1 Department of Anesthesiology, Quanzhou First Hospital Affiliated to Fujian Medical University, 362000 Quanzhou, Fujian, China
Abstract
Background: Apigenin has been reported to exhibit anti-inflammatory and
anti-oxidative activities. This study aimed to investigate the protective role of
Apigenin on chemotherapy-induced peripheral neuropathy (CIPN). Methods: CIPN
mouse model was established using Paclitaxel treatment. Hot plate and tail prick
latency tests were performed to examine the allodynia and hyperalgesia behaviors.
Anti-inflammatory and anti-oxidative effects of Apigenin on CIPN were determined
by enzyme-linked immunosorbent (ELISA) assay, Western blot, and qRT-PCR. Nuclear recruitment of nuclear factor
erythroid 2-related factor 2 (NRF2) was analyzed to evaluate the underlying
mechanisms of the protective effects of Apigenin. Results: Apigenin significantly
alleviated CIPN-induced nociceptive behaviors of CIPN mice. It also decreased the
TNF-
Keywords
- Apigenin
- CIPN
- microglia
- inflammation
- NRF2
Chemotherapy-induced peripheral neuropathy (CIPN) is one of the most common side effects of chemotherapy drugs, which results in a variety of symptoms including pain, hyperalgesia, tingling, losing sensation, numbness, paresthesia and gait disturbance [1, 2]. CIPN affects the life quality of patients and jeopardizes the patient’s survival [1]. CIPN is neuropathic pain that caused by axonal degeneration. Multiple agents, including glutathione, amifostine, and vitamin E, were evaluated as prevention strategies for CIPN, with no agent demonstrating efficacy. Moreover, adjunct therapy with topical agents, tricyclic antidepressants, and anticonvulsants, such as pregabalin and gabapentin, have shown limited efficacy [3, 4, 5], which suggests that the mechanism of CIPN is different from the typical neuropathic pain. Although American Society of Clinical Oncology (ASCO) recommends duloxetine as the only potential treatment for CIPN [6], unfortunately, several side effects of duloxetine have been reported, including nausea, fatigue, dry mouth, drowsiness, constipation, losing appetite, and dizziness. Furthermore, other drugs approved and licensed for neuropathic pain have been reported have a broad range of debilitating side effects [7]. In addition, traditional analgesics, such as non-steroidal anti-inflammatory drugs and opioids, are clinically ineffective in reducing neuropathic pain, while opioids have addictive side effects, and lead to seizures and respiratory disturbances [8].
Microglia are important neural-specific immune cells that influence brain
development, maintenance of the neural environment, and response to neural injury
that associated with the development of neuropathic pain [9]. Previous studies
have shown that microglia exacerbated neuropathic pain through triggering receptor expressed on myeloid cells 2/DNAX-activating protein of 12 kDa (TREM2/DAP12)
signaling [10]. Neurons interact with microglia through purinergic signaling
during neuropathic pain [11]. Astrocytes interference with microglia can lead to
neuropathic pain by regulating the stromal cell-derived factor 1/C-X-C motif chemokine receptor 4 (the CXCL12/CXCR4) axis [12]. Xu et al.
[13] demonstrated that nicotinamideadenine-dinucleotide phosphate oxidase (NOX2)-induced oxidative stress can increase the plasma
membrane translocation of protein kinase C
Apigenin belongs to Apigenin family that presents in fruits and vegetables, which has been used as an herbal medicine in China [16]. In mice, Apigenin inhibited the BV-2 microglia proliferation, resulting in apoptosis [17]. In addition, Apigenin also suppressed the production of prostaglandin E2 (PGE2) and nitric oxide (NO) in BV2 microglia via inhibiting NO synthase type 2 (NOS2) and cyclooxygenase type 2 (COX2) protein levels, and oral Apigenin had protective effects on neuronal injury in mice with cerebral artery occlusion [18]. In view of the regulatory effects of Apigenin on microglia function and neuropathic pain, we explored whether Apigenin can alleviate neuropathic pain by regulating the function of microglia in this study.
Eight-week-old CD-1 mice (male) were obtained from GemPharmatech (Nanjing, China). All mice were housed in polypropylene cages under 12–12 hour light-dark cycles at 24 °C with 70% humidity. Mice were fed with standard chow diet and sterile filtered water. The animal study and treatment protocols were approved by Quanzhou First Hospital Affiliated to Fujian Medical University. The CIPN mouse model was established as described previously [19]. Mice were divided into six different groups randomly. One group of mice was intraperitoneally (i.p.) injected with saline, which was set as normal control (NC). The mice in CIPN groups were injected 2 mg/kg/day Paclitaxel (PTX, #T7191, Sigma, St. Louis, MO, USA) from Day 0 to Day 6. The daily administration of Apigenin (#10798, Sigma, St. Louis, MO, USA) was started at Day 4. Three doses (25, 50, 75 mg/kg) were used in this study. The treatment ended at Day 20. Gabapentin (GABA, #1287303, Sigma, St. Louis, MO, USA) injection (100 mg/kg) was set as positive control. The pain behaviors of treated mice were monitored during the treatment, and surgical spinal cord tissues were collected after the treatment for the following analysis.
The basal pain behaviors of the normal control and CIPN mice related to allodynia and hyperalgesia were assessed by hot plate and tail prick latency tests. Hot plate test was carried out to measure the response latencies by using the Perspex cylinder as described previously [20, 21]. Tail flick test was carried out to assess the anti-nociceptive effect of certain treatment through detecting the response latencies. The plantar test device (7370 plantar test) was used to performed tail flick test as described previously [22, 23]. Tests were carried out on Day 0 (started PTX administration), Day 6, Day 11, and Day 18.
Mice were euthanized using CO
The inflammatory factors (TNF-
Total RNA was extracted from the surgical spinal cord tissues, and isolated with
Beyotime RNAeasy™ Kit (Beyotime, Shanghai, China). The cDNA was
synthesized using the Vazyme Script RT SuperMix (Vazyme, Nanjing, China). ABI
StepOne™ System (Applied Biosystems, Foster City, CA, USA) was
used to perform qRT-PCR with Vazyme SYBR Green Master Mix (Vazyme, Nanjing,
China). The 2
Total protein of surgical spinal cord tissue was extracted using RIPA buffer
containing phosphatase/protease inhibitor cocktail (Sigma, St. Louis, MO, USA).
