offspring. Several key confounding factors need to be taken into account in this context, including male and female infertility, environmental and lifestyle conditions, and age-related effects.
To mitigate the influence of these confounding factors, researchers can employ various strategies. First and foremost, the selection of an appropriate study design, such as prospective cohort studies or randomized controlled trials, is crucial to minimize confounding bias. Additionally, the inclusion of suitable control groups is essential. The use of control groups consisting of children from the general population may introduce bias due to the inherent association between infertility and adverse child outcomes [47]. It is advisable to consider couples with reduced fertility as the control group [18], or utilize sibling studies where the same mother gives birth to both ART-conceived and naturally conceived children [97, 98]. Statistical techniques, such as multivariable regression models, can be employed to adjust for confounding variables and estimate the independent effect of ART on NDD while controlling for potential confounders. Conducting sensitivity analyses by systematically varying the inclusion or exclusion of specific confounding factors can assess the robustness of the results and evaluate their impact on the observed associations. Furthermore, stratification and matching techniques based on important confounding variables such as maternal age or genetic factors can provide further insights into these factors and help reduce confounding effects.
In conclusion, by carefully considering and addressing these confounding factors through appropriate study design, rigorous statistical analysis, and comprehensive sensitivity analysis, researchers can enhance the validity and reliability of their findings regarding the association between ART procedures and NDD in offspring.
ART has been shown to potentially disrupt the epigenetic reprogramming required for normal embryonic growth and development [38, 99]. Sensitive periods in fetal brain development are highly vulnerable to environmental influences, and disruptions to the critical epigenetic processes during these stages may increase the risk of NDD associated with ART procedures. Therefore, understanding the impact of ART on epigenetic processes is crucial in elucidating the potential association between ART and NDD.
Each component of ART, including ovulation stimulation, modes of fertilization, and manipulation of gametes and embryos, may act as environmental factors leading to epigenetic modification errors. Jiang et al.’s [100] review highlights potential epigenetic disruptions in children born through ICSI in ART, including Angelman syndrome and Beckwith-Wiedemann syndrome. Embryo cryopreservation and thawing technology, which is often used in ART to store surplus embryos for future use, can also impact the epigenetic landscape of the embryo [101]. Hiura et al. [102] found that frozen-thawed embryo transfer is associated with the downregulation of four miRNAs in term placenta as compared to fresh embryo transfer and spontaneous pregnancy, indicating increased exposure of the epigenome to external influences.
Animal studies have played a vital role in assessing the safety and effectiveness of ART in humans and understanding the potential mechanisms underlying the impact of ART on NDD in offspring. These studies have provided valuable insights into the epigenetic alterations that occur in gametes and embryos as a result of procedures such as superovulation, in vitro embryo culture, and embryo manipulation. For example, it has been reported that the use of exogenous hormones in ART can alter the gene expression and epigenetic patterns of imprinted genes such as H19, Insulin-like growth factor 2 (Igf2), and Cdkn1c in the organs of mice fetuses [103]. Additionally, studies have shown increased expression of protective proteins against oxidative stress in IVF blastocysts and adult tissues of the offspring, accompanied by enhanced histone H4 acetylation at the promoter region [104]. This finding suggests a direct association between epigenetic modifications in embryos and subsequent effects on adult tissues. Furthermore, it has been observed that ICSI induces more significant epigenetic changes compared to conventional IVF, impacting gene transcription and methylation patterns of genes involved in imprinting, X-linked inheritance, and retrotransposons [105].
The association between NDD and epigenetic changes in the context of ART is complex and influenced by various factors. It has been observed that ART procedures can lead to methylation anomalies, particularly during the preimplantation stages, where there is a critical need for proper methylation maintenance. Market Velker et al. [106] have clearly indicated that the culture media used in ART do not adequately meet the requirements of developing embryos, potentially leading to an inability to maintain proper methylation and resulting in greater perturbations in genomic imprinting and metabolic marker expression. Animal studies have provided insights into this phenomenon, suggesting that blastomere biopsy, a technique used in preimplantation genetic diagnosis, can impair nervous development and function in mice [107]. This impairment may be attributed to compromised epigenetic reprogramming during early embryo development, leading to a hypomethylation state in the brain [107]. Additionally, a cord blood DNA methylation analysis in a large number of newborns has revealed widespread differences in DNA methylation, with ART-conceived newborns showing overall less methylation across the genome and differences in 176 known genes related to growth, neurodevelopment, and other health outcomes [18]. Epigenetic modifications, such as altered methylation patterns of these genes, have been associated with the development of NDD [38, 108].
