1 Bioengineering Program, University of Kansas, Lawrence, KS 66045, USA
2 Landon Center on Aging, University of Kansas Medical Center, Kansas City, KS 66160, USA
3 Neurosurgery, University of Kansas Medical Center, Kansas City, KS 66160, USA
4 Molecular & Integrative Physiology, University of Kansas Medical Center, Kansas City, KS 66160, USA
5 Rehabilitation Medicine, University of Kansas Medical Center, Kansas City, KS 66160, USA
Abstract
Background: The purpose of this proof-of-concept feasibility study was to determine if spike-triggered intraspinal microstimulation (ISMS), a form of activity dependent stimulation (ADS), results in improved motor performance in an ambulatory rat model of spinal cord injury (SCI). Methods: Experiments were carried out in adult male Sprague Dawley rats with moderate thoracic contusion injury. Rats were assigned to one of two groups: Control or ADS therapy. Four weeks post-SCI, all rats were implanted with a recording microelectrode in the left hindlimb motor cortex and a fine-wire stimulating electrode in the contralateral lumbar spinal cord. ADS was administered for 4 hours/day, 4 days/week, for 4 weeks. During therapy sessions, single-unit spikes were discriminated in real time in the hindlimb motor cortex and used to trigger stimulation in the spinal cord ventral horn. Control rats were similarly implanted with electrodes but did not receive stimulation therapy. Results: Motor performances of each rat were evaluated before SCI contusion, once a week post-SCI for four weeks (prior to electrode implantation), and once a week post-conditioning for four weeks. Basso, Beattie, and Bresnahan (BBB) locomotor scores were significantly improved in ADS rats compared to Control rats at 1 and 2 weeks after initiation of therapy. Foot fault scores on the Horizontal Ladder were significantly improved in ADS rats compared to pre-therapy ADS and Control rats after 1 week of therapy and recovered to near pre-injury scores after 3 weeks of therapy. The Ledged Beam test showed deficits after SCI in both ADS and Control rats but there were no significant differences between groups after 4 weeks of ADS therapy. Conclusions: These results show that chronic stimulation after spinal cord injury using a methodology of spike-triggered ISMS enhances behavioral recovery of locomotor function as measured by the BBB score and the Horizontal Ladder task. However, it is still uncertain if the behavioral improvements seen were dependent on spike-triggered ISMS.
Keywords
- activity dependent stimulation
- neuromodulation
- spinal cord injury
- closed-loop
- spike-triggered intraspinal microstimulation
After injury to the spinal cord, chronic motor deficits result from local necrosis of spinal cord neurons and fiber tracts, including disruption of communication between neurons in the motor cortex and spinal cord motor neurons. As a result, impairment severity is related to the function of remaining viable neurons. By focusing on spared neurons and their pathways after injury, a primary goal of current restorative research is to leverage the nervous system’s intrinsic capacity for reorganization. One innovative device-based approach utilizes activity-dependent stimulation (ADS) paradigms that record and digitize extracellular neural activity from an implanted microelectrode, discriminate individual action potentials in real time, and deliver small amounts of electrical current to another microelectrode implanted in a remote population of neurons [1, 2, 3, 4, 5]. This approach is based on mechanisms underlying neuroplasticity [6, 7]. ADS paradigms aim to strengthen the remaining pathways after injury by inducing neuroplasticity to restore or improve lost motor function.
This lack of descending motor control from the brain to neurons below the injury in the spinal cord contributes to motor dysfunction. However, even without input from the brain, studies have shown that circuits located below the injury, specifically in the lumbar segments of mammals, can fabricate coordinated patterns of leg motor activity [8, 9]. As a result, researchers have developed various neuromodulatory approaches to reconnect brain signals to the spinal cord motor neurons by “closing the loop” with brain-machine interfaces [5]. Recent studies using closed-loop interfaces between the brain and spinal cord have demonstrated improvement in locomotion [3] and functional upper limb movement [10] after spinal cord lesions; potentially via strengthened connections or enhanced plasticity in descending motor pathways.
These earlier efforts inspired us to develop an understanding of the connection between the brain and spinal cord after a bilateral thoracic spinal cord contusion that mimics those seen in clinical spinal cord injuries [11, 12, 13, 14]. Using spinal cord injury (SCI) models, we can create neuromodulation protocols that can more easily translate into clinical use for rehabilitation. This framework guided the design of a brain-machine-spinal cord interface (BMSI) that uses ADS to strengthen motor pathways that may still be intact after SCI [15]. ADS administered in a closed-loop manner uses neural activity to trigger stimulation. In our ADS paradigm, an implanted recording microelectrode in the hindlimb motor cortex records and digitizes extracellular neural activity, discriminates individual action potentials in real time, and delivers small amounts of electrical current to another stimulating microelectrode implanted in the ventral horn of the lumbar spinal cord distal to the level of the injury.
The ability of the motor cortex to evoke spinal cord activity below the level of an injury demonstrates that some descending motor pathways to the lumbar cord remain intact after a moderate spinal cord contusion [13]. Furthermore, the administration of ADS between the hindlimb motor cortex and hindlimb spinal cord has shown that ADS causes a plastic change in the intact pathways of rats with a thoracic contusion to the spinal cord [14]. The current feasibility study was conducted to determine if application of ADS therapy (i.e., spike-triggered intraspinal microstimulation) results in improved motor performance in an ambulatory rat model of SCI. The hypothesis for this study was that the application of ADS therapy will improve motor performance in an ambulatory rat model of bilateral thoracic spinal cord contusion. The goal of this rehabilitation intervention was to facilitate and direct intrinsic synaptic plasticity in spared motor pathways and circuits that would result in improved motor performance.
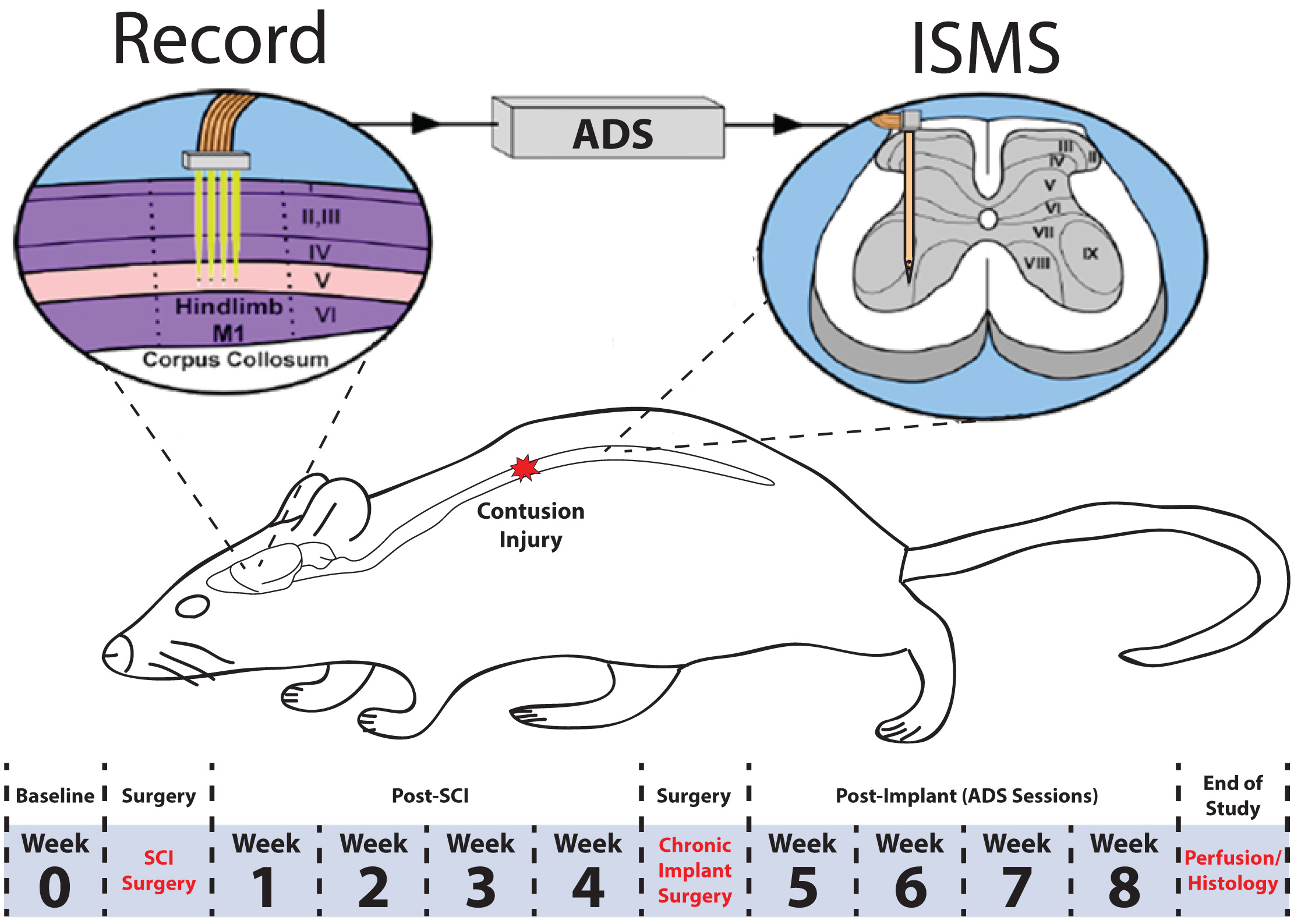
To determine if spike-triggered intraspinal microstimulation (ISMS) therapy results in improved motor performance in an ambulatory rat model of spinal cord injury (SCI), extracellular neuronal activity was recorded from the hindlimb motor cortex and used to trigger ISMS in the ventral horn of the lumbar spinal cord (Fig. 1). This procedure is hereafter called ADS. Motor performance of rats was scored on three behavioral tasks (the Basso, Beattie, and Bresnahan (BBB) behavioral assessment, horizontal ladder rung walking test, and tapered/ledged beam test) at pre-specified time points throughout the study. The observers were not blinded to the subject’s condition during the behavioral tasks.
