1 Laboratório de Propriedades Mecânicas e Biologia Celular (PropBio), Departamento de Prótese e Materiais Dentários, Faculdade de Odontologia, Universidade Federal do Rio de Janeiro (UFRJ), RJ 21941-617 Rio de Janeiro, Brazil
2 Laboratório de Morfogênese Celular (LMC), Instituto de Ciências Biomédicas (ICB), Universidade Federal do Rio de Janeiro (UFRJ), RJ 21941-590 Rio de Janeiro, Brazil
3 Programa de Pós-Graduação em Neurociência Translacional, Instituto Nacional de Neurociência Translacional (INNT-UFRJ), RJ 21941-590 Rio de Janeiro, Brazil
4 Programa de Pós-Graduação em Odontologia (PPGO), Universidade Federal do Rio de Janeiro (UFRJ), RJ 21941-617 Rio de Janeiro, Brazil
5 Department of Life Sciences & Health, Faculty of Health Sciences, Oslo Metropolitan University, 0130 Oslo, Norway
6 Department of Health Science & Technology, Faculty of Medicine, Aalborg University, 9260 Gistrup, Denmark
Abstract
Potassium (K
Keywords
- calcium-activated potassium channels
- small-conductance calcium-activated potassium channels
- SK channels
- pain
- nociception
- central nervous system
- peripheral nervous system
Small-Conductance Calcium-Activated Potassium (SK) Channels are a subtype of
Potassium (K
Molecular cloning and mutagenesis experiments have demonstrated that all K
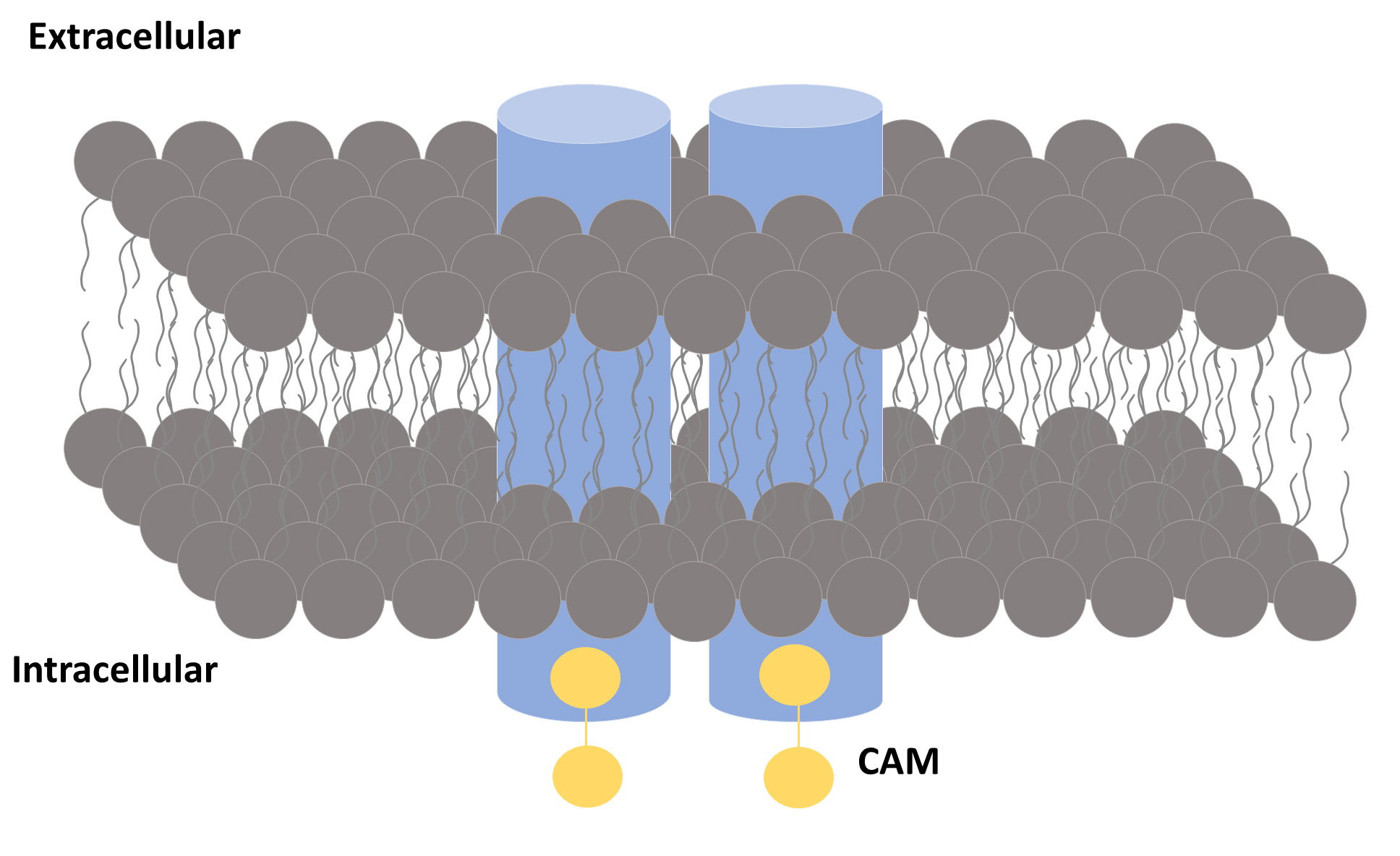
 Fig. 1.
Fig. 1.A general view of an SK channel. An SK channel is formed by four subunits (represented by blue cylinders). Each subunit binds to a calmodulin (CaM) molecule.