Epigenetic modifications and regulators are potential biomarkers for predicting disease development, screening, and monitoring therapeutic interventions [109]. Disease-specific epigenetic changes, particularly those related to DNA methylation, can be used to evaluate embryo quality and reproductive potential [110]. The advancements in single-cell multiomics sequencing technology have allowed for the development of preimplantation methylation screening as a technique used to screen embryos for DNA methylation abnormalities before implantation [111]. This technique enables a more precise and comprehensive analysis of DNA methylation patterns in individual cells of embryos, which holds the potential to improve the success rates of IVF and reduce the risk of developmental disorders in ART-conceived offspring [111]. However, additional research is necessary to fully evaluate the efficacy and safety of this technology.
In summation, the impact of ART on neurodevelopment is complex and multifactorial, and epigenetic mechanisms offer a promising avenue for understanding the molecular link between ART and NDD. Given the widespread use of ART for infertility treatment, it is crucial to investigate the potential long-term effects of ART procedures on the epigenome and health of offspring. The exploration of the connection between ART and its impact on epigenetic regulatory mechanisms presents an attractive opportunity for developing strategies to reduce the risk of NDD and improve overall health outcomes for ART-conceived children.
ART has emerged as a widely accepted and valuable set of techniques in modern medicine, providing a range of reproductive options for couples experiencing infertility. The expanding use of ART, coupled with the growing number of ART-conceived individuals, necessitates greater attention, research, and monitoring of their health outcomes.
ART is generally regarded as a safe procedure, but conflicting data exist regarding the risk of NDD in ART-conceived children, likely due to multiple factors. One well-known factor is the increased rate of multiple pregnancies associated with ART, which is a risk factor for adverse perinatal outcomes such as premature birth and low birth weight, both being risk factors for NDD. As such, future studies should involve large sample sizes, multiple centers, and adjust for confounding factors. Additionally, it is crucial to carefully select appropriate ovulation stimulation strategies and reduce the number of transferred embryos to decrease the occurrence of multiple pregnancies.
Epigenetic mechanisms provide a means of explaining the molecular link between ART and NDD. The potential effects of ART procedures on epigenetic processes and subsequent impacts on offspring growth and development are important areas of research. Preimplantation methylation screening was created with the aim of identifying embryos that possess superior genetic and gene regulatory attributes, indicating that this technology may help reduce the risk of unfavorable outcomes in children conceived through ART. The development of such techniques offers a promising opportunity to address the potential effects of ART on the health of offspring by selecting high-quality embryos that are more likely to result in healthy pregnancies and successful deliveries.
The developmental origins of health and disease (DOHaD) theory suggests that adverse events exposure during critical periods of development can have profound impacts on an individual’s health outcomes. Therefore, long-term observation and follow-up studies of ART-conceived individuals are necessary to better understand any potential long-term effects. Additionally, a multidisciplinary approach involving reproductive and neurology physicians along with researchers is essential to effectively monitor the neurodevelopment and related health outcomes of ART-conceived offspring.
In conclusion, ART has revolutionized infertility treatment, but it is important to recognize potential long-term consequences for offspring’s health and development. Future investigations should prioritize large sample sizes, rigorous adjustment for confounding factors, and a multidisciplinary approach to effectively monitor the health of ART-conceived children. Understanding the impact of ART on epigenetic processes can assist in efforts to minimize potential risks associated with these technologies.
Conceptualization: YL, YY and ZZ; Writing-original draft preparation, literature search, figures: ZZ, ZW, PY, YW, YP and YD; Writing-review and editing: ZZ, YY and YL; Critical review and final approval: YL and YY. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
Not applicable.
We gratefully acknowledge the editors and peer reviewers for their contributions to this work.
We thank the support of the Wuxi Health Commission Project (Nos. Q202111).
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.

