Academic Editor: Marco Cavallo
Background: Several studies have shown that acute exercise has a small positive effect on cognitive performance. However, it is still unclear what type of exercise has a sustained impact on cognitive performance during post-exercise recovery. Therefore, the purpose of our study was to investigate cognitive performance at the behavioral level, and their neural correlates after a 10-minute post-exercise recovery period with two different types of exercise intervention (high-intensity interval exercise (HIIE) vs. Moderate-intensity continuous exercise (MCE)). Methods: A total of 29 healthy young adults (7 women) between the ages of 19 and 33 with fair to good cardiovascular fitness were submitted to two different exercise protocols and a recovery session. Cognitive function was assessed using a digital Trail-Making-Test (dTMT). Cortical activity in the prefrontal and the motor cortex using functional near-infrared spectroscopy (fNIRS) was measured before, after acute exercise, and during recovery. The statistical analysis of fNIRS data was performed by comparing the slope and mean of the hemodynamic response. Results: High levels of hemodynamic responses were observed in the prefrontal and motor cortex on the brain during performing the dTMT while walking from pre- to post-exercise and decreased again in post-recovery, accompanied by improvement and maintenance of cognitive performance. Notably, a high hemodynamic response in the left motor area of the brain was maintained by HIIE in post-recovery compared with MCE. Conclusions: The high cortical activation in the left motor area from post-exercise to recovery for the HIIE group may be due to the additional availability of neural resources for fine motor and postural control by high-intensity exercise-induced fatigue. Additionally, the improved cognitive performance may have effectively utilized the available neural resources in the frontal lobe, depending on the condition (sitting and walking) and the two types of exercise protocol (HIIE and MCE).
It is well known that chronic (aerobic) exercise induces a positive effect of structural and functional change in our brain, leading to enhanced cognitive performance on a behavioral level [1]. Exercise-induced increased central circulation of neurotrophic and growth factors such as brain-derived neurotrophic factor (BDNF), insulin-like growth factor-1 (IGF-1), and vascular endothelial growth factor (VEGF) leads to upregulation of neurogenesis, synaptogenesis, gliogenesis, and angiogenesis [2]. For example, structural changes in the brain’s gray matter are found through processes of neurogenesis and synaptogenesis, especially for those repetitively active structures during exercise. These are cortical and subcortical motor areas (primary motor cortex and basal ganglia), prefrontal areas (dorsolateral, ventrolateral), limbic system (hippocampus), sensory areas (somatosensory cortex, auditory cortex, visual cortex, sensorimotor integration areas (parietal lobe), and other areas responsible for movement control (cerebellum) (see Lotze et al. [3] for an overview). In addition, angiogenesis may mediate the structural changes in gray and white matter volume through improved cerebrovascular function and perfusion. This is critical for neuronal growth and synapse formation, as greater blood supply is essential for providing adequate nutrients to support neuronal development [4]. These changes at the neural level are in turn associated with improvements, particularly in working memory performance (but only to a limited extent in inhibition and cognitive flexibility) in children and adolescents, and adults [5, 6].
Several meta-analyses have concluded that acute exercise has a small positive effect on cognitive performance depending on the dose-response relationship, i.e., the intensity of exercise, the timing of the measurement and complexity of the cognitive task, and the fitness level of the participants [7, 8, 9, 10]. Among these moderator variables, Chang et al. [9] revealed that the largest effect could be seen between 10 and 20 minutes after exercise and that the effect gradually disappeared after 20 minutes. However, it is still unclear what type of exercise has an immediate or delayed effect on cognitive performance during post-exercise recovery. The acute effects of exercise on cognitive abilities also play a crucial role in neurochemical research. For example, neurotrophic factors (BDNF, IGF-1, and VEGF) stimulate the growth of new neurons and enhance synaptic plasticity and long-term potentiation, leading to improved cognitive function [11]. Specifically, acute exercise increases BDNF, leading to improved cognitive performance, and furthermore, the increase in BDNF was persistent after five weeks [12]. These neurotrophic factors may help keep cognitive performance constant during sustained tasks. Also, they may have different effects depending on the type of exercise. A study by Tsukamoto et al. [13] examined the acute effects of a high-intensity interval exercise (HIIE) on cognitive performance immediately after exercise and during post-exercise recovery on a cycle ergometer in comparison to a moderate-intensity continuous exercise (MCE). Immediately after exercise, both types of exercise showed enhanced cognitive performance, but the HIIE resulted in more sustained improvement during the 30 minutes post-exercise recovery [13]. This suggested that HIIE may be more effective in maintaining cognitive performance. Moreover, a recent review by Hashimoto et al. [14] found that the type and intensity of exercise, particularly by HIIE, induces metabolic lactate, which is associated with brain health concerning the effects of chronic exercise on brain function. Despite these results, the neural activation triggered by both types of exercise during post-exercise recovery has not been generally identified.
To date, several studies have been conducted on the effects of acute exercise on prefrontal cortex-dependent executive function using electroencephalography (EEG) or functional near-infrared spectroscopy (fNIRS) with conflicting findings [15]. Studies on the effects of acute exercise on brain activity, such as event-related potentials using EEG, demonstrate that moderate-intensity exercise induces a greater amplitude of P3b [16] or P2 [17], which are related to attentional resources and positively affect cognitive performance. In addition, Kao et al. [18] compared MCE and HIIE using frontal alpha event-related desynchronization and found that only HIIE was observed to enhance information processing speed and brain activation during memory retrieval. Although exercise had a positive effect on cognition, the low spatial resolution limits the ability to determine precisely which area of the brain is involved.
fNIRS studies are still relatively new in the field of cognition and motor function research. fNIRS is a non-invasive, safe, and portable optical neuroimaging method that can indirectly measure brain activity via cortical hemodynamic responses in our brain based on neurovascular coupling [19, 20, 21]. High neuronal activation occurs in specific brain regions when performing a particular, e.g., cognitive task (such as n-back or Flanker). This neuronal activity triggers changes in local brain hemodynamics (neurovascular coupling) that induce enhanced cerebral blood flow to the activated brain regions [22, 23]. As the local supply of oxygen exceeds consumption, increased concentrations of oxygenated hemoglobin (oxyHb) and reduced concentrations of deoxygenated hemoglobin (deoxyHb) are observed in activated brain regions [21, 24]. These neuronal activity-dependent changes in oxyHb and deoxyHb concentrations can be used as indirect indicators of local brain activation [21].
Furthermore, the use of fNIRS in the field of exercise science in conjunction with methods from cognitive psychology offers an advantage. Compared to other brain imaging devices (e.g., EEG fMRI), fNIRS generally has a high tolerance towards motion artifacts. As such, fNIRS is currently more suitable for measuring brain activation during cortical hemodynamic changes during exercise and exercise-related activities in an unrestricted environment [25]. Recent fNIRS studies with higher spatial resolution than EEG have shown that acute moderate exercise enhances cerebral neural activation in the left dorsolateral prefrontal cortex (l-DLPFC) in healthy young adults associated with improved cognitive performance [26, 27, 28]. However, in these studies, a single cognitive task was set to confirm the cortical activation induced by the acute response to one specific stimulus. Since our daily activities involve continuous cognitive demands (e.g., reading a book or solving math problems at school), it is necessary to investigate the neural activation during ongoing cognitive task performance rather than acute response. Moreover, although several studies have already examined the lasting effects of acute exercise on cognitive performance on the behavioral level [13, 29, 30, 31, 32, 33], only a few have investigated these effects at the neural level during recovery from acute exercise [34, 35, 36, 37]. Herold et al. [24] also proposed studies of sustained effects on task-related cortical activation during recovery, which may add to the knowledge of hemodynamic response during recovery. Neural mechanisms need to be identified to support the effects of acute exercise on enhanced cognitive performance. Also, the changes of neural activation during the recovery period after exercise have not yet been investigated in HIIE.