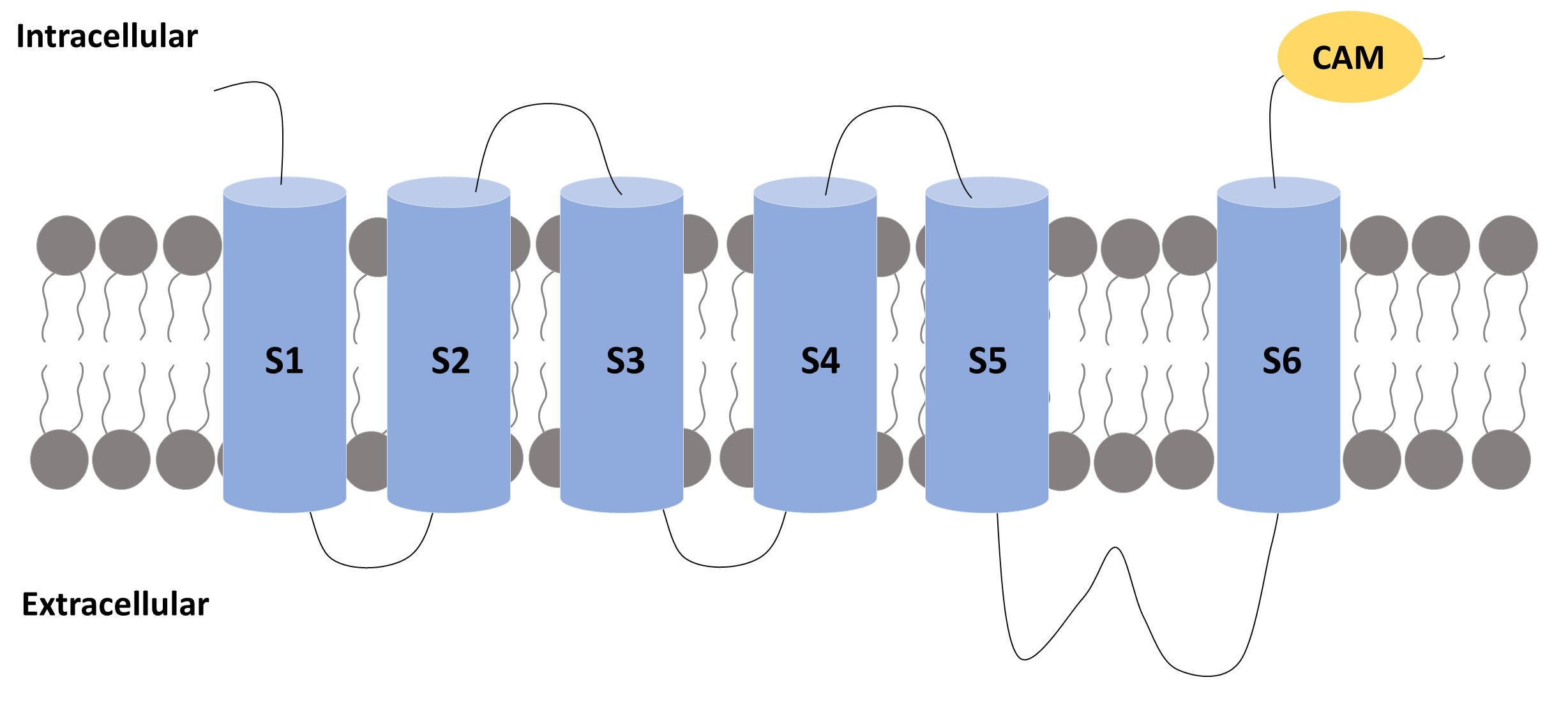
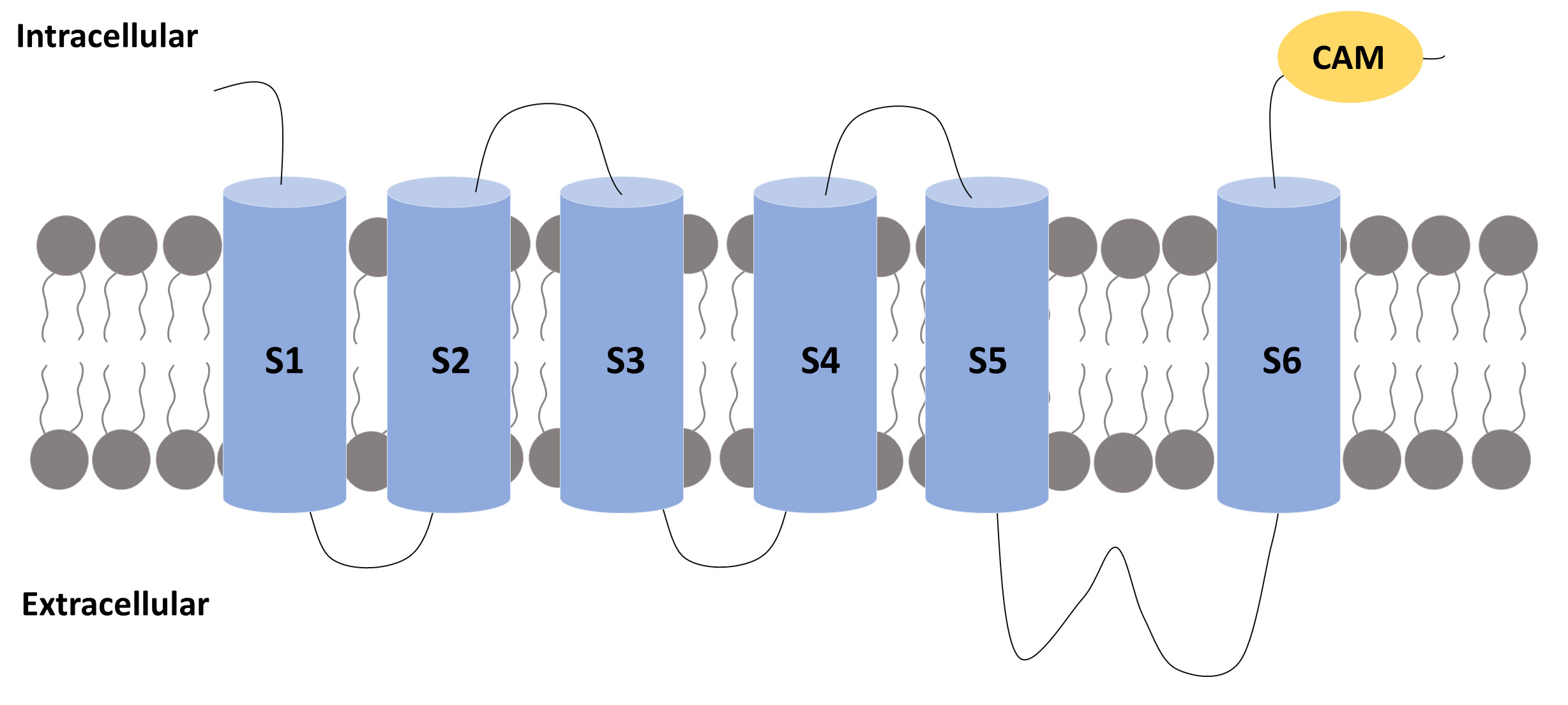 Fig. 2.
