1 Biological Sciences, Faculty of Environmental and Life Sciences, University of Southampton, SO17 1BJ Southampton, UK
2 Centre for Human Development, Stem Cells and Regeneration, University of Southampton, SO17 1BJ Southampton, UK
Academic Editors: Giovanni Grasso and Rafael Franco
Abstract
Chondroitin sulfate proteoglycans (CSPGs) present a formidable barrier to regrowing axons following spinal cord injury. CSPGs are secreted in response to injury and their glycosaminoglycan (GAG) side chains present steric hindrance preventing the growth of axons through the lesion site. The enzyme chondroitinase has been proven effective at reducing the CSPG GAG chains, however, there are issues with direct administration of the enzyme specifically due to its limited timeframe of activity. In this perspective article, we discuss the evolution of chondroitinase-based therapy in spinal cord injury as well as up-to-date advances on this critical therapeutic. We describe the success and the limitations around use of the bacterial enzyme namely issues around thermostability. We then discuss current efforts to improve delivery of chondroitinase with a push towards gene therapy, namely through the use of lentiviral and adeno-associated viral vectors, including the temporal modulation of its expression and activity. As a chondroitinase therapy for spinal cord injury inches nearer to the clinic, the drive towards an optimised delivery platform is currently underway.
Keywords
- chondroitinase
- CSPGs
- gene therapy
- spinal cord injury
- viral vectors
Chondroitinase ABC (ChABC) is a bacterial enzyme from Proteus vulgaris which has shown significant potential in the treatment of spinal cord injury (SCI). It degrades chondroitin sulphate proteoglycans (CSPGs), molecules found to be upregulated after SCI specifically within and around the glial scar, that have been shown to actively suppress axon regeneration and plasticity [1]. The glial scar formed after SCI consists of microglia, astrocytes, oligodendrocyte precursor cells and meningeal cells, of which astrocytes, oligodendrocyte precursor cells and meningeal cells secrete inhibitory CSPGs [2, 3, 4]. CSPGs are a principal constituent of the extracellular matrix (ECM) of the central nervous system (CNS), specifically CSPGs are present in perineuronal nets (PNNs), a structure which plays an important role in controlling plasticity in the adult CNS [1]. The major CSPGs in the central nervous system (CNS) are aggrecan, versican, neurocan, brevican, and phosphacan [5]. The first four belong to the lecticans (or hyalectans) family, binding to hyaluronan and tenascins which serve to reinforce the ECM; while the latter phosphacan is an extracellular splice variant of the receptor-type protein tyrosine phosphatase beta [5]. The majority of evidence suggests that CSPG-induced inhibition is due to the glycoaminoglycan (GAG) chains [1, 6]. Specifically, the synthesis of CSPGs starts with an initial serine residue of the core protein, which is then added with an additional xylose, two galactose and a glucuronic acid [7]. This process initiates the formation of GAG chains. The GAG chain is further polymerized by the addition of repeating N-acetyl galactosamine (GalNAc) and GlcA and is modified by chondroitin sulfotransferases. The ChABC enzyme has dual endolyase and exolyase subtypes whereas the endolyase digests the sulphated GAG chains of CSPGs, and the exolyase degrades the releasing polysaccharides into disaccharides [5]. Therefore, considering CSPGs are a major barrier to axonal regeneration, use of ChABC provides a promising treatment for SCI.
Various in vivo studies across different animal species have demonstrated the potential effect of ChABC administration on axonal sprouting, regeneration and growth [5, 8, 9, 10]. During that time, the approach to ChABC administration has evolved with initial studies focusing on applying the enzyme in single or repeated doses. For example, Barritt and colleagues reported in a rat model of SCI that bolus intrathecal injections of the ChABC enzyme when delivered immediately after a C4 dorsal column crush lesion injury and subsequently on day 2, 4, 6, 8, 10 after SCI, significantly promoted plasticity of corticospinal, serotonergic, and primary afferent fibres proximal to the lesion site [11]. Another study carried out by Mondello and colleagues utilised intralesional injections of ChABC concurrent with the T10 spinal hemisection injury on cats which was repeated every 2 days for 2 weeks [12]. This approach significantly increased the number of rubrospinal tract axons extending below the lesion and improved locomotor recovery in horizontal ladder walking and ipsilateral hindlimb placement, compared to controls [12]. Furthermore, a study using thoracic dorsal column crush injured YFP-H transgenic mouse reported that ChABC enzyme administration via intracerebroventricular infusion had a neuroprotective effect on cortical layer V projection neurons at 4 weeks post-injury [13]. Therefore, there is strong evidence to suggest that use of the ChABC enzyme is effective for improving outcomes after SCI.