The purpose of our study was to investigate the immediate and sustained effects of two different types of exercise intervention (HIIE vs. MCE) on cognitive performance at the behavioral level, and their neural correlates after a 10-minute post-exercise recovery period. In the present study, a newly developed, accurate, and validated digital version of the Trail-Making-Test (dTMT) was used as the most commonly used neuropsychological test to measure executive function, and the cortical activation of the prefrontal and motor cortex was measured using fNIRS [38]. In addition, the performance of the dTMT was conducted in a block design (30 seconds of task performance and 30 seconds of rest). It is likely that cognitive and motor control demands increase with block duration, especially for the dTMT-B condition, which requires more complex cognitive processes such as cognitive flexibility, set-shifting, inhibition, and working memory. For this reason, only the most demanding condition of the dTMT tasks (dTMT-B) was used for the statistical analysis in the present study. Although there is still no gold standard for fNIRS statistical analysis of changes in hemodynamic response over time, Mandrick et al. [39] suggested that the slope method appears suitable for identifying changes in hemodynamic response with the cognitive workload. In this regard, we also compared the mean value of the hemodynamic response over time with the slope value. In addition, the present study incorporated data from a previously published paper investigating the effects of acute exercise on fine motor-cognitive performance while walking [40]. The hemodynamic response data were reprocessed and reanalyzed along with the other data.
Despite initial research on the effect of acute exercise of mostly moderate intensity, but not high-intensity, on executive control, the relationship between time course and cognitive benefits has been little studied. Therefore, we hypothesized that a single acute bout of moderate-intensity exercise would significantly improve performance on executive tasks and related changes in brain activation patterns immediately after training than would high-intensity exercise. However, we hypothesized that this effect would reverse in favor of HIIE after 10 minutes. Specifically, during post-exercise recovery, the cortical activation will decrease accordingly. This may be due to the ability to use neural resources through exercise efficiently, and this effect may persist for some time. Specifically, in HIIE, cortical activation is much greater in the motor area due to the fatigue caused by the high-intensity exercise.
The data presented here are part of a larger study of the effects of acute training on cognitive performance [40]. The focus of this article is to compare the changes in individual cortical activations during a fine motor-cognitive task (dTMT-B) in a seated position after a 10-minute post-exercise recovery period with two different types of exercise intervention. Our goal was to confirm the intervention effects of post-exercise recovery between sessions. Another previously published study examined the acute effects of two exercise protocols on hemodynamic response during dTMT while walking [40].
Sample size calculations were estimated using G*Power (Version 3.1.9.2)
(Math.-Nat. Faculty, Düsseldorf, Nordrhein-Westfalen, Deutschland) [41] and
were based on small to medium effects in prior studies of acute exercise in
healthy adults [9]. A total sample size of 22 is required to detect a small to
medium effect in a two-group design with a 5% risk of type 1 error (
A total of 32 healthy young adults (7 women) between the ages of 19 and 33 participated in the present study. Inclusion criteria were right-handedness, at least 2 hours of exercise per week, and fair or good fitness (men: 43.5 mL/kg/min and above; women: 33.6 mL/kg/min and above), respectively [42]. Exclusion criteria were an abnormal resting pulse or blood pressure and cardiovascular or neurological diseases. Furthermore, participants should not have any fine motor impairments as this would hinder the performance of the cognitive task. Injuries that interfered with walking on the treadmill or scalp injuries that would interfere with measuring brain activity by fNIRS constituted another exclusion criterion. In order to recruit participants, our University Sports Club shared flyers, both manually and via e-mail, through which participation in the experiment was handled. Initially, 3 participants were excluded from the experiment, two for not reaching the required fitness level and one for the technical problem (failed recording for the fNIRS data). Thus, data from 29 participants were considered for the final analysis. The study was conducted in accordance with the Declaration of Helsinki and was approved by the university’s local ethics committee. We obtained written consent to participate in the experiment from all participants.
As a randomized controlled trial design using repeated measures (five sessions) in a two-armed parallel-group (between subjects), two laboratory visits were required for study participants (i.e., sessions (1) and (2); see Fig. 1), with an interval of at least two days between visits. During each visit, the experiment lasted approximately 1.5–2 hours. Before the experiment, participants received several instructions: They were not to exercise for 24 hours, not consume caffeine or alcoholic beverages for the last 12 hours, and sleep for approximately 7 hours the night before [43]. Fig. 1 shows the entire study procedure.
 Fig. 1.
Fig. 1.Schematic illustration of the design and testing procedures consisting of two test days for the HIIE and MCE groups. Cortical hemodynamic activation was measured using functional near-infrared spectroscopy (fNIRS) while participants performed the digital Trail-Making Test (seated or during running on a treadmill).
On the first day, after the participants gave their written consent to
participate in the experiment, they completed a questionnaire on demographic
information. Then the fNIRS system was prepared, and sources and detectors were
attached to the head cap of the participants (see 3.3 fNIRS). After preparation,
an initial resting value of 2 minutes was measured in a seated position. The
Mental and Physical State and Trait Energy and Fatigue Scales Questionnaire
questionnaire (only Part II; MPSTEFS) was then completed to determine
participants’ physical and mental energy/fatigue [44]. The first dTMT session
(session 1: on (1) of Fig. 1) followed while participants were comfortably seated
at the table. After a 20-minute rest, the second dTMT session ((2) of Fig. 1)
was performed again while sitting comfortably. Participants then completed the
questionnaire in the MPSTEFS Part II again. At the end of the first day, we
removed the fNIRS system and determined the individual fitness level
(VO
On the second day, the cap with the sources and detectors of the fNIRS was placed on the head of the participants. An initial fNIRS measurement was recorded for 2 minutes at rest. Then answers to the MPSTEFS questionnaire were collected. Next, participants walked on the treadmill for 2 minutes at a speed of 5 km/h (including collecting data with fNIRS), followed by a dTMT session during walking (session 3: on (3) of Fig. 1). After completing the dTMT, the fiber optic cables of the optodes were disconnected from the fNIRS system and secured to the participants’ backs to complete the remainder of their exercise sessions without interruption (HIIE or MCE). At the end of the intervention, we reconnected the cable of the optodes to the fNIRS system. Next, while walking at a speed of 5 km/h, the cortical activation was measured without additional tasks for the first 2 minutes, immediately followed by the second dTMT session (session 4: on (4) of Fig. 1). The heart rate (HR) was recorded with a Polar H1 heart rate sensor (Polar Electro Europe AG, Switzerland) before, during, and after exercise. Participants’ fatigue immediately after the two types of exercise protocols was collected with the BORG scale (RPE, ratings of perceived exertion) [46]. Subsequently, participants completed the MPSTEFS questionnaire again. The third dTMT session assessed the sustained effects of each exercise protocol after a 10-minute break, but in a comfortable sitting position in this session (session 5: on (5) of Fig. 1).