Nuclear and cytoplasmic protein was extracted by using the nuclear extract kit
(Active Motif, Shanghai, China). The 12% SDS-PAGE gel and nitrocellulose
membrane (Bio-Rad, Hercules, CA, USA) were used for Western blot as described
previously [24]. Primary antibodies of CD11b (ab133357, 1:1000 dilution in
blocking buffer (5% nonfat milk)), CD68 (ab283654, 1:1000 dilution), and CD206
(ab64693, 1:1000 dilution) were purchased from Abcam (Cambridge, MA, USA).
Primary antibodies of NRF2 (#12721, 1:1000 dilution), HDAC1 (#34589, 1:1500
dilution),
The Prism 8.0 (GraphPad, San Diego, CA, USA) was used to assess significant
differences among different treated groups in this study. Results were shown as
mean
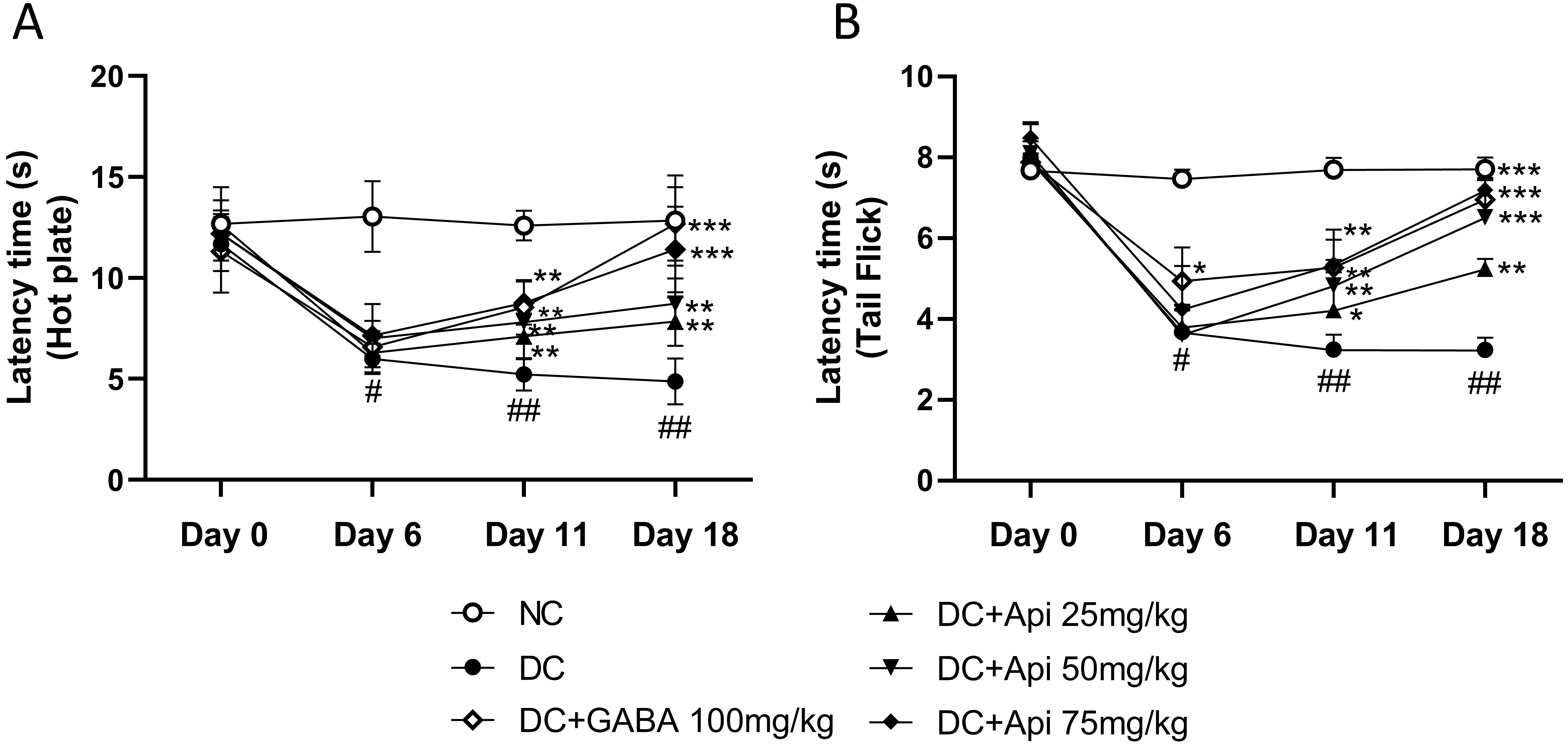
To investigate the therapeutic effects of Apigenin on chemotherapy-induced pain, we established the CIPN mouse model using the PTX treatment as described previously [8], and then performed hot plate and tail prick latency tests. As shown in Fig. 1A, CIPN reduced latency to half in all treated groups except the NC group which did not receive PTX chemotherapy at Day 6. Latency increased significantly in the CIPN-treated groups over time. At Day 11, the latency of GABA group (as a positive control) and Apigenin treated group increased significantly compared to that of disease control (DC) group (Fig. 1A). At Day 18, the latency of high dose of Apigenin treated group significantly increased compared to DC group. The group receiving the medium dose of Apigenin had increase in latency during this period too, which indicated the dose-dependent effect of Apigenin administration in hot plate test. To further assess the modulation of pain sensation and relief of mechanical hyperalgesia by Apigenin treatment, we performed tail prick latency test, and similar results to hot plate test were observed (Fig. 1B).