Fig. 2.A subunit of an SK channel. A subunit of an SK channel
comprises six transmembrane segments (S1 to S6) with the pore located between
segments 5 and 6. The S4 segment is the one that confers voltage sensitivity to
the K
Studies were conducted to evaluate clusters of conserved negatively charged
residues found between S2–S3 and in the C-terminus. No change was found in the
S2–S3 cluster neutralization. However, neutralizations in the C-terminus blocked
the channel activity. It was also found that this domain could be modelled as a
series of three or four
Each CaM molecule binds two calcium ions to activate an SK channel. Biochemical experiments confirmed that the C-terminus interacts with CaM independently of the presence of calcium, while a subdomain only interacts with the C-terminus domain in the presence of calcium [14]. This site on the C-terminus is called the CaM-binding domain (CaMBD) [15].
The CaMBD binds two CaM. Each CaM wraps around three
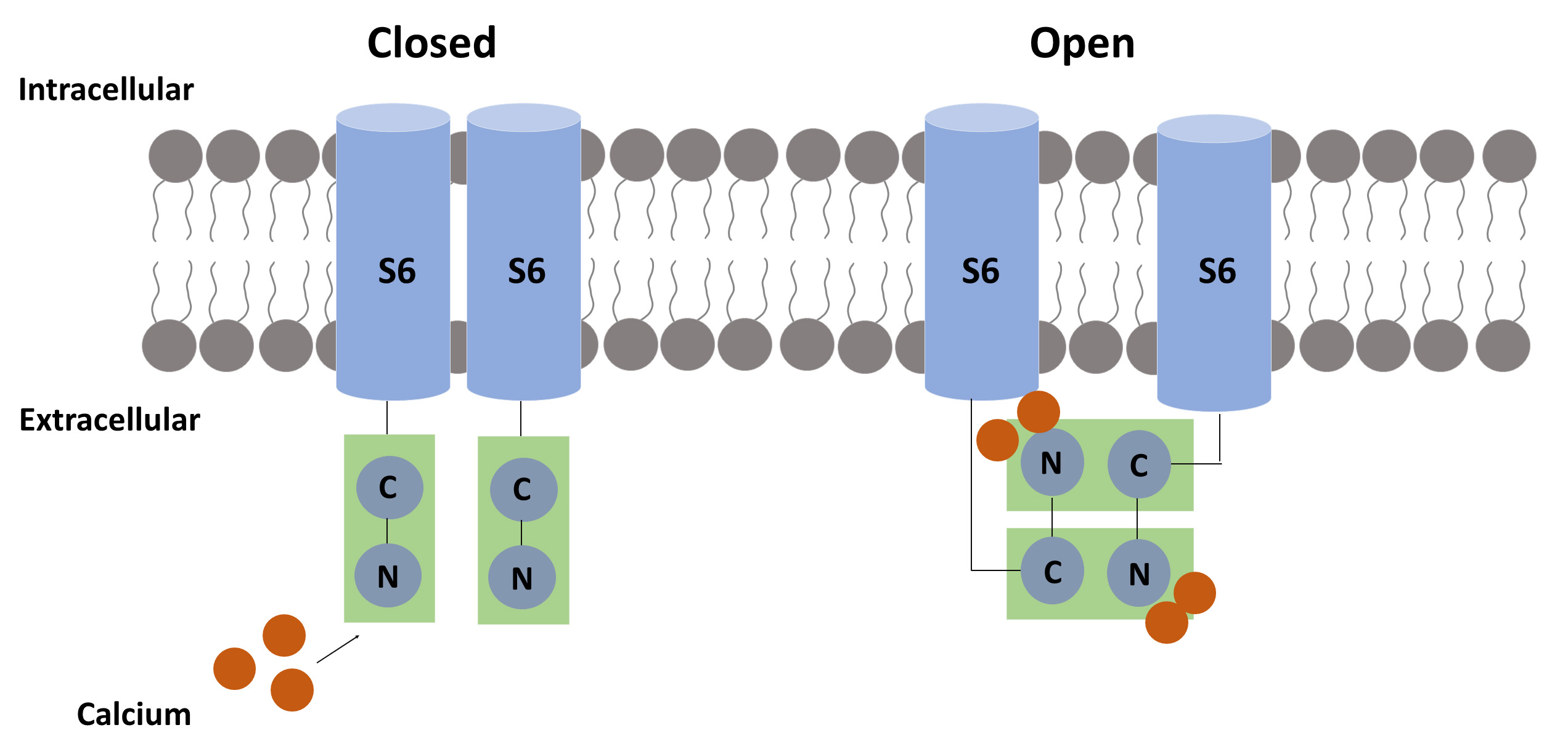
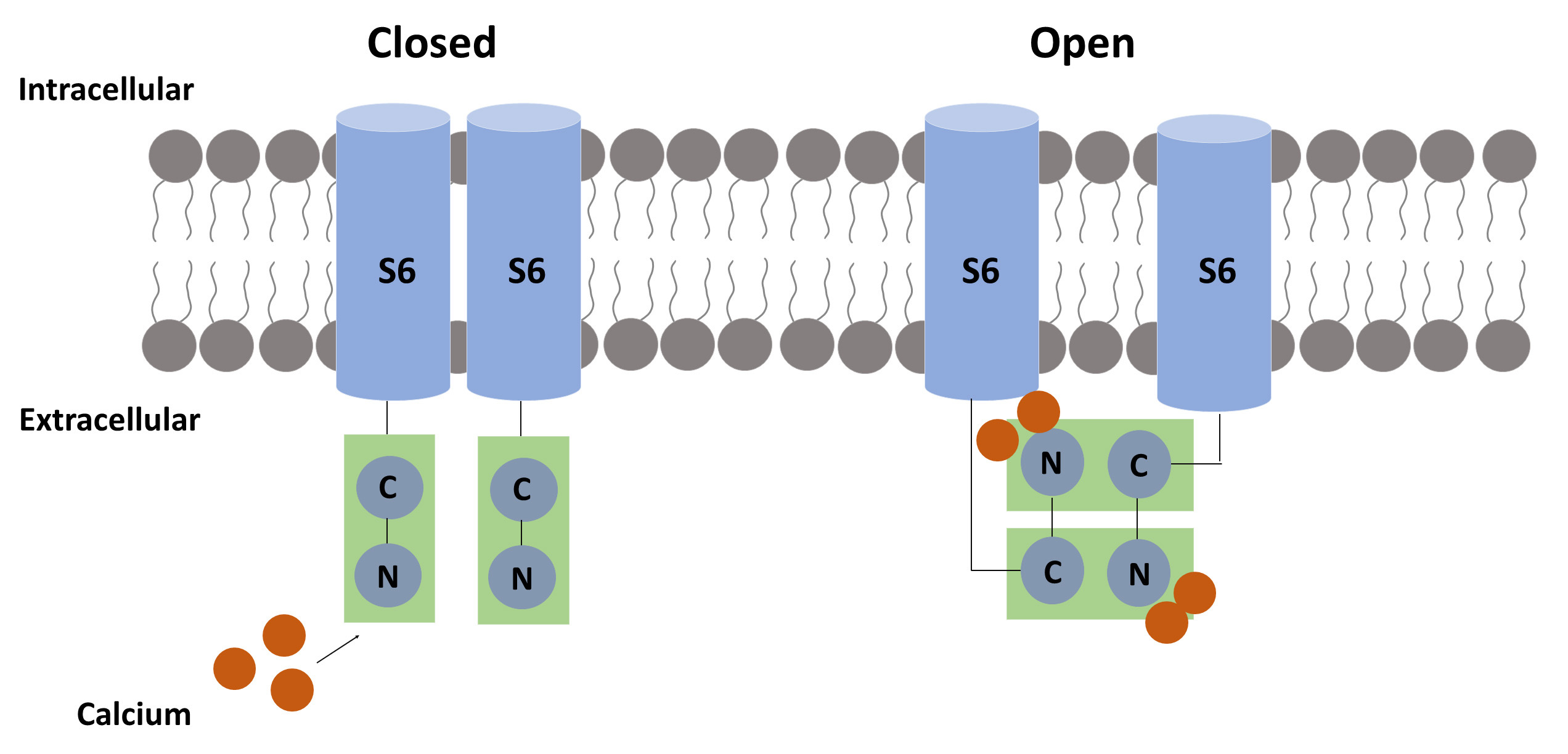 Fig. 3.
Fig. 3.The suggested calcium-related mechanism for pore opening. Each calmodulin (CaM) molecule binds two calcium ions to activate the SK channel. When calcium binds to the N-terminal of CaM, a hydrophobic region is exposed, allowing the CaM-binding domain (CaMBD) to link to another CaMBD, causing a conformational change that results in pore opening.
Experiments performed in skeletal muscle cells have demonstrated that the
process of after-hyperpolarization is mediated by SK channels that lack voltage
sensitivity. It was also demonstrated that they differ pharmacologically. For
instance, some Ca
Overall, SK channels are classified into three isoforms: SK1, SK2, and SK3,
based on their gene origin and their sensitivity to the bee venom, apamin [21, 22]. An overall view of these subtypes is presented in Table 1 (Ref. [23]).
Detailed information on each SK channel is provided in Tables 2,3,4 (Ref. [23]).
These three small-conductance channels are blocked by apamin, distinguishing them
from all other Ca
| IUPHAR | HGNC | Other |
|---|---|---|
| KCa2.1 | KCNN1 | SKCa1; SK1 |
| KCa2.2 | KCNN2 | SKCa2; SK2 |
The third column refers to other commonly used nomenclatures.
| Channel name | KCa2.1 |
|---|---|
| Molecular information | Human: 543aa, NM_002248, chr. 19p13.1, KCNN1 |
| Mouse: 580aa, NM_032397, chr. 8 | |
| Rat: 536aa, NM_019313, chr. 16p14 | |
| Conductance | 9.2 pS (symmetric K |
| Activators | Ca |
| Blockers | UCL1684 (1 nM), apamin (8 nM), tamapin (42 nM), leiurotoxin/scyllatoxin (325 nM), dequalinium (400 nM), leiurotoxin-Dab7 (6 |
| Channel distribution | Brain (amygdala |
| Mutations and pathophysiology | Not established |
| Pharmacological significance | Modulators of SK channel subtypes may have potential use in the treatment of myotonic muscular dystrophy, gastrointestinal dysmotility, memory disorders, epilepsy narcolepsy, and alcohol intoxication |