In the HIIE protocol, the participant is exposed to a loading interval four
times over 4 minutes and an unloading interval three times over 3 minutes. The
interval exercise lasted for a total of 25 minutes. The exercise intensity for
the high-intensity intervals is 90% of the VO
The external dose load, such as intensity or duration of exercise, is essential
to trigger neurobiological processes leading to neuroplasticity and changes in
cognitive function [47]. Therefore, it is important to adjust the dose in studies
focusing on the intensity of the exercise intervention. Hence, the duration of
the MCE protocol was determined as the duration required to accomplish the same
total calorie consumption as the protocol of HIIE [13, 48]. In the MCE protocol,
the participant is subjected to 30 minutes of moderate load. This protocol’s
exercise intensity was consistently 60% of the previously determined
VO
One of the most commonly used neuropsychological tests to assess executive function is the Trail-Making-Test (TMT) [49]. Its original paper-pencil version consists of two conditions. In the TMT-A, which measures information processing speed and visuospatial abilities, 25 circles randomly distributed from 1 to 25 must be connected in ascending order (i.e., 1-2-3-. . .). In the TMT-B, the most challenging condition measuring inhibitory control, cognitive flexibility, and working memory, participants must connect circles of numbers and letters in ascending, alternating order (i.e., 1-A-2-B-3-C. . .). Although the TMT is widely used, it is usually administered in its single-pencil-on-paper form (paper-pencil version). However, for this version, there are insufficiently controlled parallel versions for repeated use to rule out learning effects [50, 51].
Evaluating executive function in a neuroscientific method requires enough
repetitions of measurement to acquire an adequate signal-to-noise ratio of the
data [52]. For this reason, we used a newly developed accurate and validated
digital version of the Trail-Making-Test (dTMT) [38]. The dTMT, which was
performed using a Samsung Galaxy Note Pro (12.2-inch diagonal LED-backlit
Multi-Touch display with IPS technology; portrait alignment) with a resolution of
2560
 Fig. 2.
Fig. 2.The Design of experimental task for dTMT. Each condition appears randomly three times over 30 seconds, and dTMT lasts a total of 9 minutes, including a break between conditions.
Our version of the dTMT provides the option to run a block of tasks with a fixed time instead of performing the task with 25 circles completed at once. This is because enough test repetitions are required to evaluate neural correlates for the task [52]. Each dTMT assessment lasts 9 minutes, with a task block alternating 30 seconds of task completion followed by 30 seconds of rest (presentation of a black fixation cross on a white background). The task sequence of the dTMT conditions (dTMT-M, dTMT-A, dTMT-B) within every 9 minutes’ block was pseudo-randomly designed such that the same condition never appeared twice in succession. All conditions were displayed three times each. Using a pseudo-randomized stimulus sequence minimizes the task condition predictability and avoids the top-down effects of condition-related expectations or attention. In order to examine the effects of exercise on behavioral-level cognitive performance, we established in a pilot study that learning effects had already decreased over the first two sessions of the experiment. Also, the program’s mathematical algorithms of dTMT can continuously generate a new dTMT trial that will never match the previous sheet. Participants were instructed to complete as many circles as possible for the task performance, paying attention to error-free execution. Another was presented if they completed a sheet before the end of a 30-second block. Even if 30 seconds passed before they completed a sheet, the data was automatically saved, and the remaining blocks were displayed with a fixed cross in the center of the tablet.
The Bruce protocol [45] was carried out on a treadmill (h/p/cosmos
pulsar® 3p, Nussdorf-Traunstein, Germany), to evaluate
participants’ cardiorespiratory fitness (VO
Demographic information, type and duration of sports activities per week, and
level of sports performance (i.e., their experience in participating in
competitions) were obtained by questionnaire. Body mass index (BMI, kg/m
In the present study, concentration changes in the amount of oxygenated and deoxygenated in the right and left: frontopolar area (FPA), dorsolateral prefrontal cortex (DLPFC), and motor cortex (M1) were recorded using a portable continuous optical fNIRS system (NIRSport 88, NIRx Medical Technologies LLC, New York, NY, USA). The setup consisted of 16 light sources and 16 detectors spaced approximately 3 cm between optodes to effectively compromise depth sensitivity and signal-to-noise ratio. The center of the NIRScaps for optode placement (EASYCAP GmbH, Herrsching, Germany) was placed over the vertex (Cz) according to the international 10–20 system by marking the midpoint between the nasion and the inion and the left and right preauricular points (see Fig. 3), to ensure experiment consistency of placement between participants and experimental sessions.
 Fig. 3.
Fig. 3.Configuration for fNIRS Measurement.
The data sampling rate of this fNIRS system is 7.81 Hz and includes two wavelengths of infrared light (760 and 850 nm) [56] to measure oxygenated and deoxygenated hemoglobin (oxyHb and deoxyHb) using the continuous-wave procedure (i.e., emitting infrared light from a source with a constant intensity and frequency) [57]. Data of oxyHb and deoxyHb were recorded on a tablet (Microsoft Surface Pro2 128 GB) with the NIRS Star 14 Software (NIRSport, NIRx Medical Technologies LLC, Glen Head, NY, USA). For each 30 seconds block of alternating task and rest, triggers were set using the NIRS Stim Software (NIRSport, NIRx Medical Technologies LLC, Glen Head, NY, USA).
In the present study, only the behavioral and neural data of dTMT-B condition were analyzed between the first and last session to investigate the extent to which two types of exercise affect the maintenance or improvement of cognitive performance at a behavioral and neuronal level after recovery on post-exercise (see Fig. 1). Besides, some data (e.g., demographic and physiological data, MPSTEFS, and behavioral and neural data on pre-and post-exercise) have already been reported in the work of [40]. However, behavioral and neural data were reprocessed and analyzed concerning the research question under investigation here.