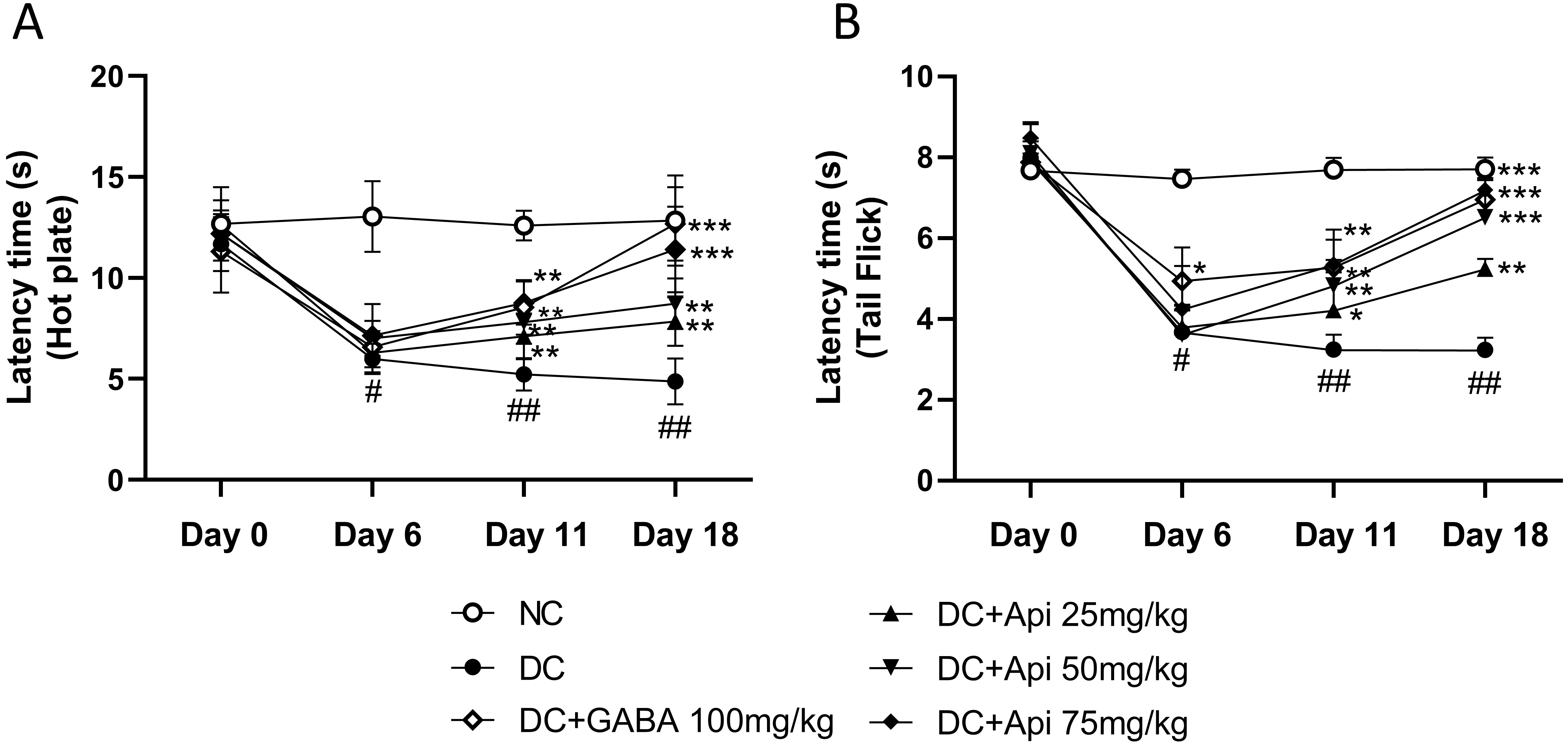 Fig. 1.
Fig. 1.Apigenin alleviated CIPN-induced nociceptive behavior of CIPN
mice. Nociceptive behavior was determined by the latency time measured by hot plate (A) and Tail Flick (B). *p
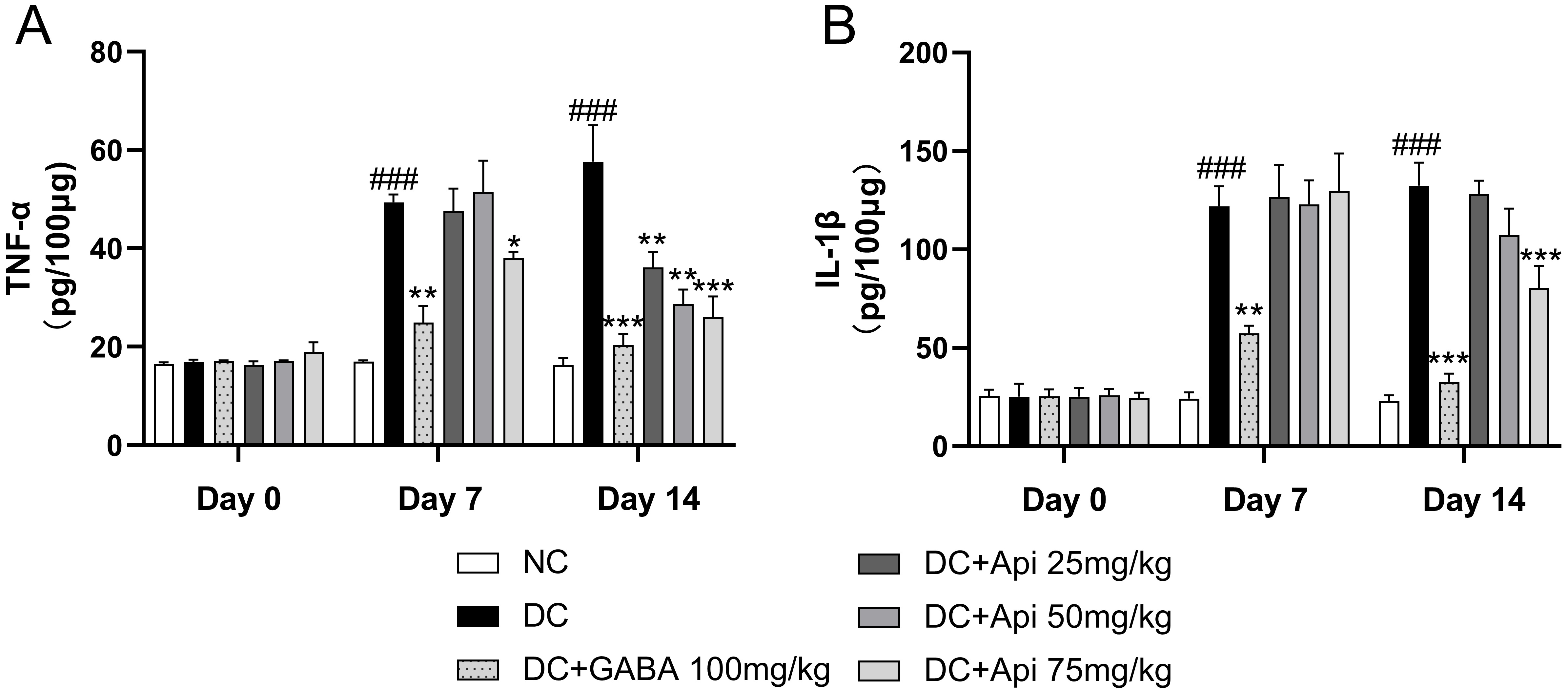
Next, we examined the effects of Apigenin treatment on the levels of the
pro-inflammatory cytokines TNF-
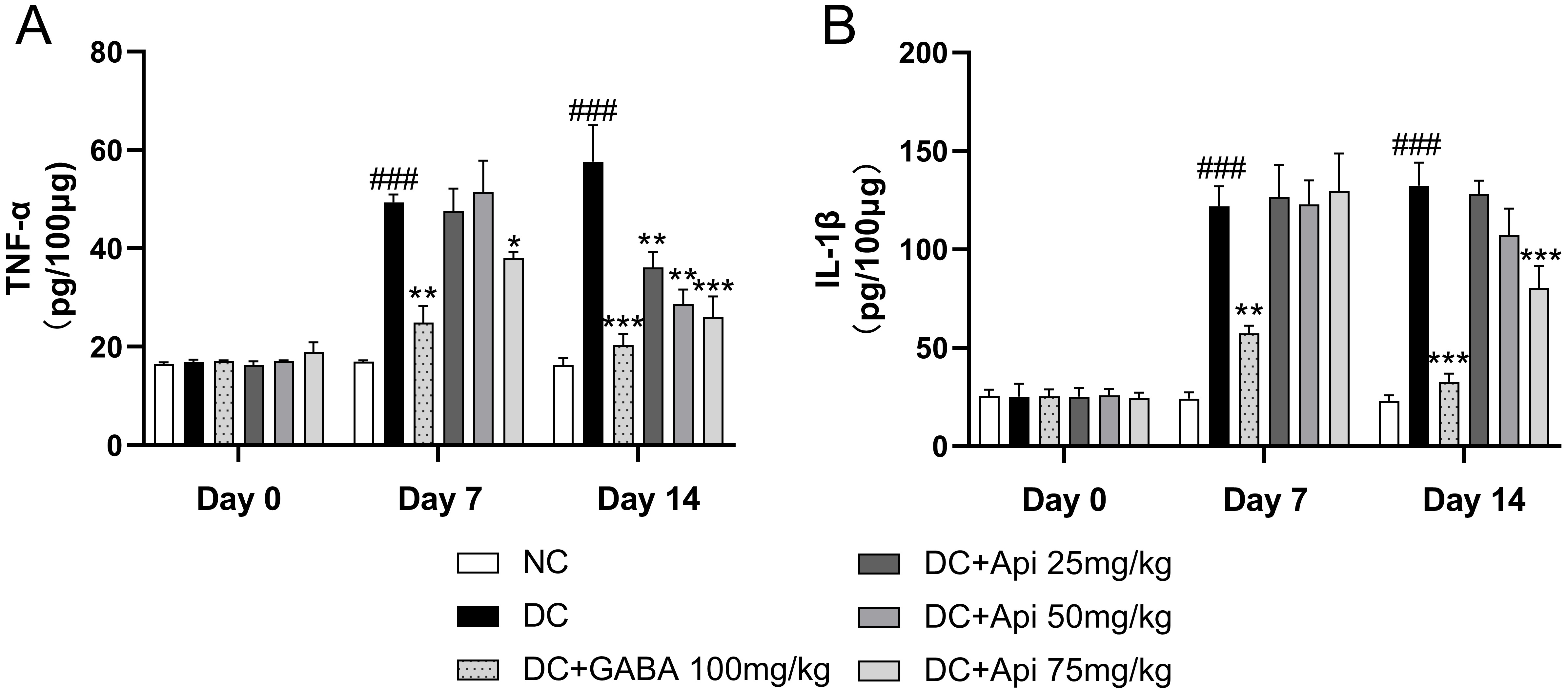 Fig. 2.
Fig. 2.Anti-inflammatory effect of Apigenin treatment was evaluated
through the measurements of TNF-
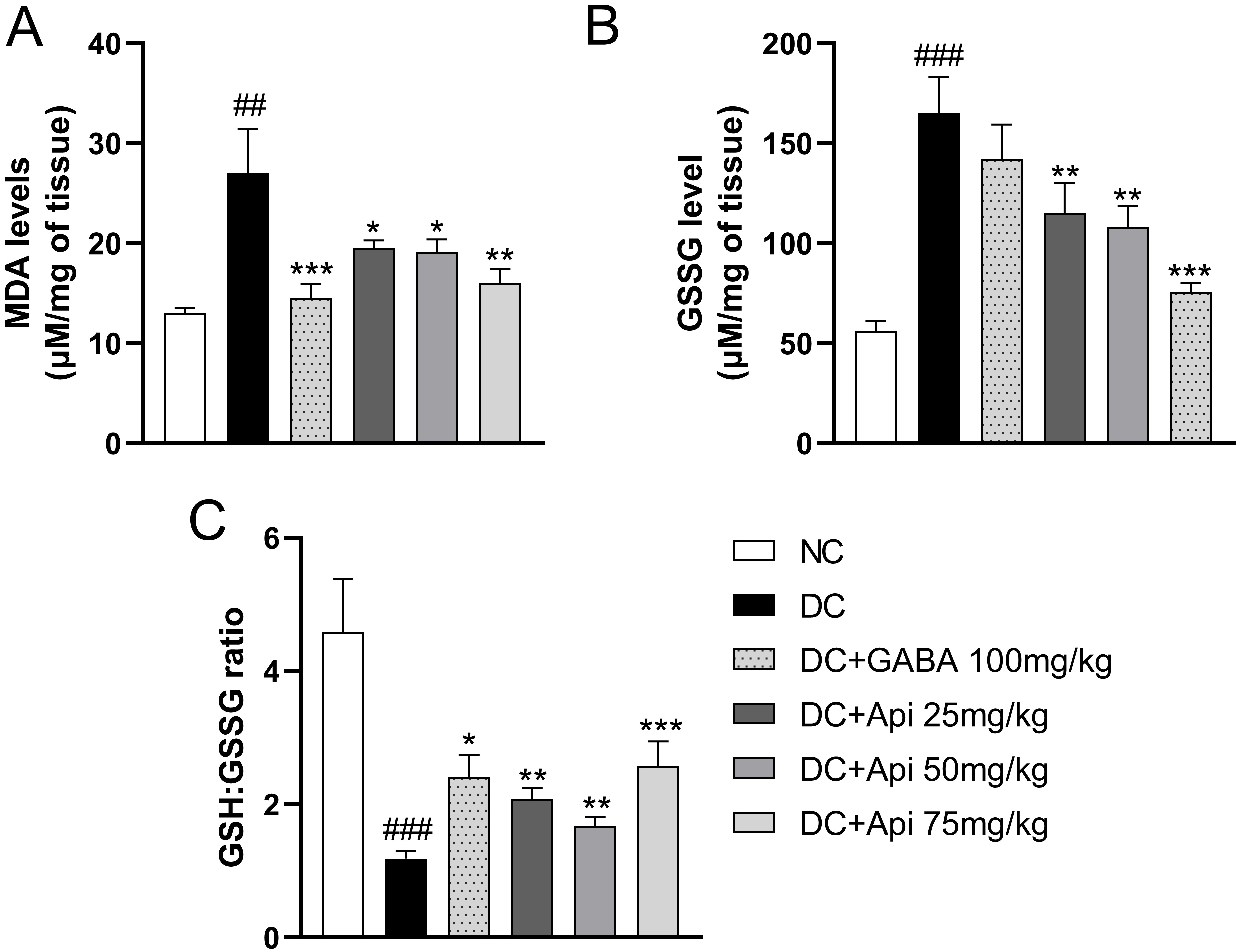
The elevated oxidative stress is another important feature of CIPN [25]. Therefore, we examined GSSG and MDA levels and GSH:GSSG ratio in surgical spinal cord tissue to assess the effects of Apigenin treatment on oxidative stress in CIPN mice. In comparison with the control group, the lipid peroxidation in the CIPN group was enhanced, and the level of MDA was significantly increased, while GABA and Apigenin treatments significantly decreased MDA levels in each treatment group (Fig. 3A). Interestingly, GSSG level was significantly increased in CIPN group compared that of the control group. GABA treatment showed a slight decrease in GSSG levels, however, GSSG levels were significantly decreased in all Apigenin treated groups compared to DC group (Fig. 3B). Likewise, GSH:GSSG ratio was significantly elevated in all treatment groups compared to the DC group (Fig. 3C). The above data indicated that Apigenin alleviated CIPN-induced oxidative stress of CIPN mice.