| Channel name | KCa2.2 |
|---|---|
| Molecular information | Human: 579aa, NM_021614 (transcript variant 1), chr. 5q22.3, KCNN2 |
| Mouse: 574aa, NM_080465, chr. 18 | |
| Rat: 580aa, NM_019314, chr. 18q11 | |
| Conductance | 9.9 pS (symmetric K |
| Activators | 1-EBIO, chlorzoxazone, zoxazolamine, NS309 (30 nM), riluzole (2 |
| Blockers | Tamapin (24 pM), apamin (60–200 pM), leiurotoxin/scyllatoxin (200 pM), leiuritoxin-Dab7 (3.8 nM), PO5 (22 nM), Tskappa (80 nM), Pi1-OH ( |
| Channel distribution | Brain (spinal cord |
| Mutations and pathophysiology | Dominant-negative suppression of KCa2.2 channels in deep cerebellar nuclei in a transgenic mouse causes cerebellar ataxia |
| Pharmacological significance | Modulators of SK channel subtypes may have potential use in the treatment of myotonic muscular dystrophy, gastrointestinal dysmotility, memory disorders, epilepsy, narcolepsy, and alcohol intoxication; KCa2.2 openers have been proposed for the treatment of cerebellar ataxia |
| Channel name | KCa2.3 |
|---|---|
| Molecular information | Human: 736aa, NM_002249 (transcript variant 1), chr. 1q21.3, KCNN3 |
| Mouse: 731aa, NM_080466, chr. 3 | |
| Rat: 732aa, NM_019315, chr. 2q34 | |
| Conductance | Not determined |
| Activators | 1-EBIO, riluzole (3 |
| Blockers | Leiurotoxin/scyllatoxin (1.1 nM), apamin (10 nM), PO5 (25 nM), Tskappa (197 nM), Pi1-OH (330 nM), and Pi1-NH2 (250 nM), UCL1684 (9.5 nM); with micromolar affinity: bicuculline, amitriptyline, fluoxetine, desipramine, imipramine, nortriptyline, fluphenazine, promethazine, chlorpromazine |
| Channel distribution | Brain (substantia nigra |
| Mutations and pathophysiology | Longer polyglutamine repeats are over-represented in schizophrenic (especially negative-symptom form) individuals and patients with anorexia nervosa and spinocerebellar ataxia; a four-base deletion has been found in a patient with schizophrenia that truncates the protein just before the S1 segment and causes dominant-negative suppression of endogenous SK channels; protein and mRNA levels are increased in skeletal muscle following denervation and patients with myotonic muscular dystrophy; involved in the endothelium-mediated vasodilation (EDHF response); conditional knockout of KCa2.3 leads to hypertension and bladder instability |
| Pharmacological significance | Modulators of SK channel subtypes may have potential use in the treatment of myotonic muscular dystrophy, gastrointestinal dysmotility, memory disorders, epilepsy, narcolepsy, hypertension, and urinary incontinence |
The SK1 channel is moderately sensitive to apamin, and its gene is located on chromosome 19, whereas the SK2 channel has the strongest affinity for apamin and is located on chromosome 5. SK3 is moderately sensitive to apamin, and its gene is located on chromosome 1 [21].