Statistical analyses were performed using SPSS v. 27 (IBM Corp., Armonk, NY,
USA). First, we explored the dependent variables for missing data points,
normality of distributions (tested by Kolmogorov–Smirnov tests), and the
presence of outliers. The effect sizes for all analyses are expressed using
partial Eta
For the demographic variables, continuous variables (e.g., age, height, weight,
BMI, exercise duration, VO
We compared the subjective fatigue parameters of the MPSTEFS questionnaire using
3
First, the data from dTMT had to be extracted from a text file stored on the
tablet via Matlab 2018b (MathWorks, Natick MA USA). Then, we selected three
variables (number of connected circles, time spent inside circle [working
memory], and speed between circles [inhibitory control]) from the data of dTMT
for the behavioral analysis, and we averaged three blocks of three selected
variables in each condition. In particular, the variable “number of correctly
connected circles” was used as an indicator of the dTMT-B in the present study
because the task had to be performed continuously for a given duration of 30
seconds. As mentioned earlier, we statistically analyzed only the dTMTB
condition, the most challenging task. In the statistical analysis step at the
behavioral level, we obtained a comparison between all sessions using 5
The analysis of the neural data was conducted with the NIRS Toolbox [59], an open-source program installed in Matlab 2021a (MathWorks, Natick, MA, USA). The relative coefficient of variation (CV in %) was first estimated with unfiltered raw data to check the data quality as a signal-to-noise ratio, a data processing method commonly used in NIRS measurements using multi-channel [60, 61]. CVs above 15% for each channel computed during each experimental session were excluded for the subsequent analysis [62]. After removing poor channels, data were processed using a low-pass filter with 0.2 Hz [57] to remove physiological components (e.g., heartbeats: 0.5 to 2.0 Hz, and respiration: 0.2 to 0.4 Hz). From these data, they could be converted to optical density. The converted data were then corrected for motion artifacts by attenuating outlier variations using temporal derivative distribution repair (TDDR) [63]. These preprocessed optical densities were finally converted into oxygenated hemoglobin (oxyHb) as well as deoxygenated hemoglobin (deoxyHb) concentration using the modified Beer-Lambert law [59]. The data of all channels were processed into time-series data by calculating the inter-trial mean of each dTMT condition. Each time-series data was corrected using the baseline period’s average value for two seconds before the onset of a task block (baseline correction). Furthermore, task contrast was performed by subtracting the time-series data for the resting conditions between task conditions from task conditions. Changes in hemodynamic responses for each condition were plotted as topographic on brain image over time during 30 seconds of task execution. Each channel of source-detector was averaged over six different regions of interest (ROI) in a further step for statistical analysis. The areas divided into six ROI consisted of the left frontopolar area (l-FPA) with source-detector pairs (S3-D2, S3-D5), the right frontopolar area (r-FPA) with source-detector pairs (S6-D5, S6-D7), the left dorsolateral prefrontal cortex (l-DLPFC) with source-detector pairs (S1-D1, S1-D2, S1-D3, S2-D2, S4-D3, S4-D4), and the right dorsolateral prefrontal cortex (r-DLPFC) with source-detector pairs (S5-D6, S7-D7, S8-D7, S8-D8), the left motor area (l-M1) containing all channels above the left motor cortex, and the right motor area (r-M1) containing all channels above the right motor cortex. We employed the freeware MNE-NIRS (v. 0.1.2; https://mne.tools/mne-nirs/) [64] from the MNE toolbox (v. 0.24.0; mne.tools) in Python [65] and MATLAB based toolbox NFRI (https://www.jichi.ac.jp/brainlab/tools.html) to visualize the topographic on a brain image [66]. It is believed that the oxyHb response can be used as a more sensitive indicator of changes in regional blood flow [67, 68]. For this reason, we used only changes of oxyHb levels for further statistical analysis. The use of ROIs as a factor in ANOVAs may lead to unintended statistical bias because the optical properties may differ systematically between ROIs [24]. Therefore, for statistical analysis, ANOVAs were performed separately for each ROI. To account for this, alpha values were corrected for the number of ROIs using the false discovery rate (FDR-corrected) to perform multiple comparisons [69]. The emphasis was also placed on the effect sizes when evaluating statistical significance due to the small number of participants in each group in the present study [70].
The parameters extracted from the fNIRS statistical analysis were the mean parameter and the slope over time (from onset to 32 sec) of the conditional block (see Fig. 4). The time of 32 sec was chosen to account for the fact that the maximum range of concentration changes is reached within a few seconds after the end of the task. According to the systematic review by Herold et al. [24], the methods used for statistical analysis of fNIRS data are very diverse (mean value, area under the curve, slope or GLM analysis, etc.). Therefore, for comparing the two parameters (slope and mean) in the present study, we followed the study of [39], which suggests that the slope method seems to be suitable for detecting changes in hemodynamic response over time with the cognitive workload.
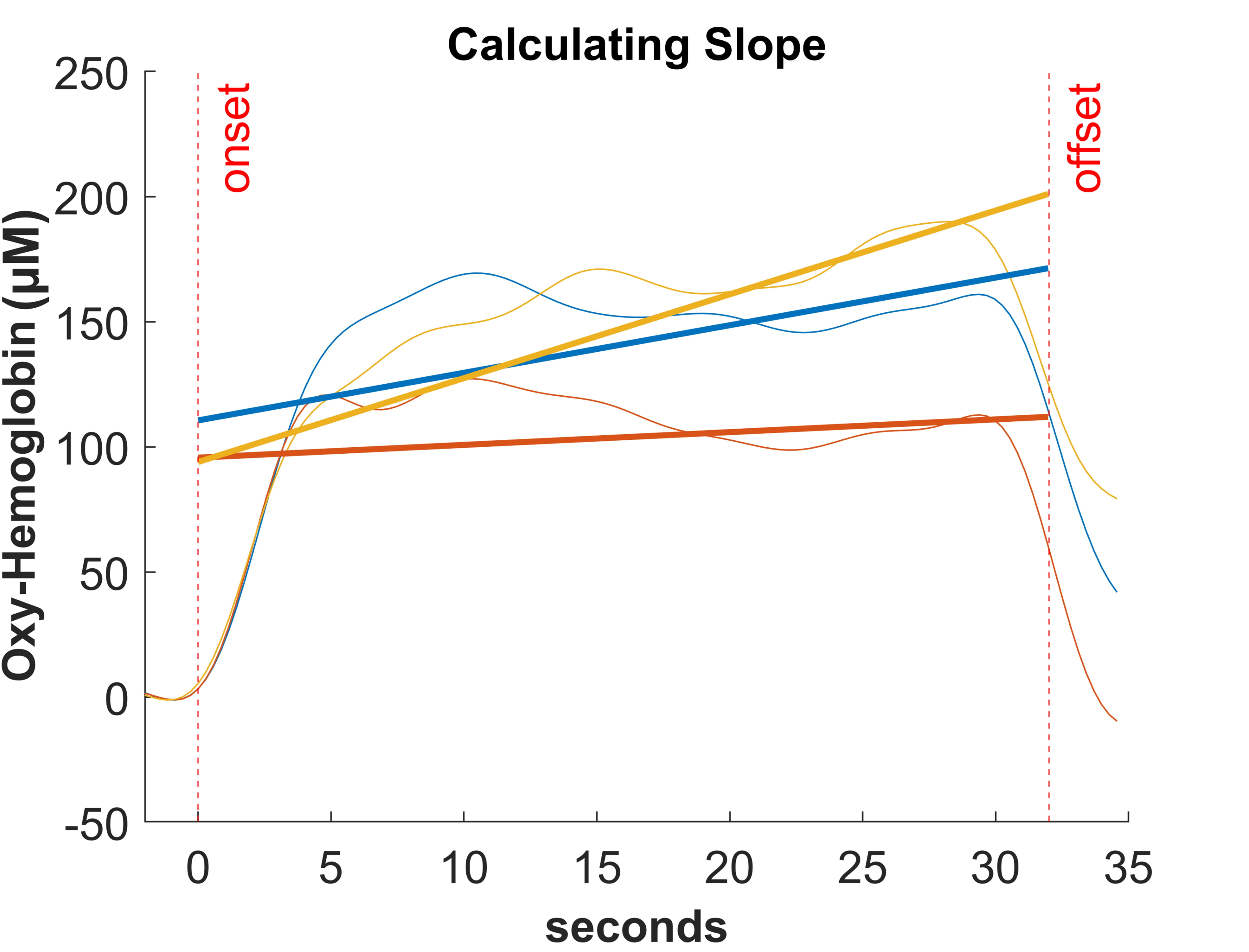 Fig. 4.