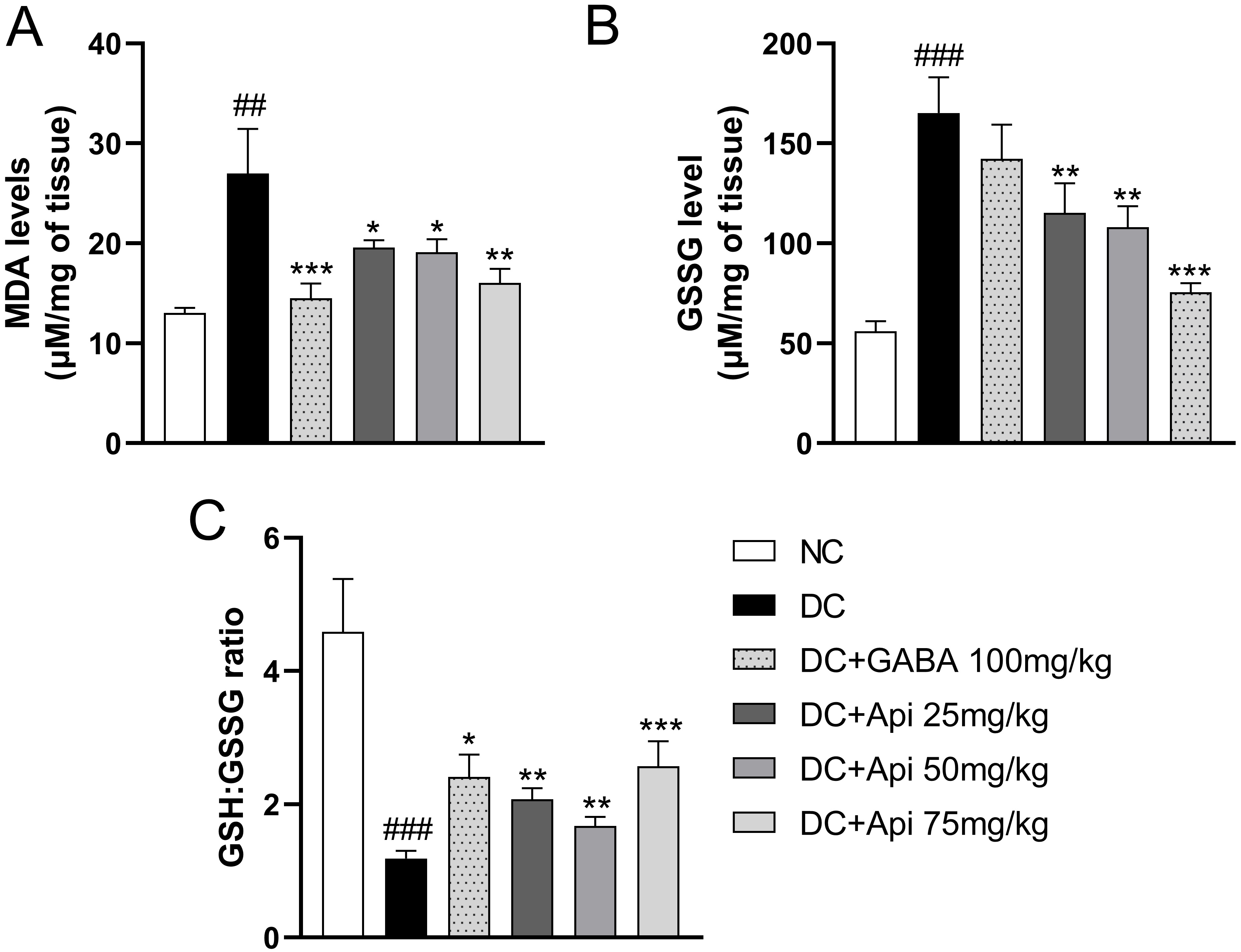 Fig. 3.