One point to consider with the ChABC enzyme is that it is thermally unstable which limits its use in vivo. Using western blot analysis, Lin and colleagues reported that a single dose of the ChABC enzyme to rat brain following cortical stab injury was present for at least 10 days in vivo, however, the level consistently decreased and diminished by 20 days [6]. Additionally, immunoblotting with anti-1F6 showed that at 7 days most of neurocan reappeared indicating the glycanated CSPG had returned. It has also been shown in vitro that the average thermostability of ChABC examined by immunoreactivity of chondroitin 6-sulfate (C6S), and fluorophore-assisted carbohydrate electrophoresis (FACE) and/or high-performance liquid chromatography (HPLC) was significantly diminished at 3 days at 37 °C and 1 day at 39 °C [14]. This represents normal body temperature and moderate fever of human, respectively [14]. Therefore, considering the future long term therapeutic use of ChABC, there has been a dire need to develop more clinically viable strategies to modulate the thermostability of ChABC in vivo.
Considering eventual translation to human patients, repeated administration of chondroitinase into spinal cord would significantly increase the risks of inflammation, infection or further tissue damage and should therefore be avoided. Several groups have investigated the use of stabilizing agents, such as trehalose, sucrose and/or BSA, to prolong ChABC enzymatic activity [14, 15, 16, 17, 18]. For instance, Hettiaratchi and colleagues stabilized ChABC using combined N1000G site-directed mutagenesis and covalent modification with poly- (ethylene glycol) chains (PEGylation), and fused to the Src homology domain (SH3). The PEGylated ChABC-SH3 significantly enhanced the stability and activity after incubation at 37 °C for 2 days when compared to the unmodified version in vitro [18]. An in vivo study using a rat model of stroke injury showed that PEG-N1000G-ChABC-SH3 cortical injection at 7 days following stroke injury significantly reduced the CSPG expression in the peri-lesional area at 14 days and 28 days post-stroke [18]. In a further study reported by Hettiaratchi and colleagues, wild type ChABC was computationally re-engineered by introducing 37, 55, and 92 amino acid changes to produce a more thermally stable mutant [19]. All of the engineered mutants were more stable when compared to wild type, however the mutant with 37 mutations was more active with a significantly longer half-life than the wild-type enzyme. A fusion construct with the SH3 was produced (ChABC-37-SH3), leading to higher protein yield, longer half-life and increased enzymatic activity when compared to other mutants, which was found to be sustained over 7 days in vitro [19]. Moreover, Lee and colleagues used trehalose to thermally stabilize ChABC. A dimethylmethylene blue (DMMB) assay demonstrated that trehalose stabilized ChABC and maintained its activity up to 15 days when compared with the non-stabilized enzyme which was completely inactive by 5 days [17]. Still others have sought to modify the delivery of ChABC by use of indwelling catheters reducing the need repeated surgical procedures. This approach involves the initial implantation of tubing directed into the intrathecal, subdural, or epidural space. The tubing can then be externalised for ease of subsequent administrations, or it can be attached to an osmotic mini-pump implanted usually into the flank of the animal to maintain a constant infusion of a compound such as ChABC. Intrathecal catheters with externalised tubing have been used by various groups, for instance to deliver ChABC every other day for 2 weeks following spinal cord injury in rats. In these cases there was significant improvement in both axon growth and behavioural recovery [20, 21, 22, 23]. Others using indwelling catheters with attached osmotic mini-pumps to deliver ChABC have demonstrated successful removal of CSPGs together with improved plasticity and better cell transplantation survival and integration [24, 25, 26]. Specifically, Karimi-Abdolrezaee and colleagues administered ChABC intrathecally by intrathecal catheter to injured rats 6 weeks after SCI compression surgery at thoracic level 7 [25]. After 7 days of ChABC treatment, neural stem/progenitors cells (NPCs) supplemented with growth factors were intraspinally introduced. In groups receiving a combined application of ChABC and NPCs, there was a significant improvement on locomotor function recovery, reduction on SCI-induced mechanical hypersensitivity and promotion on axonal preservation and plasticity when compared with untreated injured rats [25].