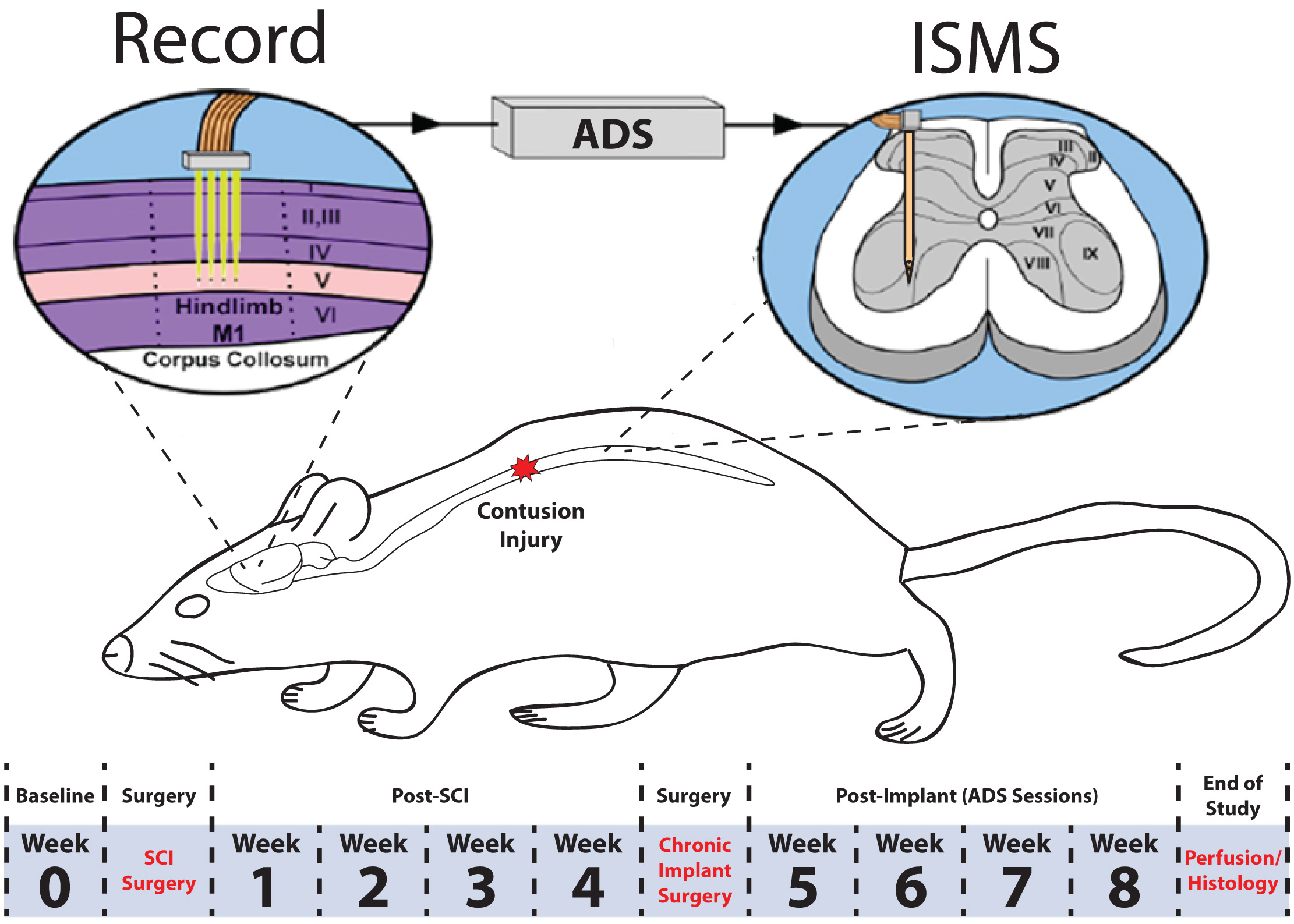 Fig. 1.
Fig. 1.Overview of experimental design. A 175 kDyn spinal cord contusion was administered at the T8 vertebral segment. Four weeks after injury, rats were implanted with a recording electrode in the hindlimb motor cortex and a fine wire electrode in the ventral horn of the lumbar spinal cord. Behavioral tests were conducted before (i.e., baseline) SCI and once per week after each surgery for the duration of the study. Activity dependent stimulation was administered for four weeks post-implant in the ADS group. No stimulation was administered in the control group. ADS sessions occurred during weeks 5–8 for 4 hours/day and 4 days/week.
Twenty adult male Sprague Dawley rats were selected for this study. At the
beginning of the study, body weights ranged from 305 to 416 g (mean
The experimental timeline is displayed in Fig. 1. First, rats were acclimated to each of the behavioral tasks. Then, baseline performance was assessed. One to three days after baseline assessment, a surgical procedure was conducted to produce a bilateral contusive injury at the T8 vertebral segment. During weeks 1–4 post-SCI, behavioral assessment was conducted once per week to evaluate the effects of the SCI on motor performance and the extent of any spontaneous recovery. Then, a second surgical procedure was conducted to implant a recording electrode in the hindlimb motor cortex and a fine wire electrode in the ventral horn of the lumbar spinal cord. After implants, ADS therapy sessions were conducted during weeks 5–8. During the ADS therapy period, behavioral assessment was conducted once per week at the end of the week, i.e., after each of the four ADS sessions. At the end of the 8-week study, the rats were humanely euthanized, and the spinal cords assessed for the extent of the injury.
Both surgical procedures (SCI surgery; chronic implant surgery) followed the same general protocol. After an initial stable anesthetic state was established using isoflurane anesthesia and the scalp and back shaved, isoflurane was withdrawn, and an initial dose of ketamine hydrochloride (100 mg/kg IP)/xylazine (5 mg/kg IP) was administered. The anesthetic state was maintained with subsequent doses of ketamine (10 mg IP or IM) and monitored via pinch and corneal reflex responses. Additional doses of ketamine were administered if the rat reacted to a pinch of the forepaw/hindpaw, had a positive corneal reflex, or exhibited increased respiration rate. At the conclusion of the surgery, 0.25% bupivacaine HCl was applied locally to the skin incision site. Buprenex (Mckesson Medical-Surgical, Irving, TX, USA; 0.01 mg/kg, S.C.) was administered immediately after surgery and 1 day later. All animals were monitored daily until the end of the experiment. Before and after surgery, rats received a S.C. injection of 30,000 U of penicillin (Combi-Pen 48, Bimeda, Oakbrook Terrace, IL, USA).
Spinal cord contusion procedures followed those described in our previous studies [12, 13, 14]. Animals underwent a T8 laminectomy and 175 kDyn moderate impact bilateral contusion injury using an Infinite Horizon spinal cord impactor (Precision Systems and Instrumentation, LLC, Fairfax Station, VA, USA). Displacement distance reported by the impactor software was recorded and used as an initial quantitative marker for successful impact. Starting the first day after surgery, daily penicillin injections (G benzathine and G procaine; 45,000 IU; Combi-Pen 48, Bimeda USA, Oakbrook Terrace, IL, USA) were given in 5 mL saline throughout the first week to prevent infections and dehydration. Bladders were expressed twice daily until animals recovered urinary reflexes. From the second week onward, animals were supplemented with vitamin C pellets (BioServ, Frenchtown, NJ, USA) to avert urinary tract infection.