Fig. 4.Example for calculating slope for oxyHb while performing the dTMT over time.
In the statistical analysis, we analyzed 5
Table 1 shows the demographic and physiological characteristics. No significant differences between the two exercise groups were found in the statistical analysis. Besides, the types of sports that subjects participate generally varied, including team sports (e.g., Basketball, Volleyball), endurance (e.g., rowing, track athletics, cycle, swim), individual sports (e.g., apparatus gymnastics, boxing, taekwondo), racket sports (e.g., tennis, table tennis).
| HIIE | MCE | statistical analysis | |
| (n = 14) | (n = 15) | ||
| M (SD) | M (SD) | ||
| Age (years) | 26.0 (3.98) | 24.2 (4.04) | t |
| Sex | m: 11; w: 3 | m: 11; w: 4 | |
| Height (cm) | 176 (9.34) | 177 (9.42) | t |
| Weight (kg) | 70.6 (10.5) | 70.2 (12.0) | t |
| BMI (kg/m |
22.7 (2.29) | 22.4 (2.70) | t |
| max. HR (bpm) | 189.1 (6.72) | 189.3 (5.59) | t |
| VO |
55.2 (6.37) | 56.2 (7.11) | t |
| Exercise duration (min per week) | 175 (144) | 227 (130) | t |
| Participation in competitions | International: 0% | International: 13.3% | |
| National: 7.1% | National: 20% | ||
| Regional: 21.4% | Regional: 20% | ||
| Local: 42.9% | Local: 26.7% | ||
| No: 28.6% | No: 20% | ||
| Note. M, mean; SD, standard deviation. | |||
Table 2 shows the physical/mental energy/fatigue components using a visual
analog scale that yielded 12 separate ratings for Physical Energy, Physical
Fatigue, Mental Energy, and Mental Fatigue before and after exercise. A 3
(“session”: before (1), before (3), and before (5))
| MPSTEFS components | Before (1) | Before (3) | Before (5) | |||
| HIIE | MCE | HIIE | MCE | HIIE | MCE | |
| (n = 14) | (n = 15) | (n = 14) | (n = 15) | (n = 14) | (n = 15) | |
| M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | |
| Physical Energy | 286 (49.7) | 286 (45.3) | 301 (50.9) | 297 (59.7) | 307 (47.3) | 297 (83.9) |
| Physical Fatigue | 127 (39.1) | 115 (72.6) | 151 (60.7) | 133 (65.2) | 176 (92.3) | 149 (108) |
| Mental Energy | 301 (45.7) | 298 (50.5) | 299 (52.8) | 313 (49.1) | 309 (64.0) | 289 (67.9) |
| Mental Fatigue | 138 (66.2) | 111 (66.7) | 154 (64.8) | 108 (41.8) | 160 (71.9) | 156 (78.0) |
| Note. M, mean; SD, standard deviation. | ||||||
First, we performed 5
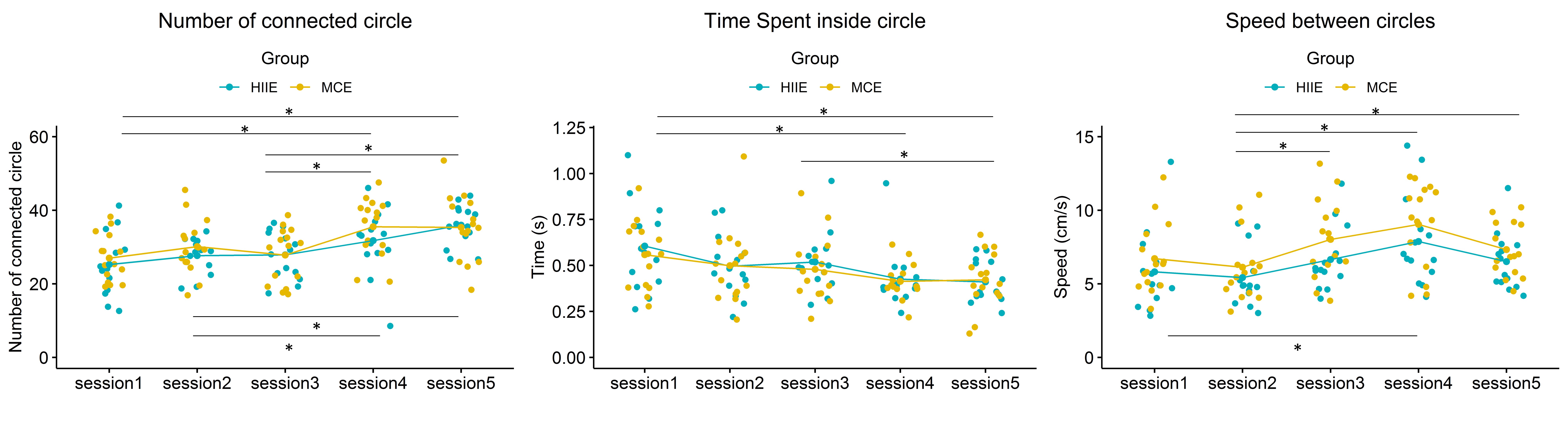 Fig. 5.
Fig. 5.
Behavioral data extracted from the dTMT-B by group. * p
In summary, for the behavioral data of the dTMT, task speed performance (speed between circles) increased during walking. Still, overall task performance (number of connected circles) remained unchanged between sitting and walking. Overall, cognitive performance improved significantly in both groups in post-exercise and was maintained in post-recovery.
Fig. 6 shows the hemodynamic responses during the performance of the dTMT for 30 seconds over a session. As shown in Fig. 6, for each condition (during sitting and walking), changes in oxyHb levels can be observed across sessions (from first to fifth session) during task performance. High cortical activation can be found after HIIE, and compared to MCE, high cortical activation was maintained to some extent after recovery.
 Fig. 6.
Fig. 6.Change of OxyHb for 30 seconds over session by group.