More recently however, the aim has been to use gene therapy to deliver ChABC. Using lentivirus or adeno-associated virus to deliver ChABC has the advantage that sustained long-lasting release can be achieved with a single injection, as transduced cells continuously synthesize chondroitinase, minimizing side effects from repeated injections [27]. Lentiviruses (LV) have been used as gene therapy vectors since the 1990s and can successfully transfer genes to non-dividing cells including neurons in the CNS [28]. Although there is some concern regarding LV-induced immune responses, purified lentiviral vectors for use in vivo have been proven safe in that they haven’t been shown to induce a significant systemic immune response [29, 30, 31]. Early studies showed that injections of lentiviral vectors in the CNS transduced glia initially and neurons predominating from 2 weeks onwards [29, 31]. More recently however refinements in targeting specific cell types have been developed with selected promoters [28]. Therefore, lentiviral vectors are a reasonable choice for the delivery of chondroitinase to the rodent CNS. However, the bacterial chondroitinase gene is not effective when transfected into mammalian cells, as the synthesized protein passes through the eukaryotic secretion pathway and the N-glycosylation site becomes glycosylated. This process interferes with folding and secretion of prokaryotic proteins that is not adapted for the glycosylation in structurally appropriate locations [32]. Thus in a study by Muir and colleagues, modification of N-glycosylation site on ChABC was performed and thereby achieved efficient secretion of ChABC by mammalian cells [32]. Using the modified chondroitinase gene, Zhao and colleagues performed intraspinal injections of lentivirus expressing ChABC in a C4 spinal cord dorsal crush injury in rat. ChABC activity was found to last up to at least 8 weeks and it enhanced axon sprouting and short-range regeneration of corticospinal (CST) axons [27]. Bartus and colleagues carried out further in vivo studies in rats with a T10/11 contusion injury using intraspinal injections rostral and caudal to the injury with either the ChABC enzyme or LV-ChABC [33]. They demonstrated that LV-ChABC led to larger scale CSPG digestion at 3 days and 2 weeks post-LV injection when compared with administration of the ChABC enzyme. Interestingly, LV-ChABC treated animals showed a significant improvement of locomotor function on the horizontal ladder test at 10 weeks post-injury which correlated with histology showing a significant increase in tissue preservation and reduction of apoptosis [33]. LV-ChABC has also been used effectively in combination with cell transplants. For example, Carwardine and colleagues genetically modified canine olfactory ensheathing cells (OECs) to secrete ChABC using a lentiviral vector [34]. Their results demonstrated that OEC transplants-secreting ChABC were effective in digesting CSPGs and there was a significant increase in the number of CST axons at 2–3 mm caudal to the lesion when compared with OEC transplants [35]. Additionally, combined treatment of transplanted enteric neural stem cells (ENSCs) and LV-ChABC into the lesion site of T10 contusion-injured rats significantly decreased the size of the lesion cavity and improved locomotor function recovery [36]. Together these results suggest that LV-ChABC is effective in enhancing sensorimotor function, plasticity and neuroprotection following SCI and furthermore when combined with cell transplantation, has the potential to confer a synergistic effect when compared to a single treatment.
Overall, these results suggest that LV-mediated expression of chondroitinase presents a powerful approach of gene delivery for promoting spinal cord regeneration after injury. It generates high levels of long-term expression/secretion of chondroitinase in both glia cells and neurons. However, for human gene therapy, AAVs are widely used in the clinic [37]. Therefore, AAV delivered ChABC would be another viable option to treat SCI, although in many cases AAVs exclusively target non-dividing cells such as neurons and have a comparatively smaller cloning capacity than AAVs. In a study by Alves and colleagues, rats received a single injection of AAV5-ChABC and/or AAV-GFP in the left vibrissal motor cortex. These injections led to long-term expression of ChABC for at least 12 weeks along with widespread secretion of ChABC from neuronal projections [38]. Beyond that proof-of-principle study, targeting AAVs to specific cell types may prove more advantageous for controlling expression/secretion. For example, Carstens and colleagues developed an AAV encoding ChABC using the Cre-LoxP system in order to degrade perineuronal nets (PNNs) with cell specificity [39]. They reported that ChABC was expressed selectively in hippocampal CA2 neurons using tamoxifen-inducible CA2 Cre-expressing mice. AAV-ChABC degraded PNNs sufficiently in CA2 regions with a reduction of PNN staining observed in the adjacent hippocampus, specifically CA1 and CA3 [39]. This suggests that expression/secretion of AAV-ChABC extended beyond the cell bodies of CA2 neurons, trafficked and secreted at the projection sites, similar to that observed by Alves and colleagues.