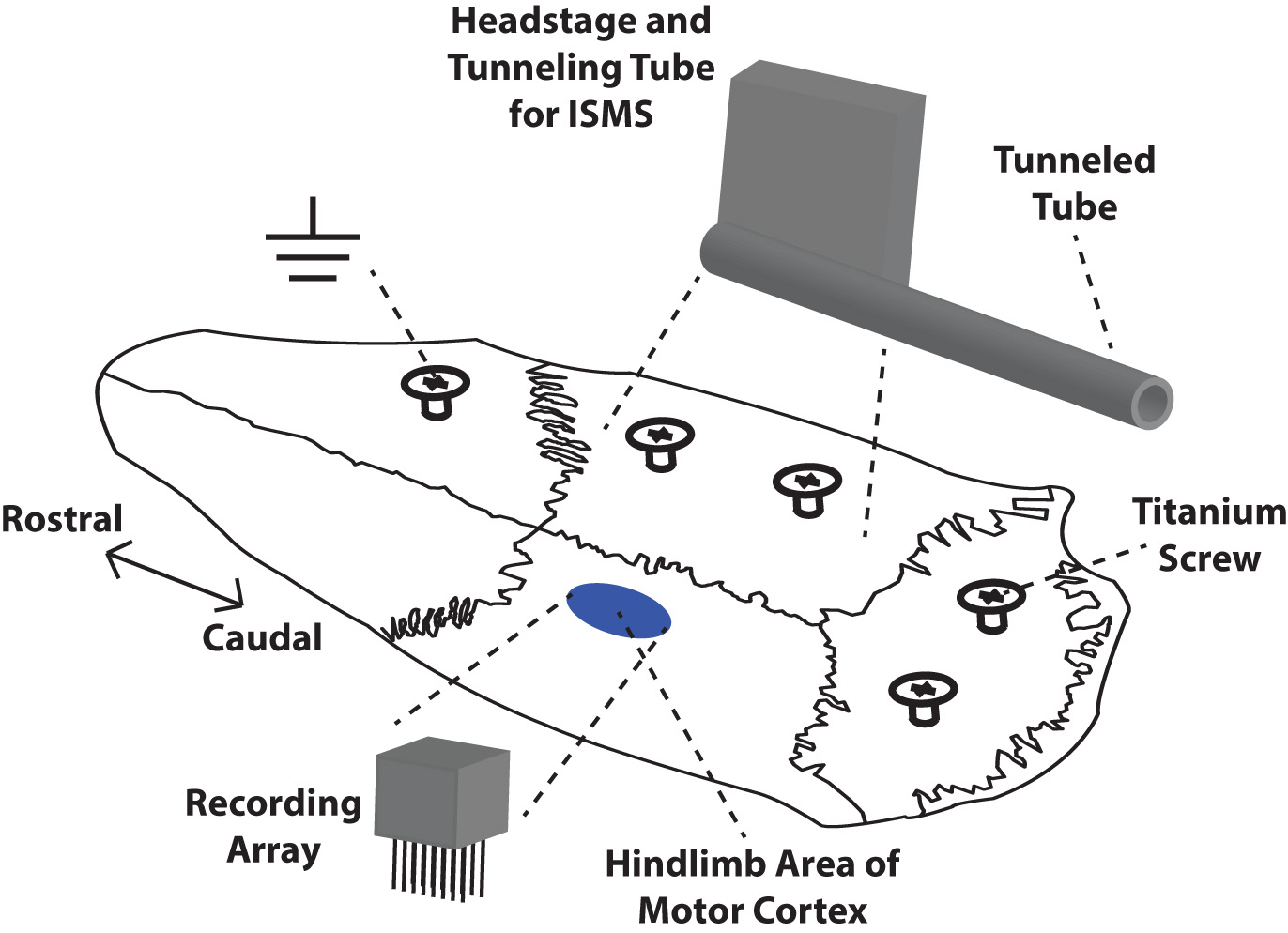
Rats were placed in a Kopf small-animal stereotaxic frame (David Kopf Instruments®, Tujunga, CA, USA) and the incisor bar was adjusted until the heights of lambda and bregma were equal (flat skull position). The cisterna magna was punctured at the base of the skull to reduce edema during mapping and implantation. Before the craniectomy, five titanium screws were placed at various positions on the skull as shown in Fig. 2. These screws served as anchors for a head cap made of dental acrylic that encased the chronic recording array, headstage, and supporting hardware on the skull.
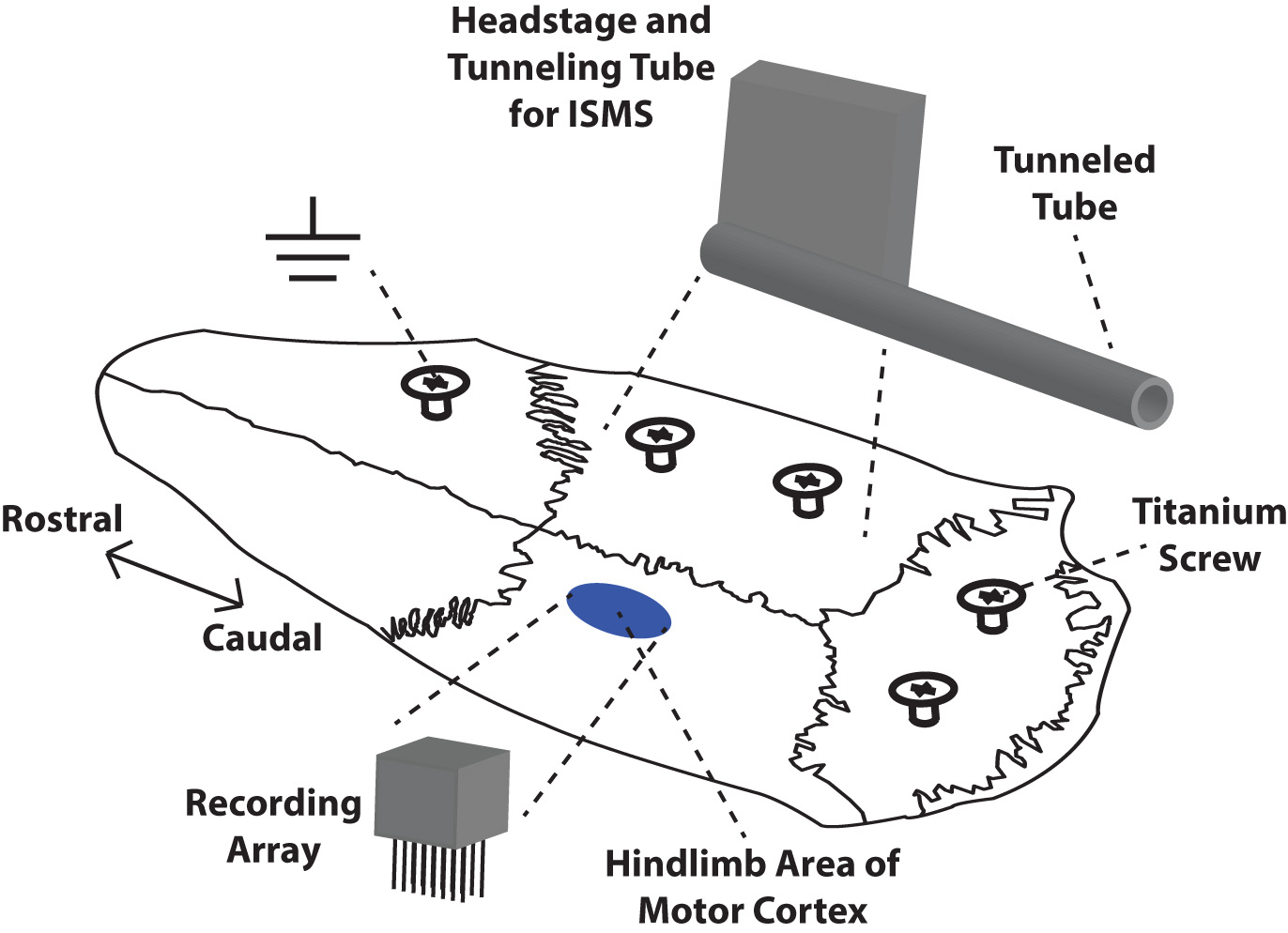 Fig. 2.
Fig. 2.Placement of chronic microelectrode array, headstage, and supporting hardware on the skull of a rat. Dental acrylic was used to build a protective cap and anchor the array and headstage to the skull via five titanium screws. The recording array was implanted in the left hindlimb motor cortex. The headstage for the stimulating electrode was mounted on the right side of the skull while the microwires were tunneled via a tunneling tube to the lumbar spinal cord. The ground wires for both electrodes were wrapped around the rostral-most titanium screw.