Modulators of SK channel subtypes can be used to treat many diseases due to their different effects. For instance, NS309 promotes endothelial protection [24]; chlorzoxazone improves motor coordination [25]; DCEBIO reduces the number of evoked spikes, the firing frequency and increases the afterhyperpolarizing potential of infralimbic neurons, suggesting the potential use of SK channels modulators to treat patients with anxiety disorders [26]; 1-EBIO decreases epileptiform activity and positively affects cerebral ischemia [27, 28], while riluzole improves symptoms related to cerebellar ataxia [29] and significantly reduces acute arthritis [30]. On the other hand, apamin acts by inhibiting SK channels. It suppresses hepatic fibrosis [31, 32], attenuates cytokine production [33], and may reduce attentional deficits related to Alzheimer’s disease [34].
SK channels are encoded by the KCNN gene family [35], which is abundantly conserved in different species, suggesting an orthologous relationship. The KCNN1 and KCNN3 genes exhibit alternative splicing [36, 37], a mechanism that increases the complexity of gene expression [38]. A pre-mRNA containing introns and exons can generate different mRNA. In theory, 32 transcripts can be formed by the KCNN1 gene and 20 have already been found in the mouse brain [36]. However, the function of alternative splicing in SK channels has not yet been clarified.
SK channels are widely distributed in humans and animals, in central nervous system (CNS) and in peripheral tissue, among them, epithelium, smooth muscle and endothelium [39]. The aim of this study in characterize the SK channels throughout the brain. SK1 and SK2 channels are co-expressed by the neurons of the neocortex, cerebellum, hippocampus, and brain stem, and SK3 channels are located in the midbrain and hypothalamus [39]. Stocker and Pedarzani [40] performed a study using in situ hybridization to characterize the distribution pattern of SK channels in the rat brain [40]. They found that SK1 channels are mainly expressed in the neocortex. The CA1–3 layers of the hippocampus and the thalamic reticular nucleus have high expressions of both SK1 and SK2 channels. In contrast, the supraoptic nucleus has predominantly SK3 channels, while the inferior olivary nucleus has equivalent expressions of SK2 and SK3.
Rimini et al. [41] evaluated the distribution of SK channels in the CNS and peripheral tissues through real-time reverse transcription polymerase chain reaction. In their evaluation, all SK channels were expressed in the human brain and, only SK1 was not detected in the peripheral nervous system. SK3 exhibited the highest expression among the SK channels, followed by SK2 and SK1, respectively. SK1 was mainly found in the amygdala, the hippocampus, and the caudate nucleus. SK2 was primarily expressed in the pituitary gland and the liver. The highest expression of SK3 was detected in the substantia nigra of the midbrain. An immunocytochemistry analysis found that SK3 is specifically localized in the presynaptic terminals of the neuromuscular junctions [42]. Although the SK channel distribution has been well-mapped in previous studies, there are still controversies as to the cellular distribution of these channels.
All SK channels are expressed in the spinal cord and the dorsal root ganglion (DRG). SK1 and SK2 are preferentially located in the cytoplasm and cellular membrane of small cell bodies of DRG neurons. In contrast, SK3 is found in the cellular membrane and granular cytoplasmic clusters of small and large DRG neurons [43]. In addition, it has been demonstrated that SK1 and SK2 are expressed in both peptidergic and non-peptidergic nociceptors [43].
SK3 expression is higher in the laminae I–III of the spinal cord [44]. However, SK3 is also expressed in ependymal cells. This finding suggests that this channel might be expressed in other epithelial cell types [45]. SK3 is also expressed in motoneurons and may determine the apamin-sensitive after-hyperpolarization in this cell type [46, 47].
SK3 is found in both peripherin-positive and peripherin-negative neurons, indicating that this specific channel could play a role in the process of the transduction of nociceptive signals. The participation of SK channels in pain and nociception is further discussed. This immunoreactivity of SK3 in peripheral neurons is also compatible with the distribution of SK3 throughout the Rexed laminae (I–III) of the spinal cord [44].
It has been reported that SK3 channels are also found in non-neuronal glial
cells, such as the satellite glial cells (SGCs). A study that evaluated the role
of trigeminal ganglion SGCs in the mechanisms of trigeminal neuropathic pain
using a model of chronic constriction of the infraorbital nerve reported a
co-labeling of SK3 and glial cell markers, e.g., the inwardly rectifying K
The expression of SK3 in peripheral neurons and SGCs has been a matter of debate. According to some studies, SK3 is a specific marker of SGCs in vivo [49, 50]. SK3 immunolabeling has also been found in early-cultured cells [49]. Our group has evaluated changes that occur in SK3 expression in cultured trigeminal ganglion cells, and found important variations [51]. It was possible to detect a high expression of SK3, with some cells double-staining with ßIII-tubulin at 4h in culture. This was followed by a decrease in SK3 expression at 24 h and an increase at 48 h, indicating great plasticity of SGCs, especially in vitro. We hypothesized that a differentiation process occurred in the time frame that was analyzed. Therefore, the specific expression of SK3 in peripheral neural cells in vitro and in vivo must be explored in-depth with further studies.