Fig. 3.Apigenin alleviated CIPN-induced oxidative stress and
inflammation of CIPN mice. Effects of Apigenin treatments on the oxidative
stress status in surgical spinal cord tissue were evaluated through MDA level
(A), GSSG level (B), and GSH:GSSG ratio (C). Data are represented as mean
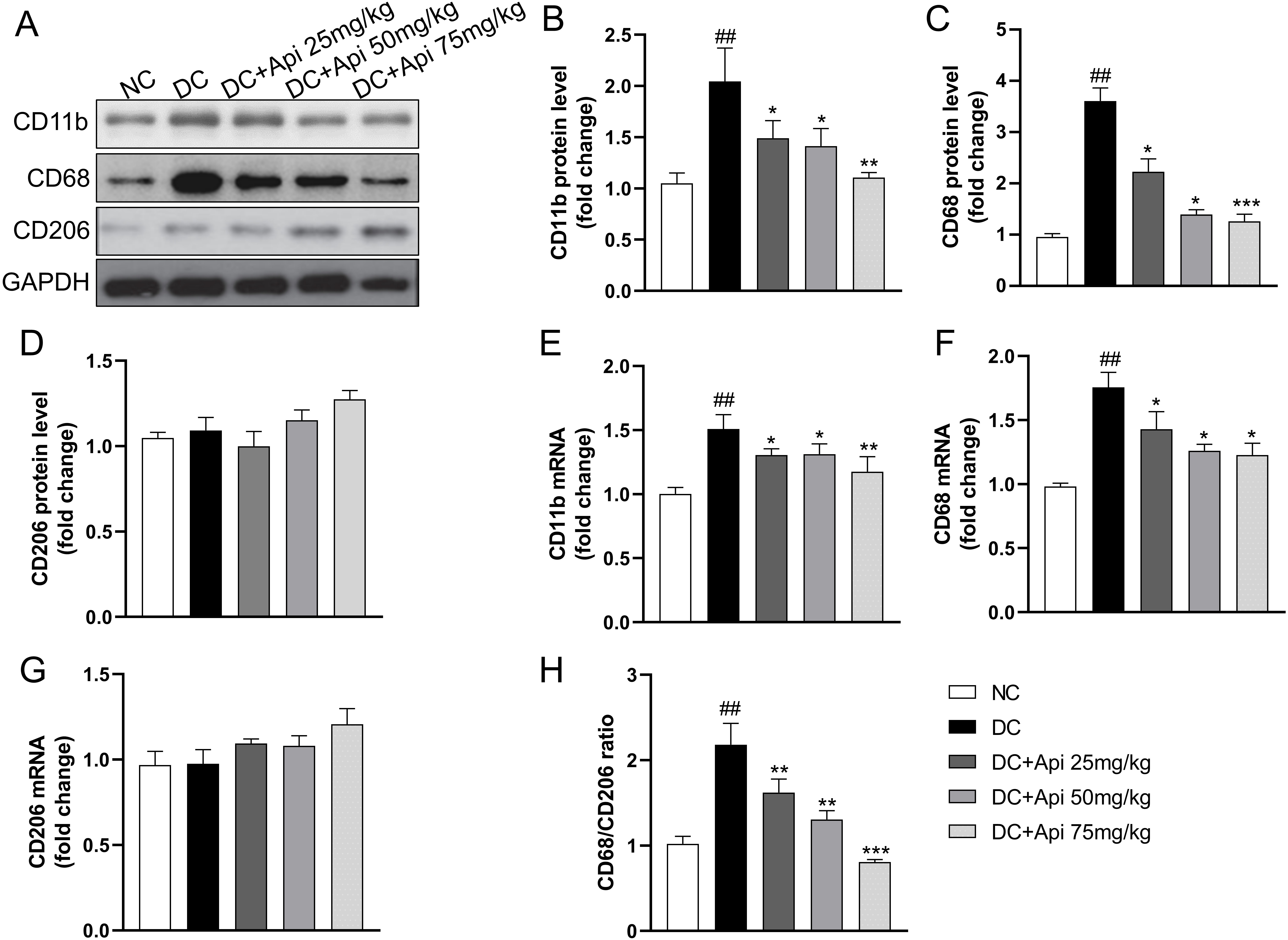
To further explore the underlying mechanism of Apigenin on CIPN, we assessed changes in pro-/anti-inflammatory phenotypes of microglia from protein and gene levels through detecting the expression of microglia markers in the surgical spinal cord tissue. CIPN promoted the expression of CD11b, CD68 protein, but had no significant effect on CD206, Apigenin administration significantly decreased the expression of CD68 and CD11b (Fig. 4A–D), which was further verified at the gene expression levels (Fig. 4E–G). Notably, the CD68/CD206 ratio was elevated in the CIPN group and significantly decreased after Apigenin administration (Fig. 4H). These data suggested that Apigenin suppressed pro-inflammatory and anti-inflammatory phenotypes of microglia markers in the surgical spinal cord tissue of CIPN mice.
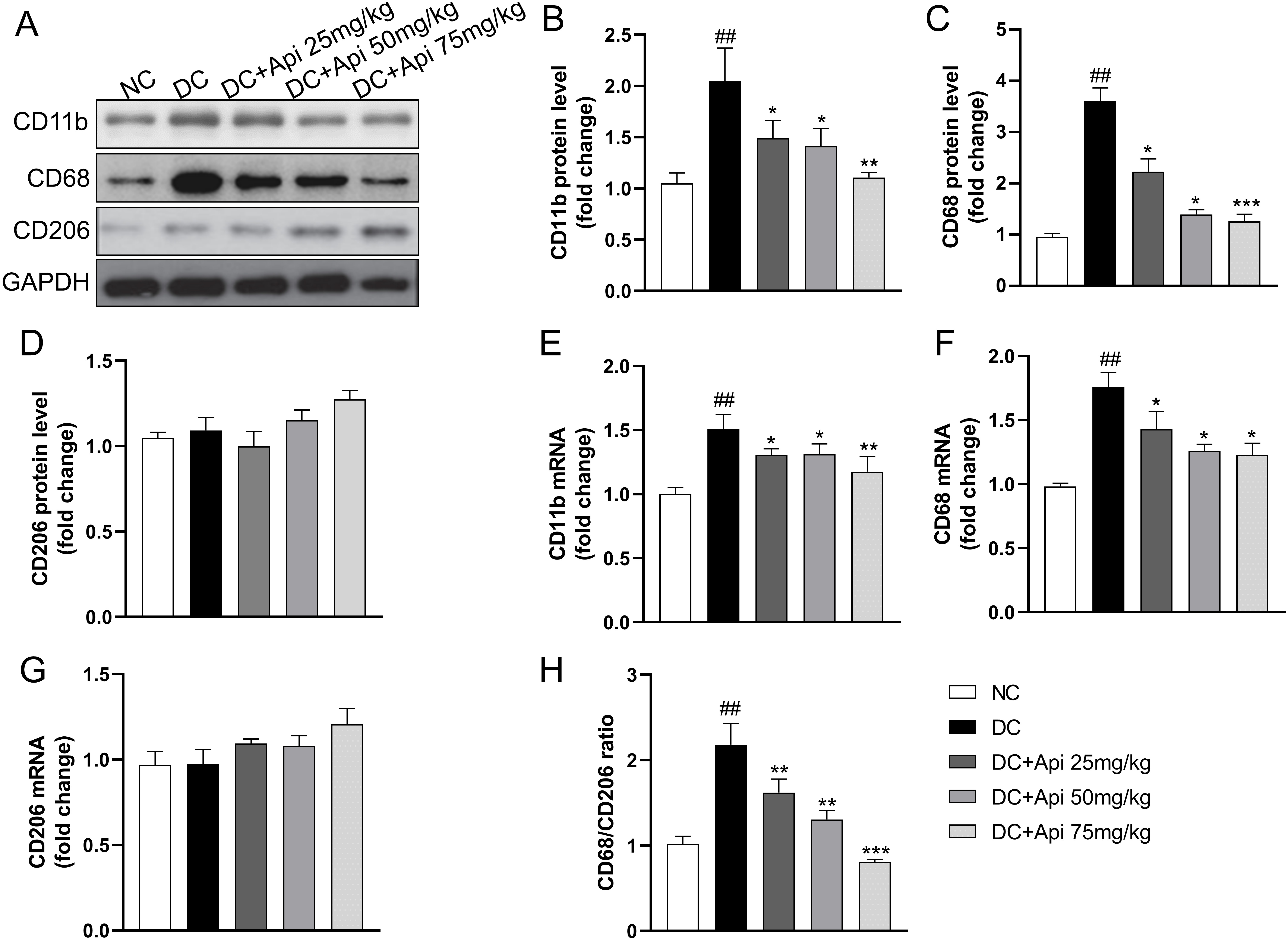 Fig. 4.