For placement of the recording array in the cortex, a craniectomy was performed over the hindlimb area (HLA) of motor cortex in the left hemisphere. The location of the craniectomy was guided by previous rat cortical motor mapping studies [11, 16]. After the dura over the cranial opening was incised, a small motor map was derived using intracortical microstimulation (ICMS) techniques detailed in our previous studies [11, 16]. Since hindlimb movements cannot be evoked using ICMS after the SCI, the location of the hip representation of HLA was inferred by proximity to surrounding representations. Based on our prior ICMS studies, the hip representation is bordered laterally by the trunk area and caudally by the forelimb area of motor cortex.
To record spikes (i.e., action potentials) in the hip representation of HLA, a
chronic microwire array (Tucker-Davis Technologies, Alachua, FL, USA) was
inserted into the defined area of the cortex. The recording array was a
12-channel (2
For placement of the stimulating electrode in the spinal cord, a laminectomy was
performed on the T13 vertebrae similar to previous studies [13, 14]. The dura
mater was left intact to minimize cerebrospinal fluid leakage. A custom-made
fine-wire stimulating electrode was fabricated from 30-
The microwire implantation procedure in the hindlimb spinal cord followed the procedure developed by Bamford et al. [17]. The microwire implant was sterilized using ethylene oxide (EtO) gas. The Omnetics connector (i.e., headstage) of the microwire implant was positioned on the right hemisphere of the skull next to the recording electrode (Fig. 2). The polyethylene tube and microwires were tunneled subcutaneously to the hindlimb spinal cord. Dental acrylic was used to anchor the recording and stimulating electrodes to the skull and screws. Once the acrylic was dry, the polyethylene tube of microwires was sutured to the T12 vertebral process with 6-0 polypropylene suture (PROLENE® blue monofilament, taper point, C-1; 13 mm 3/8c; Ethicon) and fixed further with a small amount of surgical glue (Vetbond Tissue Adhesive; 3M, St. Paul, MN, USA) being careful to glue the polyethylene tube directly to the bone and not the surrounding tissue. Before microwire insertion, a small hole was made in the dura mater using the tip of a 30-gauge hypodermic needle. Microwire insertion began by gently holding the wire with fine forceps near the tip. While avoiding major blood vessels, the tip then penetrated the superficial dorsal layers of the cord, and the remaining portion of the wire was inserted vertically until reaching the 90° bend (~2.27 mm of wire which reaches the ventral horn of the rat hindlimb spinal cord). Only one microwire was used for the study, and when the implanted wire was deemed functional the remaining three microwires were removed.
The target for microwire placement in the spinal cord was a hip site, allowing
functional pairing between the recording electrode in cortex and the stimulating
electrode in spinal cord. The rostro-caudal placement of the microwire in the
hindlimb spinal cord was guided by previously derived ISMS-evoked movement maps
in rat [18]. The medio-lateral placement of the microwire was on the right side
of the spinal cord, approximately 0.8–1.0 mm from the central blood vessel. A
low-amplitude current (one biphasic pulse with 200
During ADS therapy, electrode leads/connectors of the headstages were tethered
to the TDT neurophysiological recording and stimulation system (Tucker-Davis
Technologies, Alachua, FL, USA). Rats were placed in a 30-cm
The Basso, Beattie, and Bresnahan (BBB) locomotor rating scale was used as the primary behavioral outcome, as it is a sensitive measure of locomotor ability after SCI [19]. Briefly, rats ran along a straight alley with the home cage and bedding at the end to encourage the rats to transverse the open field. Two examiners observed each rat to minimize any bias and to reduce the risk of missing behaviors. Scores were recorded for the left and right sides of each animal. If SCI rats did not exhibit a deficit post-injury or did not have a BBB score between 13–15 at 4-weeks post-SCI, they were removed from the study. All rats that survived the SCI surgery (n = 16) fell into the BBB score inclusion criteria. Rats that did not survive the electrode implant surgery (n = 4) were excluded from all motor behavioral analysis. Rats assigned to the ADS group (n = 6) and the Control group (n = 6) were included in all motor behavior analyses.
To quantify skilled locomotor movements, rats were trained on the horizontal ladder rung walking test apparatus (Otto Environmental, Greenfield WI, USA). This is considered to be a sensitive test to show deficits in corticospinal tract connectivity [20]. Two patterns were used during this experiment to prevent the rats from learning the pattern and to modify the difficulty of the task. Pattern A consisted of a regular pattern with the rungs spaced at 2 cm intervals, while Pattern B consisted of an irregular pattern with the rungs spaced between 1 and 3 cm (pattern as follows: 1 cm spacing, 2 cm spacing, 3 cm spacing, then repeating this pattern). All rats were recorded crossing the horizontal ladder five times per session for each pattern.
Rats were video recorded traversing the horizontal ladder using a Sony digital
camera. Each recording was viewed using frame-by-frame analysis at 30 frames/sec
and scored using procedures previously described [20]. Only consecutive steps of
each hindlimb were analyzed, so if the rat stopped, the last step before the stop
and the first step after the stop were not scored. The last stepping cycle
performed at the end of the ladder was not included in the scoring. The types of
foot and paw placement on the rungs were rated using a 7-category scale. (0)
Total miss: 0 points were given when the limb completely missed the rung and a
fall occurred. (1) Deep slip: 1 point was given when the limb was placed on the
rung and then slipped off when weight-bearing which caused a fall. (2) Slight
slip: 2 points were given when the limb was placed on a rung, slipped off when
weight bearing, but did not result in a fall and interrupt the walking. (3)
Replacement: 3 points were given when the limb was placed on a rung, but before
it was weight bearing, it was quickly lifted and placed on another rung. (4)
Correction: 4 points were given when the limb aimed for one rung but was then
placed on another rung without touching the first one. (5) Partial placement: 5
points were given when the limb was placed on the rung with either heel or digits
of a hindlimb. (6) Correct placement: 6 points were given when the midportion of
the palm of the limb was placed on a rung with full weight support. If different
errors occurred at the same time, the lowest of the scores was recorded. Error
rate in stepping was recorded as a percentage of the number of misplaced steps
divided by the total number of steps on the ladder [100
To assess deficits in balance and fine motor control, rats were trained on the tapered/ledged beam test. The ledged beam is a highly sensitive test that allows chronic deficits to be easily scored. More specifically, the ledge of the beam provides a place to step with the impaired limbs so that the rats are not induced to compensate using alternative motor strategies [21]. The rats were videotaped using a Sony digital camera, and steps for each hindlimb were scored as a full-slip or a half-slip if the limb touched the side of the beam. Steps onto the ledge were scored as a full-slip and a half-slip was given if the limb touched the side of the beam. The slip ratio of each hindlimb (number of slips/number of total steps) was later calculated and averaged over three trials. The mean of three trials was used as a single measure of the task for statistical analysis.
At the conclusion of the study (after Week 8), rats were euthanized with an
intraperitoneal injection of sodium pentobarbital (Beuthanasia-D; 100 mg/kg),
then transcardially perfused with fixative (4% paraformaldehyde in 0.1 M PBS)
and the spinal cord was removed. The spinal cord was transversely sectioned at 30
Verification that the fine-wire spinal cord implant remained in the spinal cord throughout the study was made during the dissection and removal of the spinal cord implant.
Statistical analyses of motor performance measures were conducted using JMP 11
software (SAS Institute Inc., Cary, NC, USA). For parametric data, a two-factor
repeated measures analysis of variance (ANOVA) was used to analyze effects of ADS
therapy on hindlimb function over time on the skilled-movement behavioral tasks
(Ledged Beam and Horizontal Ladder tasks). Hindlimb performance was obtained as a
percentage of foot-faults on the behavioral tasks. An arcsin transformation was
used to normalize percentage data for parametric analysis. Group comparisons were
made following a significant interaction with Fisher’s Least Significance
Difference (Fisher’s LSD) test. The nonparametric, Mann-Whitney U test
was used to compare ordinal-scaled BBB scores between the ADS and Control group.