SK channels are predominantly expressed in the nervous system. These channels are found in neurons and other cell types and contribute to a large variety of physiological functions. Functional studies show that SK channels are diverse, and their activity depends on neuronal types, developmental stages, and animal species. The activation of SK channels in the midbrain and cerebellum neurons is involved in muscle coordination and movement. In the hippocampus and amygdala, SK channels of pyramidal neurons regulate the amplitude of excitatory postsynaptic potentials (EPSPs). SK channel activation may also influence N-methyl-D-aspartate (NMDA) receptor activation, consequently playing a role in synaptic plasticity. Interestingly, blocking the activation of SK channels in hippocampus pyramidal neurons and the amygdala increases long-term potentiation (LTP), which can point to the involvement of SK channels in learning and memory. SK channels expressed in the mitochondrial membranes are proposed to be neuroprotective, for example, in protecting against glutamate toxicity or inhibition of reactive oxygen species (ROS) production [52].
It has been well-accepted that SK channels are essential for neuronal excitability. An action potential is characterized by a quick change in the voltage of the cellular membrane due to an influx of ions determined by the ionic concentration and the cellular membrane permeability [53, 54]. The action potential is followed in many cells by an after-hyperpolarization (AHP) period that prevents repetitive neuronal activation. SK channels are related to the phenomenon of after-hyperpolarization (especially in non-innervated skeletal muscle and in some nerve cells) since apamin (an SK channel blocker) abolishes the process of this phenomenon [55]. Considering that SK channels are blocked by apamin while BK channels are not, it has been proposed that small conductance calcium channels are involved in a larger variety of cells [55].
The regulation of neuronal excitability is crucial for healthy brain function. The equilibrium between depolarizing and hyperpolarizing ion channels determines the neuron’s firing activity. Potassium channels constitute the most prevalent ion channels. Since they mediate the outward potassium currents that repolarize and hyperpolarize the membrane potential, they are one of the most important elements of neuronal excitability [56].
Several categories of potassium channels mediate the after-hyperpolarization
process. This process repolarizes the neuronal membrane, limits both the
amplitude and width of the spikes, also controling neuronal activation.
Therefore, neurotoxicity related to excessive neuronal firing is avoided [57].
Because of their distinct activation-inactivation kinetics and Ca
The SK channels of the CNS neurons are activated by high concentrations of
intracellular Ca
Studies that evaluated the distribution of SK channel subunits and their pharmacological properties through specific toxins have led to the molecular identification of the channels that underlie the process of after-hyperpolarization in multiple brain regions.
High levels of SK2 subunits are associated with the presence of an apamin-sensitive after-hyperpolarization current in the pyramidal neurons of CA1 and CA3 in the hipocampus [65, 66]. The after-hyperpolarization currents in the CA1 pyramidal neurons of rats are blocked by apamin at a concentration approximately eight-fold higher than those required to block recombinant SK2 channels [67]. Given that SK1 and SK2 subunits are co-expressed in pyramidal CA1 neurons [68], and that SK2 channels are apamin-insensitive [69], the low apamin sensitivity of currents after the after-hyperpolarization is probably related to the development of heteromeric SK1/SK2 channels. In fact, there is evidence that rat heteromeric SK1 and SK2 channels are less apamin sensitive in heterologous expression systems [70]. Homomeric SK2 channels also underlie an apamin-sensitive after-hyperpolarization current that occurs in the cerebellum, which is critical for the intrinsic firing pattern of the Purkinje neurons [67, 71].
In the brainstem, the after-hyperpolarization of the motor neurons of the dorsal vagus nucleus influences spontaneous firing and is involved in the regulation of esophageal, gastric, and pancreatic functions [72] and is also blocked by apamin. The after-hyperpolarization current of the dopaminergic neurons of the midbrain is controlled by spontaneous firing activity, and SK3 channels are involved in this process [73].
The hypothalamus is another region of the brain with an increased expression of SK3 [40]. In the rostral hypothalamic basal region, SK3 expression is hormonally regulated [74]. The secretion of oxytocin and vasopressin from nerve terminals of magnocellular neurosecretory cells is partially regulated by an SK3-mediated after-hyperpolarization current that regulates phasic firing [40, 75]. SK3 channels also mediate the mAHP in the suprachiasmatic nucleus. Therefore, it regulates the firing rate, which undergoes diurnal modulation by the circadian rhythm [40, 76]. SK3 channels underlie the after-hyperpolarization in superior cervical ganglion neurons, which also regulate circadian rhythm via its connections with the pineal gland [46].
The overexpression of SK3 channels produces abnormal breathing patterns during a hypoxic challenge and compromises parturition [77]. After-hyperpolarization was selectively abolished in CA1 pyramidal neurons from knockout mice that lacked SK2 channels. However, this current was intact in mice that lacked SK1 and SK3 channels [78], which confirmed the predominant role of SK2 subunits in hippocampal pyramidal neurons [65, 66] and raised questions regarding the function of SK1. The splice variant SK3-1B, is known to suppress SK2 and SK3 channels [79, 80] and was recently used to generate a transgenic mouse model [81]. The expression of SK3-1B under the control of a neuronal promoter suppressed the expression of SK channels in several regions of the brain [81]. These transgenic mice showed severe cerebellar ataxia related to the downregulation of SK1 and SK2 expressions in the deep cerebellar nuclei neurons, resulting in the loss of the after-hyperpolarization current and, consequently, in increased spontaneous firing and hyperexcitability [81]. A strong and selective reduction in the apamin-sensitive after-hyperpolarization was also observed in layer V of neocortical neurons, where overexpression of SK1 or SK2 led to a selective enhancement of this current component [80]. These studies support the role of SK channels as regulators of intrinsic neuronal excitability and firing. Further studies are needed to evaluate and scrutinize their impact on cellular and network functions and to correlate them with specific changes in animal behavior [55].
It has been demonstrated that SK channels are important in controlling the action potential firing frequency and regulating dendritic excitability, synaptic transmission, and synaptic plasticity. In accordance with their role in modulating synaptic plasticity, SK channels exert control over several learning and memory tasks and may also participate in the mechanisms of several neurological disorders [82].
Modulating dendritic excitability and activation of SK channels by calcium influx during action potentials regulates the frequency of action potential discharge in most neurons. The location of the channel underlying this effect is unknown, but it is generally believed to be somatic near the action potential initiation site. Nevertheless, it has been shown that apamin-sensitive channels are also present in the dendritic tree and are activated by calcium surges from sources other than voltage-gated calcium channels. For example, calcium released from intracellular stores in dopaminergic [73] and cortical pyramidal neurons [66, 83] activates apamin-sensitive conductances, leading to hyperpolarizing potentials.