Fig. 4.Apigenin altered pro-inflammatory and anti-inflammatory
phenotypes microglia markers of the nerve tissue of CIPN mice. (A) A
representative blot of CD11b, CD68, CD206 of the nerve tissue. (B–D) A
quantitative analysis of CD11b, CD68, CD206 protein level. Fold increase of
pro-inflammatory phenotype markers (CD11b, CD68) (E,F) and anti-inflammatory
phenotype microglia markers (CD206) (G) mRNA. (H) Fold increase of the ratio of
CD68/CD206. Data are represented as mean
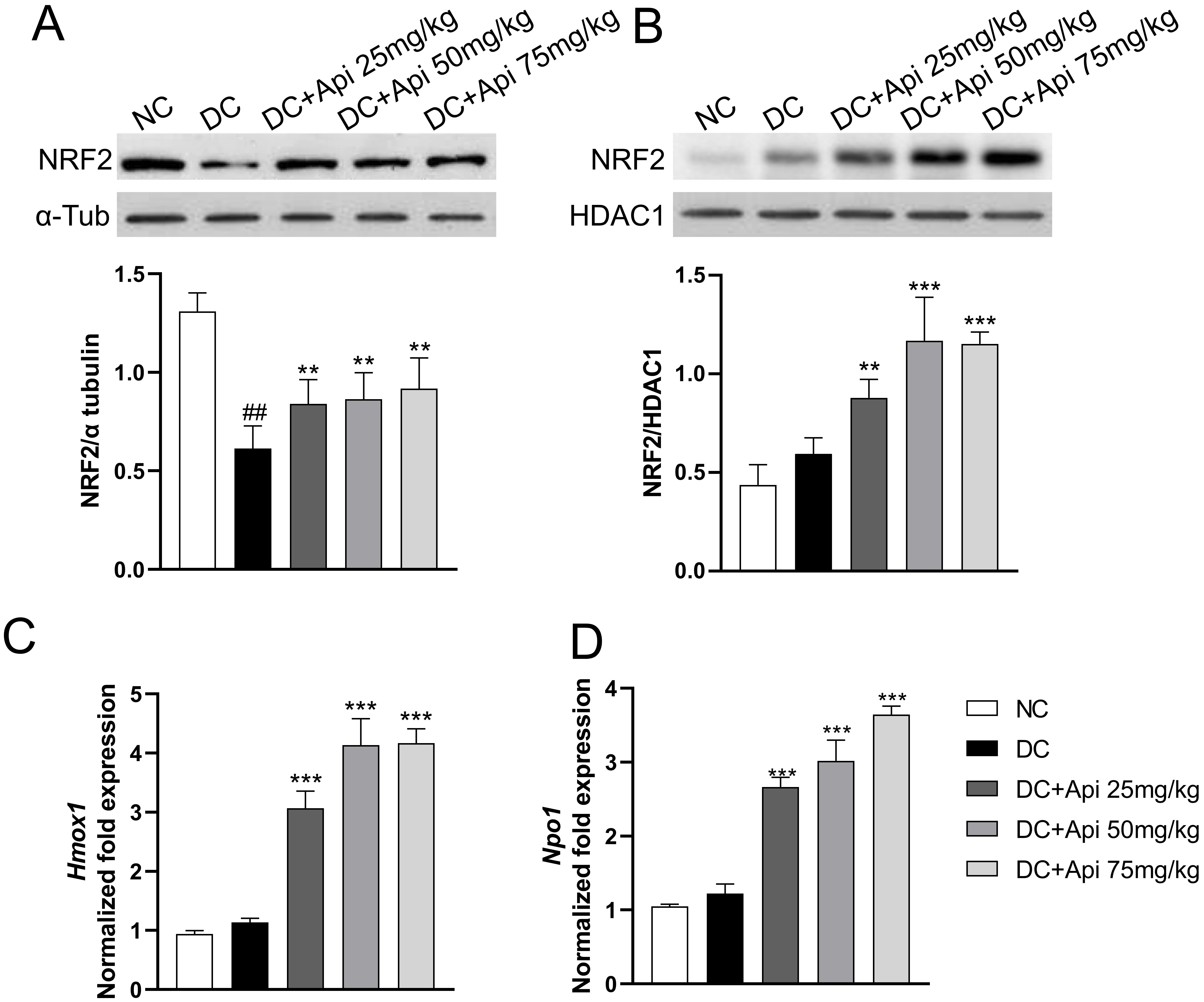
Recent studies have shown that Apigenin affected the occurrence and development of various diseases through the NRF2/ARE signaling pathway [26]. We determined NRF2 protein levels in the cytoplasmic and nuclear fractions of microglia from lumbar spinal cord of control and CIPN mice. The results showed that cytoplasmic NRF2 protein in the all CIPN groups was decreased relative to the normal group, while the nuclear NRF2 protein was increased in microglia after Apigenin administration (Fig. 5A,B). To further verify the nuclear translocation of NRF2, we detected the expression of its downstream target genes Heme oxygenase 1 (Hmox1) and NAD (P) H quinone oxidoreductase1 (Nqo1) by RT-qPCR. The results showed that the expression of Hmox1 and Nqo1 genes was significantly upregulated in Apigenin-treated CIPN mice in a dose-dependent manner (Fig. 5C,D). All these data indicated that Apigenin induced nuclear recruitment of NRF2 and activated the NRF2/ARE pathway in microglia.