Table 3 shows the statistical results for the hemodynamic response over session with the ANCOVA with repeated measures for slope and mean of oxyHb in each ROI. The slope and mean value parameter over the entire session in all ROIs are shown in Fig. 7. The slope parameter showed a significant effect of the session in all ROIs, but the mean parameter showed a significant effect with a medium effect size in r-FPA, l-DLPFC, r-DLPFC, l-M1, and r-M1. An interaction effect with a medium effect size between session and group was observed for both parameters only in the l-M1 (see Fig. 8A), indicating a significant difference with an above medium effect size between groups (Fig. 7 (1-e and 2-e)) for slope value in post-exercise while walking (p = 0.073, d = 0.694) and post-recovery while sitting (p = 0.027, d = 0.871), and a significant difference between groups for mean value in post-exercise (p = 0.024, d = 0.886) and post-recovery (p = 0.026, d = 0.873). In summary, high hemodynamic responses were observed in all ROIs during performing the dTMT while walking in pre-and post-exercise and decreased again in post-recovery. Notably, a high hemodynamic response in l-M1 was maintained by HIIE in post-recovery compared with MCE.
| Effects | l-FPA | r-FPA | l-DLPFC | r-DLPFC | l-M1 | r-M1 | ||||||||||||
| Slope | F |
p | F |
p | F |
p | F |
p | F |
p | F |
p | ||||||
| Session | 2.83 | 0.028 | 0.098 | 4.49 | 0.002 | 0.147 | 3.85 | 0.006 | 0.129 | 5.12 | 0.001 | 0.164 | 4.11 | 0.004 | 0.136 | 7.77 | 0.001 | 0.230 |
| Session |
1.07 | 0.377 | 0.039 | 1.38 | 0.245 | 0.050 | 0.398 | 0.810 | 0.015 | 0.919 | 0.456 | 0.034 | 1.90 | 0.116 | 0.068 | 1.36 | 0.254 | 0.050 |
| Mean | F |
p | F |
p | F |
p | F |
p | F |
p | F |
p | ||||||
| Session | 1.27 | 0.285 | 0.047 | 2.60 | 0.040 | 0.091 | 1.92 | 0.113 | 0.069 | 3.16 | 0.017 | 0.108 | 1.68 | 0.161 | 0.061 | 4.37 | 0.003 | 0.144 |
| Session |
0.966 | 0.430 | 0.036 | 1.54 | 0.197 | 0.056 | 0.470 | 0.757 | 0.018 | 0.892 | 0.471 | 0.033 | 2.68 | 0.057 | 0.094 | 1.36 | 0.253 | 0.050 |
| Note. Session: from first to fifth session; group: HIIE and MCE; Slope: slope of hemodynamic response between onset and 32 sec; Mean: mean of hemodynamic response for last 7 sec of task end. Bold: Above a medium effect size 0.06 in bold. | ||||||||||||||||||
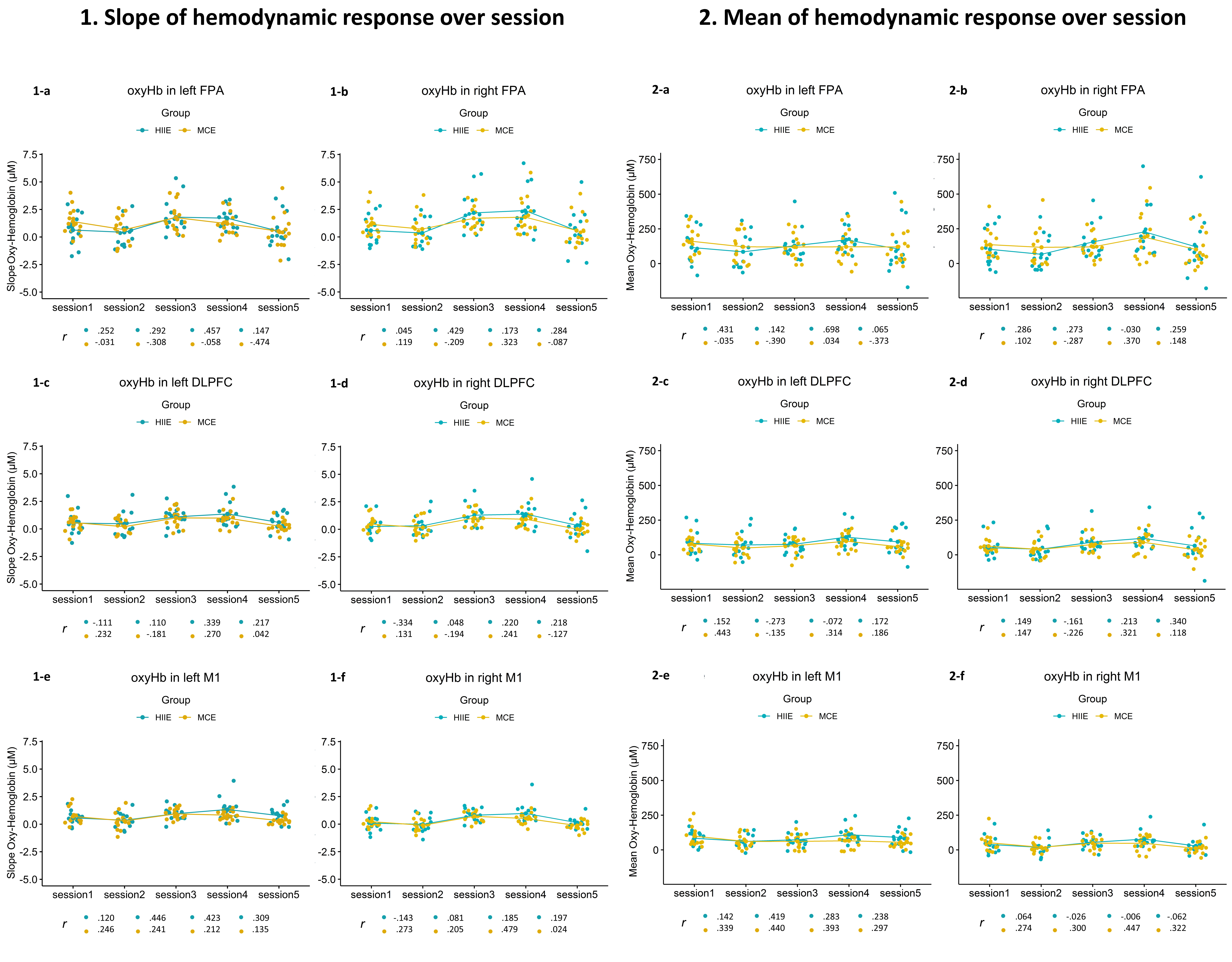 Fig. 7.
Fig. 7.Slope (1) and Mean (2) of OxyHb related to dTMT-B performance
over session by group. Individual slope and mean oxyHb changes related to dTMT-B
performance over the whole session are shown in all ROIs. Below each graph is the
correlation coefficient between the rate of behavioral data (number of corrected
circles) and the rate of neural data (slope and mean) between each session (e.g.,
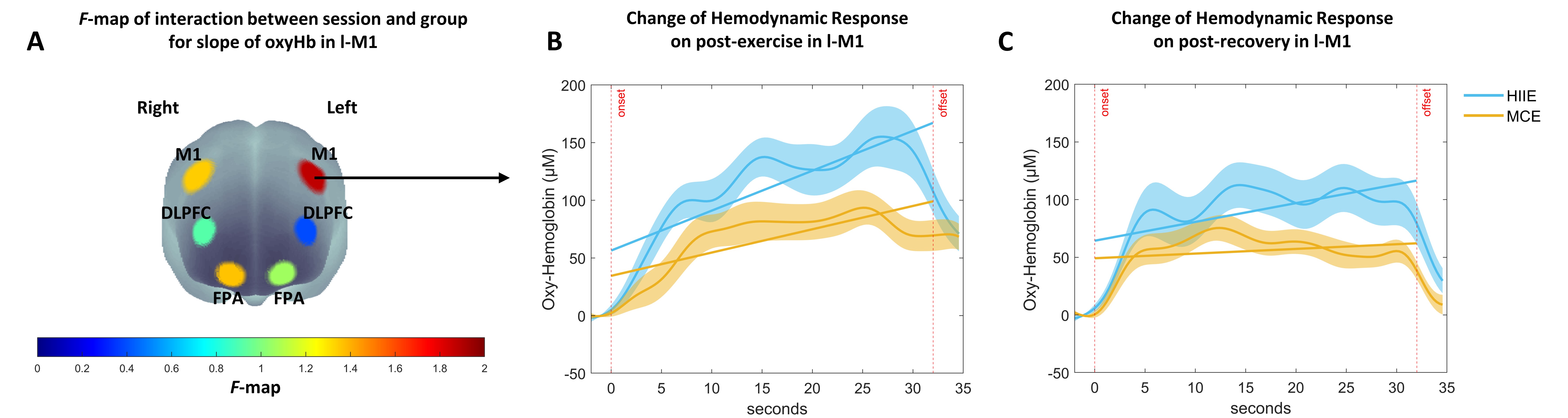 Fig. 8.