Bonferroni correction for multiple comparisons was used to control for multiple
comparisons. Parametric data are presented as mean
The average impact displacement was 1069.67
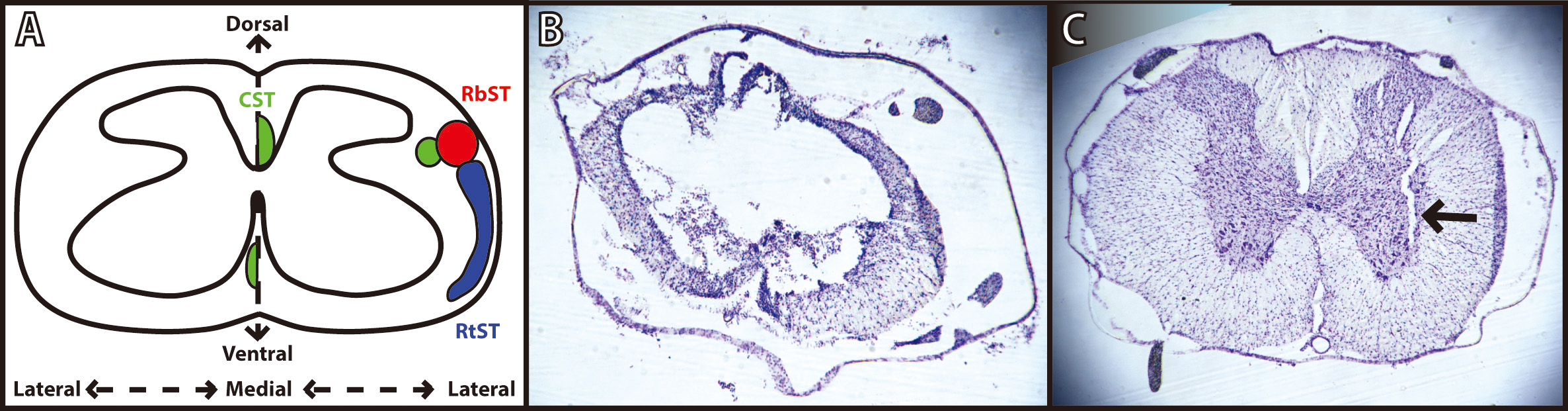 Fig. 3.
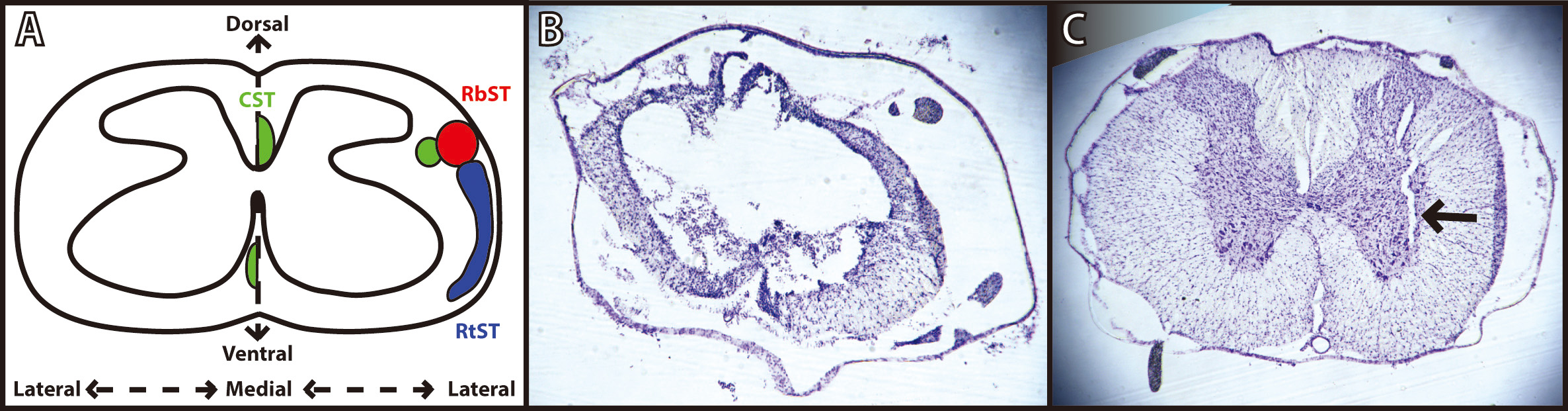
Fig. 3.Verification of spinal cord injury and microwire implantation. (A) Schematic diagram of a coronal thoracic section identifying the location of the descending corticospinal tract (CST; green), rubrospinal tract (RbST; red), and reticulospinal tract (RtST; blue). Reproduced with permission from Roger N. Lemon, Descending Pathwaysin Motor Control; published by Annual Reviews, 2008. [22]; and reproduced with permission from Kathren L. Fink, Reorganization of Intact Descending Motor Circuits to Replace Lost Connections After Injury; published by Springer, 2016. [23]. (B) Representative image of spinal cord injury epicenter under T8 vertebrae stained with cresyl violet 8 weeks after injury. (C) Representative image of microwire implant track under T13 vertebrae stained with cresyl violet 4 weeks after implantation.
The average cortical spike frequency during therapy sessions for ADS rats was
8.30
The average impedance values (mean
| Average impedances (kΩ) of implanted electrodes | ||||||
| Week of study | ISMS implant | HLA implant | ||||
| ADS | Control | p-value | ADS | Control | p-value | |
| Implantation (Week 4) | 50.4 |
39.0 |
0.6125 | 41.3 |
31.7 |
0.1088 |
| Week 5 | 51.4 |
59.8 |
0.7108 | 73.6 |
68.4 |
0.4483 |
| Week 6 | 66.8 |
79.5 |
0.7225 | 66.8 |
33.9 |
0.0025* |
| Week 7 | 59.7 |
65.1 |
0.8403 | 73.7 |
67.2 |
0.3639 |
| Week 8 | 72.2 |
63.2 |
0.7479 | 69.2 |
70.3 |
0.8896 |
| ISMS implant is the stimulating electrode located in the spinal cord, and the
HLA implant is the recording electrode located in the cortex. * = statistical
difference between groups (p | ||||||
The minimum current needed to evoke movement (i.e., movement threshold) via the
implanted microwire in the hindlimb spinal cord was recorded directly after the
implantation surgery and before each ADS session. Immediately after the
implantation surgery, the average current at movement threshold for all rats was
36.0
In contrast to the tests carried out during the microwire implantation, no movements could be evoked when tested immediately before any of the ADS sessions. The current used during these sessions was therefore set at 50% of the threshold for evoking movement, as measured immediately after the implantation surgery. A qualitative difference was observed between the rats in the ADS group and the Control group during the therapy sessions: Rats in the ADS group would actively stretch the right hindlimb or keep the right hindlimb completely extended. This behavior occurred intermittently and only when the rats were laying down and sleeping. This observed stretching and extending of the right hindlimb only occurred when the stimulation current was turned on during therapy sessions. This did not occur in the Control rats where the stimulation current remained off during therapy sessions. The rats receiving ADS therapy did not exhibit any signs of pain or distress during or after stimulation nor did this behavior interfere with normal behavior when roaming around the home cage or therapy chamber.
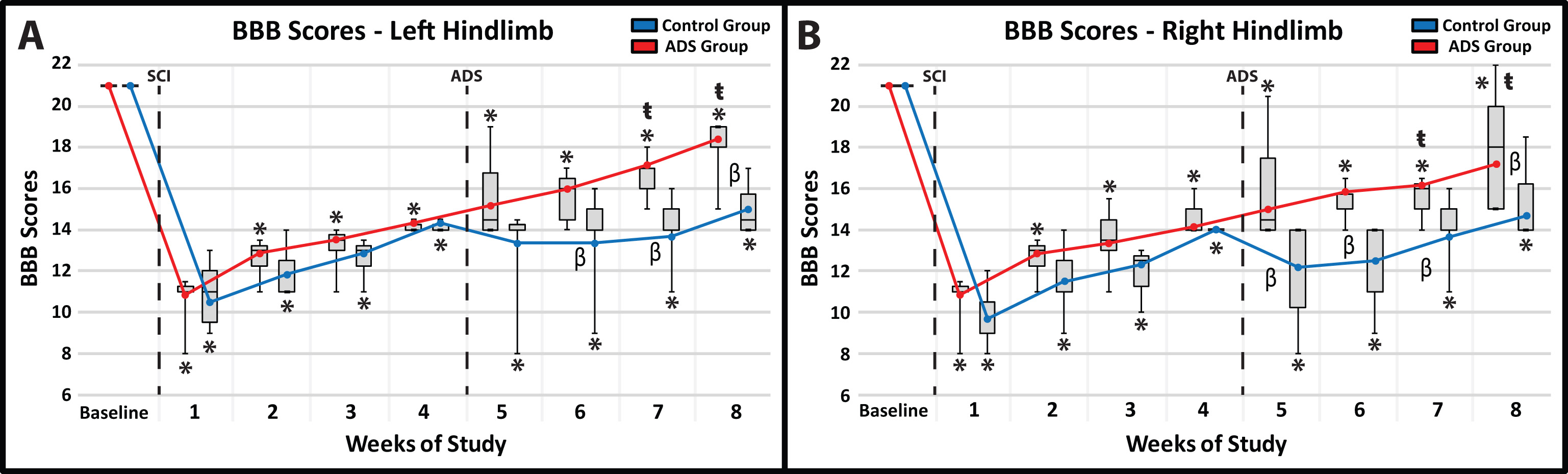
Before SCI contusion, all rats were tested to verify that there were no pre-injury hindlimb deficits. All rats had a BBB score of 21 for both hindlimbs (Fig. 4A,B) which consisted of consistent plantar stepping, consistent forelimb-hindlimb coordination during gait, consistent toe clearance, parallel paw position throughout the entire gait cycle, consistent trunk stability, and tail consistently up.
