Hypobaric hypoxia is a stressful condition known to decrease fertility both in
humans and animals. However, the mechanism by which the
hypothalamus-pituitary-gonad axis is altered remains unknown. The aim of the
present study was to analyze the effects of chronic intermittent and continuous
exposure to hypoxia on hypothalamic-pituitary-gonadal axis regulation in male
rats. Thirty adult male Wistar rats were assigned to one of the following three
groups: control group; chronic intermittent hypoxia: subjected to 600 mbar for 18
h/d five days a week; and chronic continuous hypoxia: subjected to 600 mbar for 23.5
hours/day seven days a week, for 30 days. Plasma luteinizing hormone and
testosterone concentration, hypothalamic GnRh, Kiss1 and Rfrp3
mRNA levels and PGE
Reproductive functions in adults are mainly controlled by the
hypothalamic-pituitary-gonadal (HPG) axis. Gonadotropin releasing hormone (GnRH)
secretion by specific hypothalamic neurons is regulated by several factors:
gonadal steroids, neurotransmitters (including dopamine, GABA, histamine, and
opioids) and by hypothalamic neuropeptides, such as gonadotropin-inhibitory
hormone (GnIH)/RFamide-related peptides (Rfrp) and kisspeptin, a peptide encoded
by the Kiss1 gene [1]. Rfrp3 is known to suppress the HPG axis at the
level of both the hypothalamus and adenohypophysis (AH) inhibiting GnRH and
luteinizing hormone (LH) secretion [2, 3]. Kisspeptin is a stimulatory
neuropeptide and therefore increases GnRH synthesis and secretion [4].
There is evidence that Rfrp-3 may affect the signaling to GnRH neurons from other
neuronal fibers, such as kisspeptin neurons, indicating that there exists an
intricate balance between inhibitory and excitatory neuronal signals that
ultimately modulate the activity of the reproductive axis [5]. In addition to the
neuropeptides mentioned above, prostaglandin E
GnRH is released from its neuronal hypothalamic terminals to the portal vessels and reaches the AH, a specific region of the pituitary gland, where it induces the release of LH and follicle-stimulating hormone (FSH) into the bloodstream. LH is responsible for stimulating sex steroid secretion (such as testosterone (T), estradiol, and progesterone) from the gonads of both sexes, whereas FSH is the main gametogenic hormone [8].
Hypoxia (HX) is considered a stressful stimulus and the body must develop
compensatory physiological responses in order to ensure homeostasis. Continuous
HX is experienced by populations living at high altitude and who, as a result,
have undergone phenotypic and genetic adaptations to cope with low environmental
O
The present study sought to analyze the effects of chronic intermittent and continuous exposure to hypoxia on the mechanisms that modulate HPG axis activity. The aim is to contribute to understanding of hypoxia-related male infertility.
Male Wistar rats (250–300 g) supplied by the animal housing facility of the
School of Pharmacy and Biochemistry, University of Buenos Aires (Argentina), were
used throughout. Animals had free access to food and water and were kept under
controlled light (12 h light/dark) and temperature (20–25
Plasma T concentration was determined using a rat enzyme-linked immunosorbent assay (ELISA) with antibodies and standards from DRG Instruments (GmbH, Marburg, Germany), as described by Surkin et al., [1]. Absorbance was determined at 450 nm on a microplate reader (Model 3550, BIO-RAD Laboratories, California, USA). Plasma T levels are expressed as ng/mL.
As hypothalamic PGE
Plasma LH was measured by RIA using rat LH antiserum (NIDDK-anti-rLH-S-II), antigen (NIDDK-rLH-I) and reference preparation (NIDDK-rLH-RP-3) purchased from Dr. A. F. Parlow (National Institute of Diabetes and Digestive and Kidney Diseases; Torrance, CA, USA). All samples were measured in duplicate; results are expressed as ng of LH/mL of plasma [19].
Following euthanasia, hypothalami were harvested in RNAZOL Reagent and frozen at
–80
Results are expressed as box plots, where the lower, central, and upper sides of
the box represent the first, second (median), and third quartile of the data
points, respectively; the lower and upper whiskers represent the lower and upper
value of the data, respectively. All data were processed using Infostat
(Córdoba, Argentina). The significance of the differences between means was
determined by one-way ANOVA followed by Tukey’s test. Statistical significance
was set at p
Following the findings reported by Farias et al. [14], body weight at the end of the experimental period
was lower in rats exposed to intermittent hypoxia than in the other two groups (F
| Control | CIH | CCH | |
| Body weight (g) | 512.50 ± 25.88 |
399.4 ± 11.78 |
487.30 ± 28.18 |
| Hematocrit (%) | 42.6 ± 2.30 |
52.4 ± 3.50 |
56.6 ± 2.51 |
| C, control; CIH, chronic intermittent hypoxia; CCH, chronic continuous hypoxia.