Chronic secretion of ChABC is likely not required to induce a regenerative effect, and may cause concern long-term in either the case of LV-ChABC or AAV-ChABC. The ability to finely tune and regulate the expression and secretion of ChABC would therefore be preferred. With that in mind, Burnside and colleagues recently developed a novel immune-evasive dual vector system that utilized a doxycycline-inducible regulatory switch to deliver ChABC (dox-i-ChABC) and evade T cell recognition [40]. They demonstrated that in a C5/6 spinal contusion rat model with intraspinal injection of dox-i-ChABC, sustained doxycycline administration maintained high levels of ChABC gene expression for at least 8 weeks while removal of doxycycline at 2.5 weeks significantly decreased the expression level at 8 weeks. Moreover, short-term (2.5 weeks) and long-term (8 weeks) expression of dox-i-ChABC significantly improved recovery of ladder walking behaviour as well as skilled reaching and paw grasping [40].
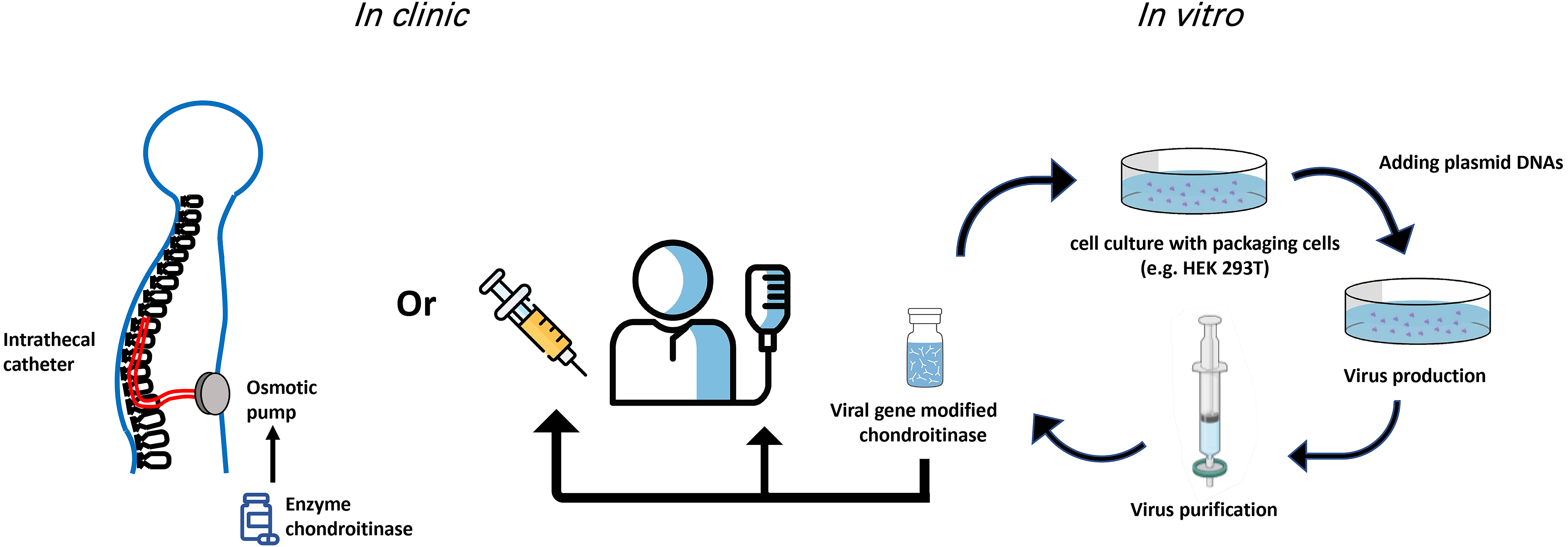
Of the current gene therapy clinical trials using viral vectors, 28% use an AAV vector delivery while lentivirus is used in 22% of trials [41]. Table 1 (Ref. [17, 18, 20, 23, 27, 28, 32, 37, 38, 40]) summarizes the advantages and disadvantages of methods used for ChABC in the spinal cord. The delivery methods of ChABC are still being optimized. In summary, a balance is required to provide a thermally stable ChABC over a time course appropriate to induce regeneration, neuroprotection, and/or functional recovery. As well, each approach of ChABC that has been used thus far (direct injection of enzyme, indwelling catheter, viral vector) has provided useful evidence to support this enzyme going forward in the treatment of SCI (Fig. 1). For example, lentivirus can integrate into host cells which brings advantages for long-term gene expression but raises a possibility to cause the insertional mutagenesis [42]. It is likely that in the near future, ChABC delivered with a viral vector will be a potent therapeutic treatment for human SCI.