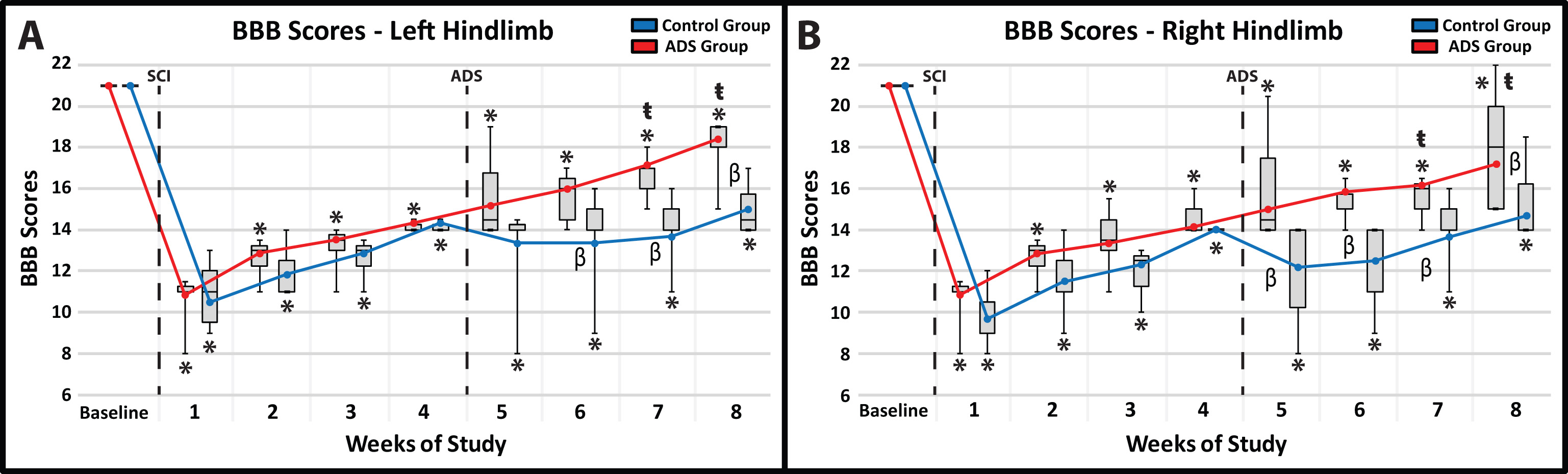 Fig. 4.
Fig. 4.Average BBB scores (mean
All rats exhibited clear hindlimb deficits immediately after SCI contusion. BBB
scores were not significantly different between the ADS and Control groups at any
time point post-SCI during weeks 1–4. In the 4 week post-SCI/pre-implant period,
BBB scores improved for each hindlimb in both the ADS and Control group, but
remained significantly lower relative to baseline BBB scores (within-group
comparisons, p
The post-implant BBB scores were significantly higher (better) in the ADS group compared to the Control group in each hindlimb. Specifically, the significant difference between groups for each hindlimb continued at three weeks (Week 7) and four weeks (Week 8) of ADS therapy (Fig. 4A,B). The final BBB scores in the ADS group (i.e., BBB score of ~17–18) represented improvement in toe clearance and in the position of the paw during gait (either parallel throughout the entire gait cycle or parallel only when the toe returns to the ground after the swing phase).
Within-group comparisons during the ADS period (weeks 5–8) compared with Week 4 (pre-ADS) show that by Weeks 7 and 8, BBB performance in the ADS group had improved significantly compared with Week 4 (Week 7: left hindlimb, p = 0.0062; right hindlimb, p = 0.0248; Week 8: left hindlimb, p = 0.0107; right hindlimb, p = 0.0088). However, despite gradual improvement over the subsequent weeks, BBB scores of the Control group were not significantly different from their Week 4 scores.
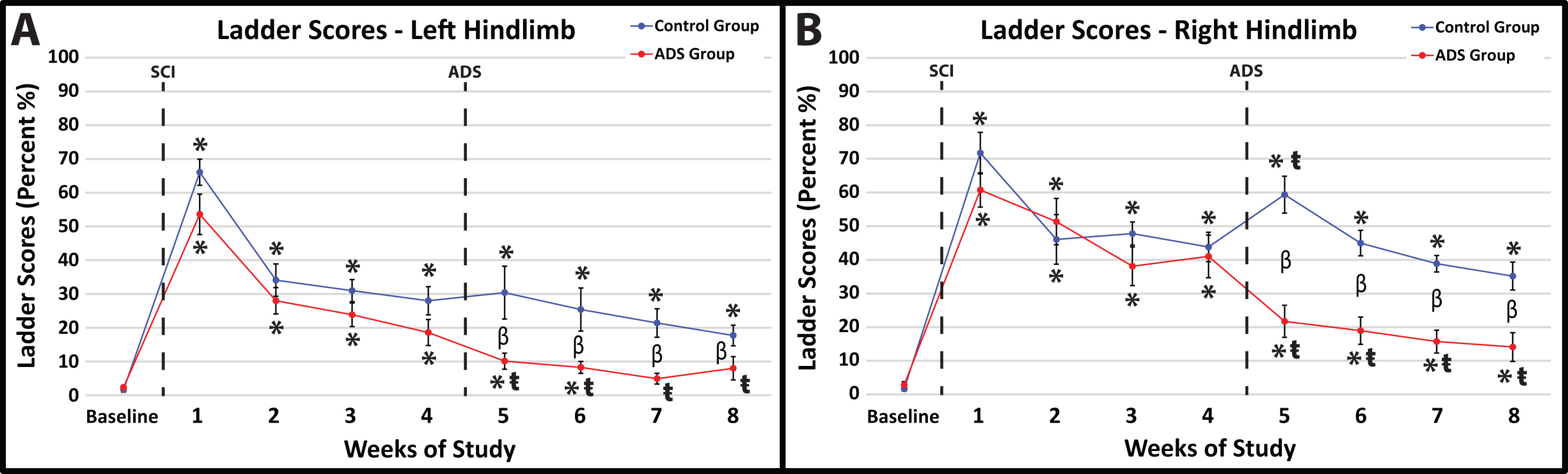
Before SCI contusion, all rats demonstrated minimal foot-faults, averaging zero to two foot-faults for each hindlimb while traversing the ladder (Fig. 5A,B).
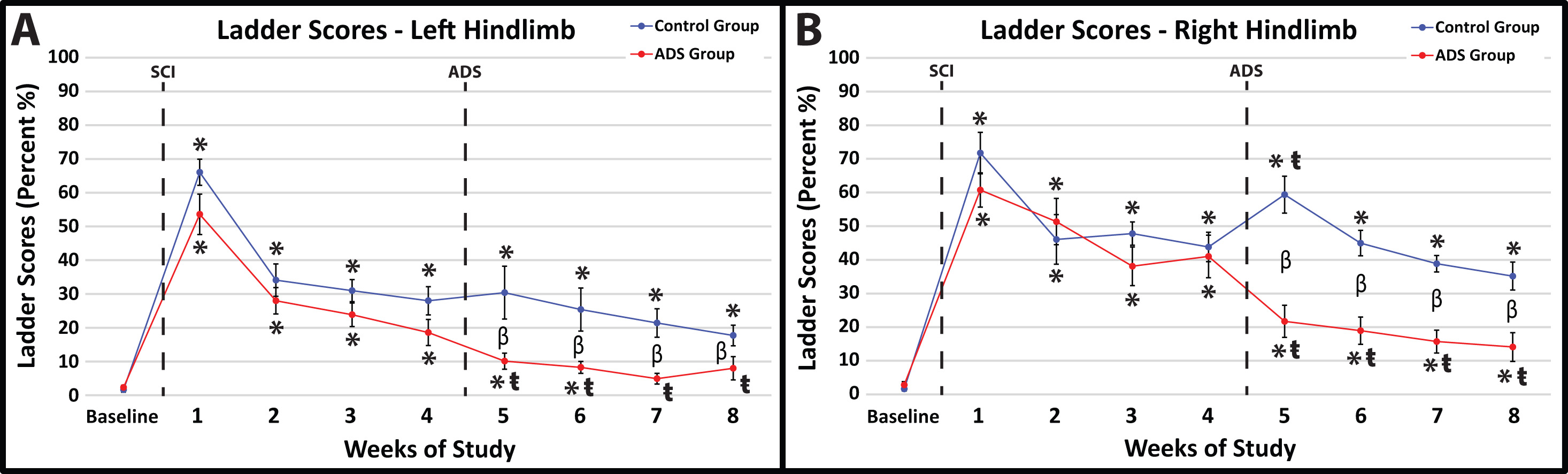 Fig. 5.