In Lamprey spinal motor neurons, SK channels can also be activated following dendritic activation of NMDA receptors, terminating the resulting dendritic plateau potential [84]. SK dendritic channels in these neurons also contribute to after-hyperpolarization and reroute excitatory inputs when triggered after-hyperpolarization. This shunting requires potential activation of SK channels, since blockade of SK channels alone does not affect individual excitatory postsynaptic potentials (EPSPs) or episodes of EPSPs [85]. Finally, activation of NMDA receptors in mitral cells of the olfactory bulb also activates dendritic SK channels, modulating dendritic excitability [86]. The regulation of synaptic transmission and the role of SK channels in synaptic transmission was first described in neurons in the substantia nigra and ventral tegmental area, where SK channels contribute to inhibitory post-synaptic potentials. Activation of SK channels follows the release of calcium from intracellular stores triggered by glutamate acting on metabotropic glutamate receptors [87].
An inhibitory post-synaptic conductance in auditory outer hair cells following activation by calcium influx through calcium-permeable nicotinic acetylcholine receptor is also mediated by SK channels [88]. Recently, SK channels have been shown to bypass fast excitatory synaptic transmission in the lateral amygdala and hippocampal pyramidal neurons [85, 88]. Calcium influx in these neurons via NMDA receptors during basal synaptic transmission activates SK channels co-localized in the spines of hippocampal and amygdala pyramidal neurons [85, 88]. The resulting hyperpolarization bypasses EPSP and increases the magnesium blockade of NMDA receptors. Apamin use reverses this effect and increases NMDA receptor-mediated calcium transients in the cephalic spine [88]. In the pyramidal neurons of the lateral amygdala, shunting of excitatory synaptic transmission by SK channels reduces the amount of depolarization during repetitive stimulation of cortical afferents, thus reducing the extent of the long-term potentiation (LTP) at these synapses [83]. Similarly, the blockade of hippocampal SK channels enhances the LTP after low-frequency tetanic stimulation of Schaffer collaterals [89] and lowers the threshold for LTP in the hippocampal CA1 pyramidal cells [90]. These effects have been attributed to a depression of the mAHP and the consequent increase of the action potential discharge. However, it has become clear that these effects are most likely due to the SK channel-mediated shunt on excitatory synaptic transmission rather than to the relatively minor regulation of the firing frequency [91]. Further defining the role of SK channels in limiting the LTP, it has been found that overexpression of SK2 channels in the hippocampus reduces the LTP in the CA1 neurons [92].
SK channels are involved in the processes of learning and memory through the modulation of basal excitatory synaptic transmission and plasticity [85]. The blockade of SK channels by a systemic administration of apamin in rats enhances learning in an object-recognition task [93]. In addition, the administration of apamin reversed the spatial navigation deficit induced by lesions in the medial septum and the hippocampus of mice in the Morris water maze spatial memory task [94, 95]. Corroborating the findings of these studies, apamin induced the expression of the immediate early genes c-fos and c-jun in the hippocampus, which are considered the initial markers for memory formation [96, 97].
Conversely, the overexpression of SK2 channels led to an impairment in the performance of rats in the Morris water maze, contextual fear conditioning, and amygdala-dependent cued fear conditioning [92, 98]. In addition, apamin enhanced learning in an appetite-motivated bar-pressing response in mice [93] and an olfactory discrimination learning task [93]. Finally, increases in the SK3 expression underlie an age-related deficit in hippocampal-mediated learning tasks [92, 99]. Together, these findings indicate that SK channels play a key role in negatively regulating learning and memory formation.
Several ion channels have phosphorylation sites for protein kinases. The phosphorylation by sites for protein kinases present in several ion channels that modulates the functioning or trafficking across those channels. However, only some studies examined the process of SK channel modulation. Despite the presence of many possible sites for the phosphorylation of the protein kinases A (PKA) and C, the biophysical evidence for the modulation of SK channels via these kinases still needs to be determined. PKA’s phosphorylation of the C-terminus of the SK2 channel has been demonstrated, and this process reduces the cell surface of this channel subtype [100]. According to previous studies, this indicates that SK channels may be regulated by membrane trafficking, similar to what occurs with voltage-dependent potassium channels [101].
As previously discussed, SK channels play a role in memory and learning.
Therefore, modulators of SK channels that improve performance in learning tasks
may be useful therapeutic agents in treating cognitive dysfunction and memory
disorders. Nevertheless, agents that block SK channels, such as apamin, currently
have a narrow therapeutic window. Therefore, there is a need for new agents that
offer fewer side effects regarding therapeutic purposes [101]. High doses of
apamin can induce epileptic-like activity, and agents that enhance the activity
of SK channels (called SK channel activators), such as
1-ethyl-2-benzimidazolinone (1-EBIO) or NS309, may be useful in treating
epilepsy. NS309 also modulates the activity of both pro-inflammatory and
anti-inflammatory cytokines by regulating the TSG-6/NF-
SK channel potentiators may be useful in treating depression, phobias, and other psychiatric disorders. For example, enhancing the activity of SK channels may raise fear conditioning thresholds [92] and apamin enhances performance in the forced swim test and increases measures of antidepressant activity [76]. It has been found that the binding of apamin to SK channels is high in the prefrontal cortex and low in the hippocampus of rats exposed to chronic unpredictable stress, indicating dysfunctional activity. In addition, this can be reversed by selective inhibition of SK3 channels with selective SK3 channel negative allosteric modulators or in knockout mice [104].
SK channels are also present in dopaminergic neurons in the midbrain and control firing patterns. Burst firing of these neurons triggers the release of dopamine, which is depleted in Parkinson’s disease. Blockade of SK channels causes the sudden firing of these neurons [105], suggesting that treating midbrain dopaminergic neurons with SK channel blockers may alleviate some symptoms of Parkinson’s disease. In fact, a preclinical study showed that the systemic administration of apamin increases the levels of striatal extracellular dopamine, which could not be detected after nigrostriatal lesions induced by the neurotoxin 6-hydroxydopamine (6-OHDA) in rats [105].