| ChABC enzyme | LV-ChABC | AAV-ChABC | |
| Advantages | Clinically safe | Target dividing and non-diving cells | Target dividing and non-diving cells |
| Larger cloning capacity (9 kilobases) | Site-specific integration | ||
| High level of the transgene expression | Very mild immune responses | ||
| High titers | |||
| Disadvantages | Thermally unstable | Medium immune responses | Small cloning capacity (4.8 kilobases) |
| Short half-life | Low risk of insertional mutagenesis and oncogenicity | Potential for constitutive secretion | |
| Need repeated administration | Potential for constitutive secretion | ||
| Improvements | Use stabilizing agents, such as trehalose, sucrose | Modification of N-glycosylation site on ChABC | Cell-specific targeting using Cre-LoxP system |
| Use indwelling catheters | Temporal modulation to regulation ChABC expression, such as dox-i-ChABC | ||
| References | Lee et al. [17]; Hettiaratchi et al. [18]; Cheng et al. [20]; García-Alías et al. [23] | Zhao et al. [27]; Parr-Brownlie et al. [28]; Muir et al. [32] | Choudhury et al. [37]; Alves et al. [38]; Burnside et al. [40] |
| All delivery methods have the potential to decrease the GAG chains on CSPGs, however viral vectors provide more sustained ChABC activity. LV, lentivirus; AAV, adeno-associated virus. | |||
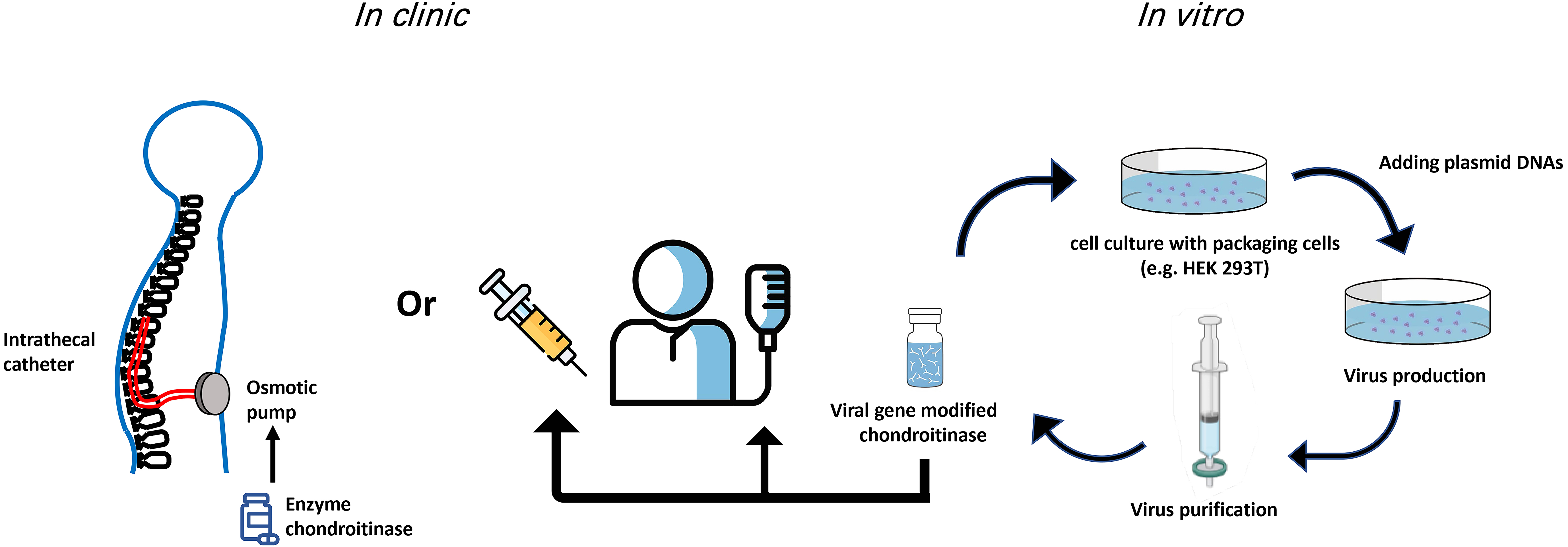 Fig. 1.
Fig. 1.Potential routes of ChABC administration in the clinic. Both gene therapy and indwelling catheters used for delivery of ChABC have provided viable improvements over single/multiple injections for delivery of the ChABC enzyme. Viral production and delivery of ChABC have the advantage that modifications to the enzyme can be implemented in vitro to temporally modulate expression and secretion of ChABC.
Both YW and MRA framed the structure, wrote, edited and finalized the manuscript.
Not applicable.
Not applicable.
MRA is supported by a research grant from the Biotechnology and Biological Sciences Research Council (BBSRC) (Grant: BB/N008189/1) and the International Foundation for Research in Paraplegia (IRP) (Grant: P182).
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.