Fig. 5.Average foot-fault scores (mean
In post-SCI Week 1, all rats exhibited the same hindlimb deficits on the
horizontal ladder after SCI contusion (no statistical difference between groups).
Within-group comparisons between Week 1 and Baseline showed a significant
increase in foot-faults for each hindlimb in both ADS and Control groups (Fig. 5A,B; p
At one-week post-implant (one week of ADS therapy; Week 5), the ADS group demonstrated improved scores compared to the Control group (Fig. 5A,B). The foot-fault scores for both hindlimbs remained significantly lower in the ADS group than the Control group for the remainder of the study (Weeks 6–8).
After one-week post-implant (one week of ADS therapy; Week 5), the foot-fault scores from the ADS group significantly improved in each hindlimb compared to Week 4 (left hindlimb, p = 0.0360, Fig. 5A; right hindlimb, p = 0.0002, Fig. 5B) and continued to be significantly improved for the rest of the study (Weeks 6–8). With the exception of Week 5 in the right hindlimb (Fig. 5B), significant differences in foot-fault scores were not observed within the Control group after implantation and remained unchanged by week 8.
Foot-fault scores from the left hindlimb of the ADS group returned to close to baseline values (three weeks post-implant; within-group comparison between Baseline and Week 7; left hindlimb, p = 0.4285, Fig. 5A) and remained at a non-significant level for the remainder of the study (within-group comparison between Baseline and Week 8; left hindlimb, p = 0.2106, Fig. 5A). This improvement was similarly seen in the right hindlimb of the ADS group; however, foot-fault scores did not become close to baseline values during any week post-implant (within-group comparison between Baseline and Week 8; left hindlimb, p = 0.0054, Fig. 5B). This recovery in foot-fault scores was not seen in either hindlimb in the Control group.
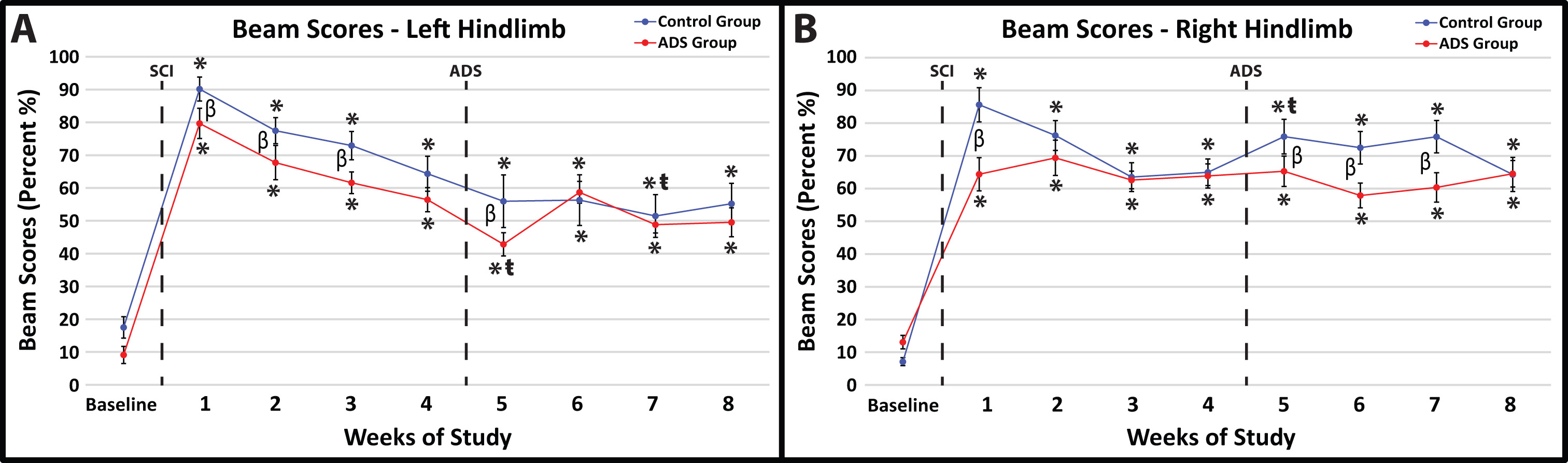
Before SCI contusion, all rats demonstrated minimal foot-faults on the ledged beam with either hindlimb (Fig. 6A,B). Baseline foot-fault scores on the ledged beam were not significantly different between groups for both hindlimbs (right hindlimb, p = 0.3285; left hindlimb, p = 0.2171).
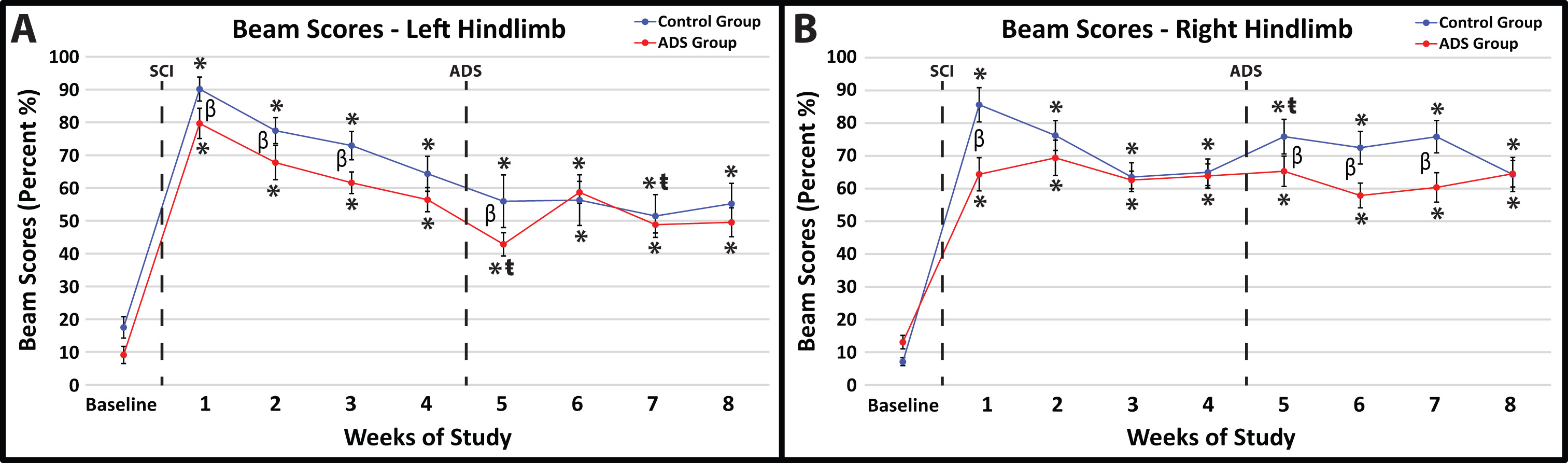 Fig. 6.
Fig. 6.Average foot-fault scores (mean
All rats exhibited hindlimb deficits on the ledged beam immediately after SCI
contusion; however, there was a significant difference between groups in both
hindlimbs one-week post-SCI (Week 1; left hindlimb, p = 0.0064, Fig. 6A;
right hindlimb, p
Significant improvements in foot-fault scores within-group relative to week 4 were not observed at Week 8 in either group (Fig. 6).
This proof-of-concept feasibility study demonstrates, for the first time, that chronic stimulation using cortical spike-triggered intraspinal microstimulation (ISMS) can enhance behavioral recovery of locomotor function after spinal cord injury, as measured by behavioral assessment. However, since tests of activity-dependence were not conducted in this study, it is still uncertain if the measured behavioral improvements were dependent on spike-triggered ISMS. Previous studies have used ADS paradigms specifically focused on recovery of forelimb function after cortical [1, 5] and spinal cord [2, 24, 25] injury and/or deactivation. Previous efforts have been made to recover hindlimb function using spike-triggered epidural stimulation to bypass a unilateral spinal cord laceration injury [3]. The present study demonstrates that an ADS approach using cortically-evoked intraspinal stimulation with the intent to reconnect the hindlimb motor cortex with the lumbar spinal cord may enhance recovery of function specific to hindlimb control. Importantly, behavioral recovery was seen after ADS (i.e., spike-triggered ISMS) was applied to bypass a bilateral spinal cord contusion injury that more accurately mimics injuries typically seen in clinical populations. However, it should be noted that a laminectomy was conducted prior to injury, which may limit the comparisons to injuries typically seen in clinical populations.