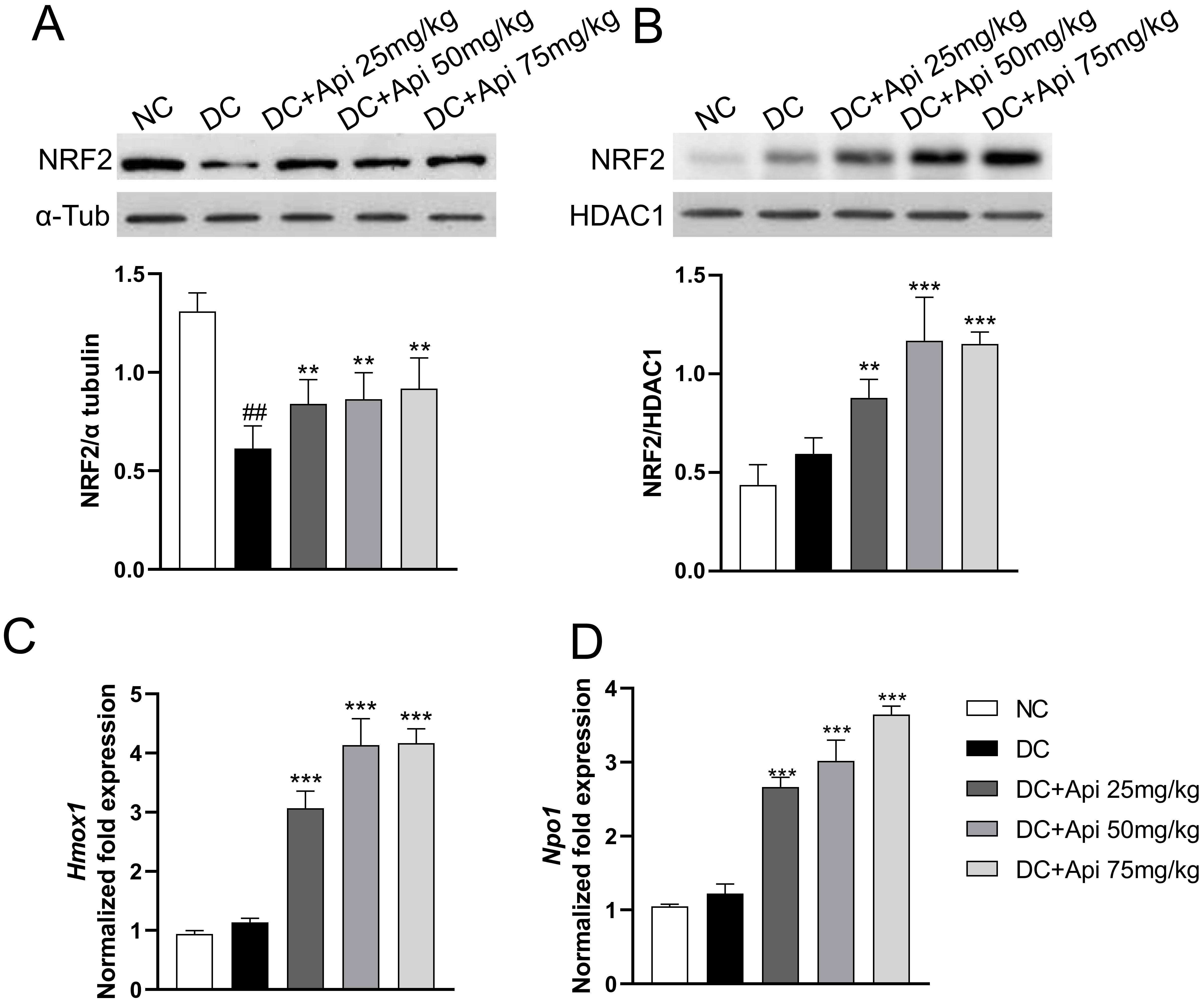 Fig. 5.
Fig. 5.Apigenin induced nuclear recruitment of NRF2. The localization
of NRF2 protein was evaluated in isolated microglia from different groups. (A,B)
Cytoplasmic and nuclear NRF2 protein expression (80 kDa) was evaluated in our
experimental conditions.
Numerous studies have investigated the effects of flavonoids on various biological phenomena and mechanisms to develop new therapeutic tools [27, 28]. Apigenin has been used as an herbal medicine in China for its anti-inflammatory and anti-oxidative activities. In this study, we established the PTX-induced CIPN mouse model, investigated the protective role of Apigenin on microglia, and revealed that Apigenin relieves neuropathic pain by regulating the function of microglia.
Microglia are important neural-specific immune cells that influence brain
development, neural environment maintenance, and neural repair [29]. Microglia
has been reported to involve in neuropathic pain in nerve injury [30]. In
neuropathic pain, neurons interact with microglia via purinergic signaling, the
interference between astrocytes and microglia also causes neuropathic pain. The
elevation of both oxidative stress level and inflammatory cytokine production
contribute to CIPN significantly [11, 12]. In line with the above findings, we
found that TNF-
Recent studies have shown that plant-derived active molecules such as Apigenin affect the occurrence and development of various diseases through the NRF2/ARE signaling pathway [26]. Western blot data showed that Apigenin administration significantly increased the nuclear NRF2 recruitment in microglia, which was further confirmed by the up-regulation of the downstream targets of NRF2/ARE signaling pathway. Given NRF2 plays an important role in regulation of the expression of antioxidant genes that exert anti-inflammatory functions [31]. We speculate that the anti-inflammation and -oxidative stress functions of Apigenin in CIPN mice are mediated by the induction of nuclear recruitment of NRF2 and activation of NRF2/ARE signaling pathway. Meanwhile, several important questions need to be addressed in following studies, such as whether the inhibitory effect of Apigenin is specifically on CIPN pain instead of all pain sensation. Also, further tail flick and hot plate tests need to be performed to establish that PTX does in fact observably lower pain threshold. The antioxidant role of Apigenin at gene expression and enzyme activity levels that related to oxidative stress regulation process could be profiled. Last, the underlying mechanism of Apigenin-induced nuclear localization of NRF2 needs more delicate experiments to elucidate.
Apigenin alleviates PTX-induced CIPN in mouse model through the regulation of inflammation and oxidative stress in microglia. This action is mediated by the Apigenin-induced nuclear localization of NRF2 and activation of NRF2/ARE pathway.
Data and materials could be obtained upon reasonable request to the corresponding author.
WJX, WQX, CCJ, ZMK, NZL conducted the experiments, analyzed the data, and wrote the manuscript; WJX conceived the study. All authors discussed the results and approved the final manuscript.
The animal study and treatment protocols were approved by Quanzhou First Hospital Affiliated to Fujian Medical University (365p2.v1).
Not applicable.
This work was funded by Fujian Natural Science Foundation (2020J011278).
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.