Furthermore, SK channels play an important role in endothelium-dependent hyperpolarization and thus have an important influence on the regulation of vasodilation. Therefore, SK channels are highly involved in regulating the vascular tone related to health and disease. For instance, it has been demonstrated that administering the SK channel activator NS309 before and during cardioplegic hypoxia protects the coronary microvasculature against cardioplegic-hypoxia and reoxygenation injury. However, this effect is reduced in diabetic coronary microvasculature [24]. Calcium-Activated Potassium Channels also influence the occurrence of preeclampsia, an important pregnancy-related complication. Li et al. 2020 [106], found that the dysfunction of SK and IK channels might be associated with deficient blood vessel formation and inadequate remodeling of spiral arteries, which are related to the development of preeclampsia.
SK channels’ role in nociception mechanisms has also been explored. In literature, some studies use drosophila models to understand nociceptive behavior. Activation of SK channels reduces nociceptive behavior in response to these stimuli [107], suggesting that these channels play essential roles in regulating the sensitivity of nociceptive neurons. Walcott et al. 2018 [107] evaluated the influence of SK channels on the thermal regulation of nociceptors, mechanical nociception behavior, and stability regulation. The nociceptor-specific requirement for SK was assessed using the SK-M transcript under the control of the ppk-GAL4 driver in the SK mutant background. This released the hypersensitive nociception phenotype of the SK mutant animals, confirming the site of action for SK in nociceptor neurons. It was found that, compared to the short isoforms, the long SK isoforms may be more important in controlling the thermal sensitivity of nociceptors. In Regulation Mechanical Nociception Behavior, the effects of SK were more specific for the nociception pathway, regulating nociceptor activity in mechanical stimuli and response to noxious thermal [107].
SK channels have been found to be capable of regulating the tonic firing of
nociceptive neurons. The behavior of substantia gelatinosa (SG) neurons, crucial
to the nociceptive transmission, was studied through whole-cell recordings. The
results showed that 1-EBIO attenuates spike discharges and increases the AHP
amplitudes of the neurons located in the SG, an effect mimicked by a high
Ca
It has been widely accepted that the expression of postsynaptic NMDA receptors
is associated with LTP and the development of chronic pain. However, the role of
expression of presynaptic expression of NMDA on chronic pain has not been fully
evaluated. One study demonstrated that presynaptic expression of NMDA receptors
in spinal cord neurons modulates the synaptic transmission in a nociceptive
tone-dependent form [109]. The results of that study indicate that this process
is determined by the influx of Ca
A recent study has also reported the expression of SK channels (in this case SK3) in the trigeminal ganglion [112]. Furthermore, a decreased expression of SK3 has been found following infraorbital nerve ligation, a classical model of trigeminal neuropathic pain in rodents. The administration of the SK channels agonist (CyPPA) significantly increased the pain thresholds (resulting in mechanical allodynia), while the administration of the SK channel antagonist apamin significantly increased the pain thresholds [112]. These findings indicate that SK channels, especially SK3, could be potential pharmacological targets in the treatment of trigeminal neuralgia and other types of trigeminal neuropathic pain. There is a considerable number of nonselective SK channel blockers such as apamin, scyllatoxin, UCL 1684, and UCL 1848, and a few SK2 selective blockers such as the peptide inhibitor Lei-Dab (7) [113], the scorpion toxin tapamin. The discovery and development of novel classes of selective inhibitors of SK1, SK2, and SK3 are crucial to determine the specific contribution of each SK channel in nociception mechanisms and pain [114].
According to one preclinical study, SK3 is confined to the SGCs and not expressed by the neurons of the trigeminal ganglion [50]. However, this concept must be taken cautiously when interpreting the results of in vitro studies, since phenotypic changes involving SK channels can occur in cell cultures of the trigeminal ganglion [51]. However, these results shed light on the expression of SK channels in the trigeminal ganglion cells and suggest that targeting SK channels could be of great value in the treatment of orofacial pain, especially those of neuropathic origin. In fact, the expression of SK and IK channels can be modulated by neuropathic factors as demonstrated by the significant increase in the percentage of SK1-positive cells after the treatment of DRG cultures with nerve growth factor and the stimulation of IK1 expression upon the administration of the neurotrophin 3, NT-3 [96]. Thus, the modulation of potassium channels with different compounds might be a promising therapy in the treatment of different types of pain.
Chronic pain results in a negative impact on the quality of life and compromises healthcare systems worldwide. The burden of chronic pain and the limited therapeutic options for these patients, drives the search for novel pharmacological therapies that could offer more efficient and safer treatments. Riluzole, a drug used in the management of amyotrophic lateral sclerosis (ALS), has shown positive antinociceptive effects in preclinical models of both inflammatory [115] and neuropathic pain [116]. Changes in regions related to somatosensory processing (e.g., thalamic nuclei and postcentral gyrus) have been found in patients with ALS. Future studies will evaluate how these findings relate to the effects of riluzole on the brain network altered in ALS and chronic pain [117]. One study that used brain slice electrophysiology and behavioral evaluation in an experimental model of neuropathic pain through spinal nerve ligation, found that riluzole has a positive effect in reducing neuropathic pain. This occurs through SK channel-dependent and independent mechanisms in the amygdala. These results also indicate that this action could be related to central amygdala output nucleus (CeA) neurons, which in turn, have high levels of SK channels [118]. The participation of the CeA in the mechanisms of riluzole-induced pain modulation through the activation of SK channels were also demonstrated by another study that used an experimental model of knee arthritis induced by kaolin/carrageenan. This study found that riluzole inhibits supraspinally pain behaviors by activating SK channels, but not BK channels in the CeA amygdala [119]. These findings suggest that riluzole is a promising compound to treat chronic pain and that this mechanism of action is probably related to the activation of SK channels located in the CeA. However, clinical studies combined with neuroimaging analysis will be necessary to verify these hypotheses. In addition to efficacy, the long-term safety of this compound, and newer compounds for chronic pain needs to be determined.
SK channels are known to translate changes in the level of the intracellular
second messenger, Ca
MFD, and PG designed the study. LGAF, CPV and CKS performed the research, developed the search strategy and literature search. MFD, LGAF, CPV, CKS, and PG wrote the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript.
Not applicable.
Not applicable.
This research received no external funding.
Parisa Gazerani is serving as one of the Editorial Board members and Guest editors of this journal. We declare that Parisa Gazerani had no involvement in the peer review of this article and has no access to information regarding its peer review. Full responsibility for the editorial process for this article was delegated to Gernot Riedel. The other authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.