Fig. 8.Change of OxyHb related to dTMT-B performance for 30 seconds over session by group. (A) F-map of oxyHb signal changes of slope reflecting the interaction between session and group for the dTMT-B performance. Among the six regions of interest, an interaction with a medium effect size can be seen in the left motor cortex (l-M1) (FDR-corrected). F-value is displayed according to color bars. (B) Time course of oxyHb changes in response to dTMT-B performance in the l-M1 in post-exercise while walking between HIIE and MCE. (C) Time course of oxyHb changes in response to dTMT-B performance in the l-M1in post-recovery while sitting between HIIE and MCE. Error bars indicate standard error.
The present study investigated the immediate and sustained effects of acute exercise with different exercise intensity on neural correlates underpinning cognitive performance in the post-recovery period. In our previous study [40], two different exercise intensities resulted in increased activation in task-related areas (FPA and M1). Accordingly, cognitive performance was improved by using the available neural resources effectively. The main focus of this present study was on the oxyHb changes related to cognitive performance (dTMT-B) during recovery in post-exercise. Since we already confirmed the differences in oxyHb levels between task conditions in pre-exercise [40], in the present study, we only investigated the dTMT-B, which is mainly used to test complex cognitive functions such as working memory set-switching over the entire session.
Demographic data were reported in our previous study [40]. Among them, there
were no significant differences between groups. Also, no differences were found
for VO
First, we conducted two sessions on the first day to effectively control for learning effects. No significant difference in cognitive performance for our three reported variables (see Fig. 5) was found between the first and second sessions. Recent studies have shown that individuals with high fitness levels show little or no improvement with acute exercise [71, 72]. However, due to the training regime we chose, the participants had to have at least an intermediate fitness level to perform the full extent of the training. In addition, it was possible to effectively control the learning effect by integrating a divide-and-combine approach [54], creating alternative sheets of the TMT task from the newly developed dTMT. Despite different conditions, there was no significant difference between the second and third sessions (between sitting and walking). In general, cognitive performance seems to be impaired during a dual-task while walking in children, adolescents, and older adults [73, 74]. However, this was not the case in our study. In comparing sitting and walking, the cognitive performance for the number of connected circles did not decrease due to the automation of gait in the treadmill task performance [75]. Notably, cognitive performance increased in the post-exercise period, which is supported by a study by Penati et al. [76] that showed better cognitive performance in fixed-speed walking on a treadmill than over-ground walking. Above all, the increased activation of the frontal lobe induced by exercise may have amplified the posture-second strategy [77] to focus more on cognitive tasks due to the automatized walking on the treadmill. As the posture-second strategy, dual-tasks while walking on a treadmill could enable resource allocation toward cognitive tasks while performing automatized walking naturally, contributing to improved motor and cognitive function. In this regard, dual-task performance on the treadmill may be a suitable alternative to improve motor and cognitive functions in clinical or rehabilitation fields.
However, significant cognitive performance increases were no longer observed post-recovery, but the performance was maintained. This sustained performance in the post-recovery period was significantly different, especially compared with the first and second session as baseline under the same condition (during sitting). According to the meta-analysis by Chang et al. [9], the greatest positive effect on cognitive performance was found after 10–20 minutes in post-exercise. This effect gradually decreased when it lasted longer than 20 minutes. In the present study, the increase and maintenance of cognitive performance in post-exercise and -recovery were independent of the intervention group. In the study by Tsukamoto et al. [13], the cognitive performance was also maintained for 20 minutes. In the case of HIIE, the performance was maintained up to 30 minutes longer than in MCE. However, in our study, only a 10-minute recovery was given, and thus a follow-up study on the post-exercise maintenance of cognitive performance should be conducted. Notwithstanding the limitations associated with the short recovery period, in the present study, in which exercise-induced changes of neural correlates related to task performance were additionally investigated using fNIRS, the comparisons between the two types of exercise should also be observed at the neural level.
To date, the effect of acute exercise on cognitive task-related neural correlates has been studied extensively using fNIRS (for a systematic review, see Herold et al. [24]). However, studies on the neural correlates of the maintenance of cognitive performance in post-exercise are still lacking. Furthermore, only few studies have investigated the sustained effects of exercise on task-related neural correlates [34, 35, 36, 37]. In general, acute exercise plays a role in increasing cortical activation concerning enhanced cognitive performance. Prefrontal cortical activation in post-exercise positively affects cognitive task performance [14]. In a recent study on the effects of exercise on cognitive performance in post-recovery, the cortical neural activation in the prefrontal area depends on age. At the behavioral level, inhibitory control for young and older adults improved immediately after 15-minutes of moderate exercise with 40–60% heart rate rebound, and, notably, was maintained even 30-minutes after recovery [34]. However, the cortical activation at the neural level appears to differ between young and older adults. In young adults, the cortical activation gradually decreased from baseline to post-exercise and -recovery periods. In contrast, in the elderly, it increased in the middle frontal lobe post-exercise and returned to pre-exercise levels in post-recovery again. In children aged 8–10 years, the cortical activation of the frontal lobe also increased in post-exercise (continuous and intermittent moderate-intensity exercise). However, it decreased steadily post-recovery, maintaining cognitive performance for 30 minutes [36].
The present study investigated the effects of exercise on various brain areas by additional measurement of the motor area, unlike other studies that are generally limited to frontal lobe measurements. Also, performing task conditions (during sitting and walking), type of cognitive task (dTMT-B), and exercise types (HIIE and MCE) should be considered when interpreting the results of the present study compared with other studies. Furthermore, from the point of view of statistical analysis, the simultaneous analysis and comparison of the mean and the slope value could be an appropriate alternative solution for the fNIRS analysis methods, for which there is no golden standard yet.