Hindlimb deficits after a bilateral, moderate thoracic spinal cord contusion, such as the injury model used in the present study, have been reported using the BBB open field rating scale to assess locomotor function. From the onset of injury, hindlimb function naturally (or spontaneously) improves until 4 weeks post-injury, when recovery typically plateaus [12, 19, 26]. For most of the behavioral tests in the pre-implant period, the control group showed more deficits than did the ADS group, though nearly all the differences for individual weeks were not significant. This raises the possibility that that the between-group differences after ADS may not be solely attributed to the ADS therapy. However, with the possible exception of the ledged-beam test, these pre-implant differences were all small and, indeed, were all non-significant at Week 4. Thus, although they remain as a caveat, we were able to proceed on the basis that all the rats in the study started with sufficiently similar motor deficits at the time of electrode implant, including the inclusion criterion of BBB score of ~14. Differences regarding the natural recovery of hindlimb motor function and the BBB score are related to the location and severity of injury to the thoracic spinal cord, but reports from different laboratories agree that natural improvement in post-injury hindlimb function plateaus around 4 weeks post-injury.
While the anatomical substrate for recovery after ADS remains speculative, and was not examined in the present study, it may be useful to consider the putative role(s) of spared pathways for design of follow-on studies. Three descending spinal cord pathways provide substantial influence on hindlimb motor function in the rat: the corticospinal tract (CST), the rubrospinal tract (RbST), and the reticulospinal tract (RtST). Crossed corticospinal axons, traveling in the dorsal columns in rats, are severely damaged using the present contusion model over a wide range of contusion severity [27]. Based qualitatively on the location of white matter observed in post-mortem histological sections, the RbST and dorsolateral CST (dCST) in the dorsolateral funiculus, the RtST and ventromedial CST (vCST) in the ventral funiculus, and many propriospinal fibers (both descending and ascending) likely remained at least partially intact after the moderate bilateral contusion injury used in this study (Fig. 3). Spared fibers in these pathways may be viable therapeutic targets for strengthening of connectivity from cortex to the lumbar cord to enhance the recovery of voluntary movement.
It has been shown that activity (spikes) in hindlimb spinal cord neurons below the level of the injury can still be evoked via cortico-reticulo-spinal pathways that remain intact after CST injury [28]. Sparing RtST fibers after SCI has been shown to result in the recovery of 7 to 8 points on the BBB locomotor score, as compared to a recovery of 1 to 2 points due to spared CST fibers [28]. Significant increases in BBB scores (roughly 3–4 points) were observed in the ADS group in the present study. This recovery in BBB scores suggests that ADS may have strengthened the cortico-reticulo-spinal pathways and resulted in improved control of open-field locomotion. However, further studies will need to be conducted to verify this assumption. Future studies should also consider the sparing of central pattern generators (CPGs) caudal to the SCI and the formation of functional neuronal connections with these spared CPGs that could help promote recovery [29, 30].
Control of interlimb coordination (forelimb-hindlimb (FL-HL) and left-right coordination) is known to be influenced by long descending propriospinal pathways [31]. The amount of propriospinal sparing is uncertain in the present study, but it has been shown that long descending propriospinal axons are almost completely lost in the cervical cord with this type of injury [27]. Nonetheless, FL-HL coordination was recovered before electrode implantation (BBB score of ~14 after Week 4) and remained present after ADS therapy, suggesting that some propriospinal axons remained intact. Since propriospinal pathways are known to receive a large number of terminals from reticulospinal neurons [32], this suggests that coordination is strongly influenced via the RtST and further supports the notion that ADS strengthened cortico-reticulo-spinal fibers. Additionally, if propriospinal pathways are disrupted after injury, recovery of FL-HL coordination may be associated with the possible sparing of RbST axons after injury [27].
The ladder rung walking task was used to assess skilled locomotion (i.e., aiming, fine-adjustment, limb coordination, and balanced weight supported stepping), which is assumed to rely more strongly on CST function [20]. The narrowed beam task was used to test weight bearing and fine motor adjustments during gait as well as to remove compensatory strategies that provide balance to non-impaired limbs [21]. Surprisingly, foot fault scores on the horizontal ladder walking task returned to near pre-injury measures in the left hindlimb after ADS therapy, as this extent of recovery was not expected; however, no improvement was observed in either hindlimb on the narrowed beam task after ADS therapy. Improvement on the narrowed beam task was not expected, as trunk stability never returned after ADS therapy, as measured in the final BBB scores. This lack of trunk stability after this thoracic injury results in an inability to balance appropriately on the beam. However, aiming, fine-adjustment, and limb coordination did improve, as reflected on the ladder rung walking task. This suggests that there may be partial recovery of the CST, especially since the CST has been shown to play a major role in the precise control over paw placement and limb trajectory [33]. Due to the extent of the injury to the descending corticospinal fibers, this seems highly unlikely and suggests that the RbST may have played a role in the recovery seen on the ladder rung walking task [27]. However, a strengthened cortico-reticulospinal tract cannot be ruled out as it has the capacity to deliver information from the cortex to the spinal cord in the absence of direct CST input [32].
Finally, the ventral CST has been shown to sprout in parallel with functional recovery after a dorsal transection in the cervical enlargement of the rat [34]. It is unknown if this sprouting occurs after a thoracic contusion. Nonetheless, it is assumed that the ventral CST remains intact after injury in this study, in addition to some sparing of the dorsolateral CST. The recovery seen after ADS therapy could involve plasticity of the ventral and dorsolateral CSTs in chorus with rubrospinal and reticulospinal fibers.
The main limitation of this study was that the fine-wire electrode that was implanted in the right side of the spinal cord may have itself caused deficits, as noted by instances of decreased performance measures in the Control group in the first tests post-implant. These deficits were noted in some rats one week after implantation (Week 5) as a decrease in BBB scores and an increase in foot-faults on the Horizontal Ladder and the Ledged Beam tasks. However, this initial deficit was not observed in any of the rats in the ADS group. This could be an indication that stimulation after implantation provided some benefit that offset deficits caused by the implant. However, since behavioral tests were not conducted immediately after implantation surgery and only after ADS therapy began, it is uncertain if the rats in the ADS group sustained a similar deficit due to the implant. To address this issue, future studies will need to conduct behavioral tasks immediately after electrode implantation and before any stimulation therapy begins.
Another limitation to this study was the inability to evoke movement via
intraspinal microstimulation 1–3 days post-implant. This could have occurred due
to one or a combination of the following factors: (1) Extensive scar tissue
formed around the microwire thus driving the maximum current needed to evoke
movement (i.e., movement threshold) well above the maximum current that was used
in this study (i.e., 100
Finally, as this was designed as a proof-of-concept feasibility study, additional groups, including open-loop stimulation groups will need to be included in future iterations to control for the possibility that intraspinal stimulation alone can produce a similar effect in this model. With the cortical spike frequency data obtained in the present study, parameters for open-loop control groups can be implemented to provide the most robust matched control groups possible. Future studies may also consider using an older age group of rats (such as ~530–570 days old or ~1.5 years old) that reflects the age of the majority of individuals living with SCI (i.e., middle aged adults), as the age range of the rats utilized in this study are roughly equivalent to an adolescent population. Future studies may also consider restricting the inclusion criteria of 13–15 BBB score at 4 weeks post injury, as this variability may be the factor in the visible but not significant differences in behavioral measures between groups. This study is a revised and refined version of a previously published dissertation [35]. Including more rats in future studies may also resolve the issue of trending but not significant differences in behavioral performance between groups. Finally, future studies may consider a different or more specific location of the implanted stimulating microwire in the spinal cord, as a different location of stimulation may promote better behavioral recovery. In this study, the location was limited to an ISMS evoked hip movement and was not specifically determined post-mortem.
Chronic microstimulation was shown to significantly improve hindlimb motor performance after a moderate thoracic spinal cord contusion injury as measured by improved BBB scores and horizontal ladder performance. The behavioral recovery shown here indicates that stimulation may have strengthened spared descending fibers (e.g., cortico-reticulospinal, rubrospinal, dorsolateral corticospinal, and ventral corticospinal fibers). These data will inform the future development of activity-dependent based stimulation therapies which have been shown to be translatable across mono- and multi-synaptic pathways as well as various injuries to the central nervous system.
Data sets will be made available upon reasonable request.
Conception and design—JAB, DG, RJN, SBF; Aquisition of data—JAB, DG, DKA, MWJ; Analysis and interpretation of data—JAB, DKA, MWJ, RJN, SBF; Drafting the manuscript—JAB; Revising the manuscript—JAB, RJN, SBF; Final approval—SBF. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
The protocol was approved by the University of Kansas Medical Center Institutional Animal Care and Use Committee (Protocol number 2020-2565).
The authors thank Erica Hoover and Brad Lamb, for exceptional technical support.
Paralyzed Veterans of America Research Foundation #3068, The Ronald D. Deffenbaugh Family Foundation, NIH/NINDS R01 NS030853, T32 Neurological Rehabilitation Sciences Training Program, and NIH/NINDS F31 NS105442.
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.