The performance of the 1st and 2nd sessions serves as a baseline to control the learning effect of the task. The results for oxyHb between the 1st and 2nd session showed decreased cortical activation only in the l-FPA with no change in cognitive performance. This appears to have been effectively utilized by reducing the neural resources due to the learning effect [78]. In comparing performance conditions, higher cortical activation in all ROIs was shown during the fine motor-cognitive task for 30 seconds while walking than sitting. In this regard, the higher cortical activation during walking compared with sitting might be due to allocating more neural resources to postural control and task performance while walking, which is why cognitive performance may have been maintained even during the dual-task condition. In particular, the slope of exercise-induced cortical activation was increased or maintained in post-exercise, which seems different between the two types of exercise. Notably, considering the mean value, there was a significant increase in the r-FPA and l-DLPFC from pre- to post-exercise, regardless of the intervention group, which is consistent with other studies showing exercise-induced increased cortical activation in the l-DLPFC [27, 79]. However, compared with these studies, more neural resources may need to be recruited in other areas due to type of exercise (intensity or duration), performing condition (sitting or walking), and type of cognitive task (Stroop task or fine-motor cognitive task) to maintain task performance in post-exercise. Thus, further studies should be conducted considering the factors mentioned above.
More importantly, depending on the characteristics of the fine-motor cognitive task, the motor area may have been significantly differently affected by the two types of exercise. After HIIE, the cortical activation was increased in both M1 areas, whereas it decreased after MCE. Notably, the rate of change for oxyHb (both parameters for slope and mean) between pre-and post-exercise (adjusting rate of change for dTMT performance as a covariate) showed a significant difference between groups. The increased activation of M1 in the HIIE group, along with an increase in the frontal lobe, could be explained by the need for more neural resources for postural control and fine-motor control, along with smooth task performance while walking on the treadmill [40]. According to the conceptual model of mechanisms of physical activity [1, 80], regular exercise induces changes in cellular and molecules such as brain growth factors, which in turn lead to structural and functional changes in the brain, leading to an increase in cognitive performance. In addition, the upregulation of VEGF by exercise in animal models increased cerebral blood flow [81, 82]. Moreover, the increased cerebral blood flow velocity is related to fitness level and healthy human aging [83]. Given the physical fitness level of the subjects participating in the regular exercise in the present study, the increase in cerebral blood flow to the frontal lobe in post-exercise, which plays an essential role in the executive function, seems reasonable. A further increase in M1 caused by HIIE could also be explained. Therefore, other studies are needed to determine whether HIIE induces high cortical activation and enhanced cognitive performance when used in different age groups (children and the elderly) or groups with varying levels of fitness (high and low) [71].
Moreover, cortical activation had returned to baseline levels (1st and 2nd sessions) in all ROIs after recovery, even when both variables for the slope and mean parameters were accounted for. Notably, interaction with the above medium effect size for both slope and mean parameters was found only for l-M1, indicating that the higher cortical activation in the l-M1 appeared to be maintained in HIIE than MCE. In addition, in oxyHb, there was no difference between groups in the rate of change between post-exercise and -recovery periods. However, the exercise-induced increased cortical activation from post-exercise was maintained until the post-recovery period, resulting in a significant difference between groups (p = 0.027, d = 0.871). In a previous study that applied moderate-intensity exercise [84], the cortical activation of the frontal lobe tended to decrease in post-recovery. Still, the cognitive performance was maintained for up to 30 minutes. In the present study, decreased cortical activation was also observed in the frontal lobe. An additional measurement of the motor cortex also revealed decreased oxyHb in the left and right M1. In particular, the left M1 was more affected by HIIE. This can be interpreted as the effect of exercise intensity. Although it was necessary to mobilize a large amount of neural resources to process the fine-motor cognitive task simultaneously with walking in post-exercise, a general downregulation of cortical activation occurred during sitting in the post-recovery period. HIIE, which may have led to fatigue due to high intensity, could affect the upregulation of the left M1 in processing the fine motor task.
Also, the change in behavioral data was already considered by performing ANCOVA
with repeated measurements between sessions and groups for oxyHb, adjusting the
Although the present study has provided novel attempts and interesting findings, some limitations are worth noting. First, the sample size in our study was small, which may indicate high inter-individual variability in exercise-induced cortical activation. Therefore, in statistical analysis, emphasis was placed on the effect size to compensate for this. Nevertheless, a large number of participants is required for generalization. Second, although various moderator parameters between acute exercise and cognition are involved, it is necessary to observe the acute and sustained effects of cortical activation induced by exercise on cognitive function through studies that consider various fitness levels (age group or high/low fitness). For this reason, given the participants’ high fitness levels, we probably did not observe any differences in cognitive performance at the behavioral level due to the effective use of neural resources by the two types of exercise. Finally, our study’s recovery duration was very short compared to studies investigating the sustained effect of acute exercise-induced neural correlates on cognitive function. Since the regular accumulation of acute exercise results from chronic exercise, the sustained effect of acute exercise by prolonging the recovery duration could be a bridge to show the effect of chronic exercise.
In the present study, a novel comparison was attempted between two types of exercise with different intensities from pre-exercise over post-exercise to post-recovery. The results showed increased cortical activation in all ROIs from pre- to post-exercise and a decrease in the post-recovery period along with the increase and maintenance of cognitive performance. Also, on the behavioral level, no difference was found between groups. HIIE, however, showed an increase and maintenance of cortical activation in the left motor area from post-exercise to -recovery, likely due to an additional availability of neural resources for the fine motor and postural control due to the high intensity of exercise. Furthermore, it appears that the available neural resources of the frontal lobe were effectively utilized to increase or maintain cognitive performance depending on the conditions (sitting and walking) and the two types of exercise (HIIE and MCE). Further studies are needed to complement the limitations mentioned above in future studies. From the perspective of fNIRS statistical analysis, two variables (slope and mean) were considered to interpret the hemodynamic response data. This is a step towards exploring the advantages and limitations of both parameters and a proposal for a new alternative to fNIRS analysis.
BDNF, Brain-derived neurotrophic factor; deoxyHb, Deoxygenated hemoglobin;
DLPFC, Dorsolateral prefrontal cortex; dTMT, Digital Trail-Making-Test; EEG,
Electroencephalography; FDR, False discovery rate; fNIRS, Functional
near-infrared spectroscopy; FPA, Frontopolar area; HIIE, High-intensity interval
exercise; HR, Heart rate; IGF-1, Insulin-like growth factor-1; M1, Motor cortex;
MCE, Moderate-intensity continuous exercise; MPSTEFS, Mental and Physical State
and Trait Energy and Fatigue Scales; oxyHb, Oxygenated hemoglobin; ROI, Regions
of interest; RPE, Rating of perceived exertion; TMT, Trail-Making-Test; VEGF,
Vascular endothelial growth factor; VO
NS designed the research study. SYP performed the research. NS provided help and advice on the analysis. SYP and NS analyzed the data. SYP and NS wrote the manuscript. Both authors contributed to editorial changes in the manuscript. Both authors read and approved the final manuscript.
The study was conducted according to the Helsinki Declaration and approved by the university’s local ethics committee. All participants gave written informed consent to participate in the experiment.
We are grateful to the participating participants from the general university sport of the University of Stuttgart, Germany. Special thanks are given to Eunike Radic and Berkin Yanarsönmezat, who collected the data as part of their thesis at the University of Stuttgart.
This research received no external funding.
The authors declare no conflict of interest.