Results expressed as mean | |||
Analysis of hormonal secretion by the HPG axis showed lower LH and testosterone
concentration in rats exposed intermittently to hypoxia than in controls (LH:
C: 0.68
 Fig. 1.
Fig. 1.Intermittent exposure to hypoxia for 30 days decreased LH and
Testosterone concentration. Hypothalamic GnRH mRNA levels (A), LH
concentration (B), and Testosterone concentration (C) in rats exposed to chronic
hypoxia for 30 days. C, control; CIH, chronic intermittent hypoxia; CCH,
chronic continuous hypoxia. Results are shown as boxplots. *p
Analysis of the neural factors that regulate HPG axis activity showed higher
Rfrp3 mRNA levels in hypothalamic neurons of the CIH group compared to
those observed in the other two groups (C: 0.14
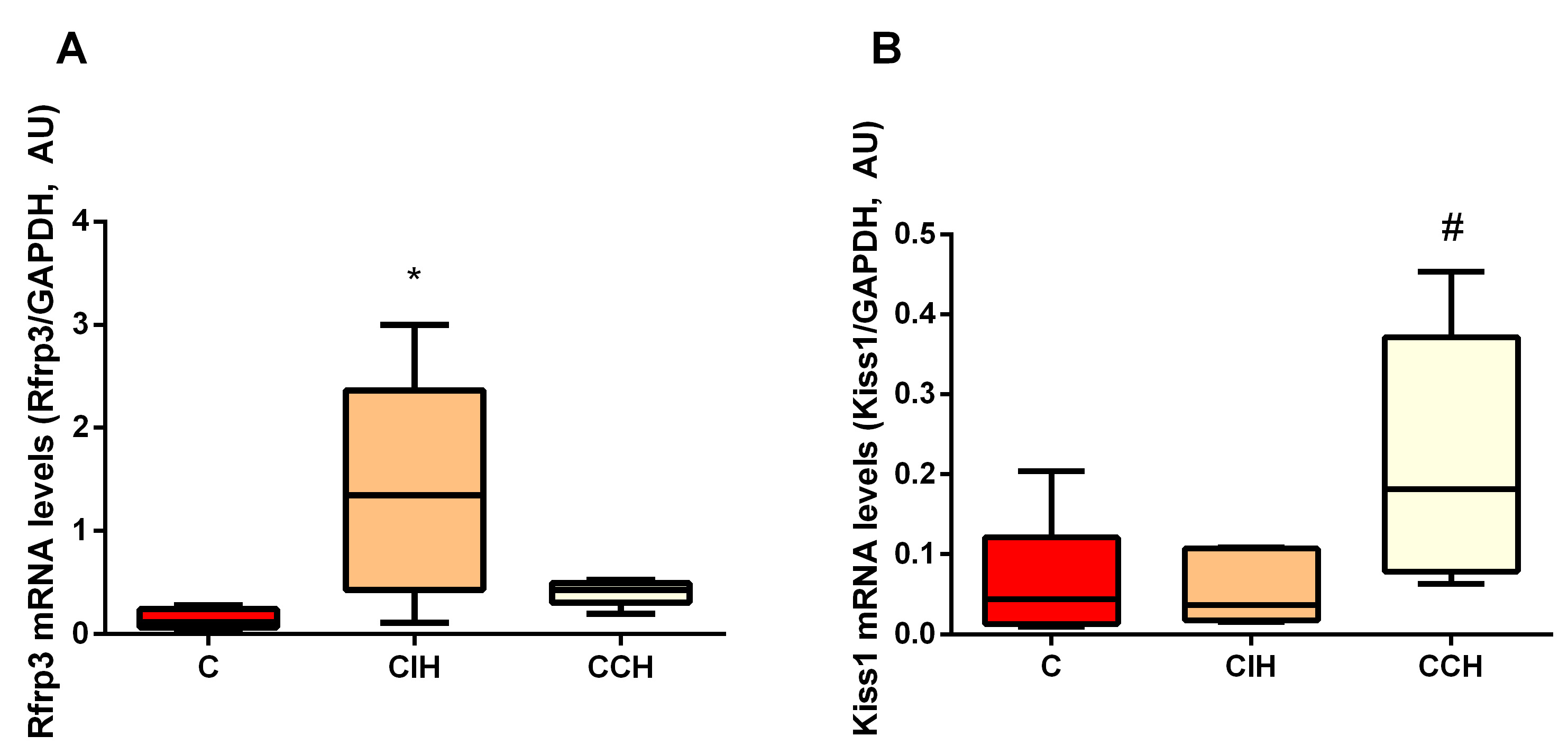 Fig. 2.
Fig. 2.Intermittent hypoxic exposure resulted in increased
Rfrp3 mRNA levels. Hypothalamic Rfrp3 (A) and
Kiss1 (B) mRNA levels in rats exposed to chronic hypoxia for 30
days. C, control; CIH, chronic intermittent hypoxia; CCH, chronic
continuous hypoxia. Results are shown as boxplots. *p
Hypothalamic PGE
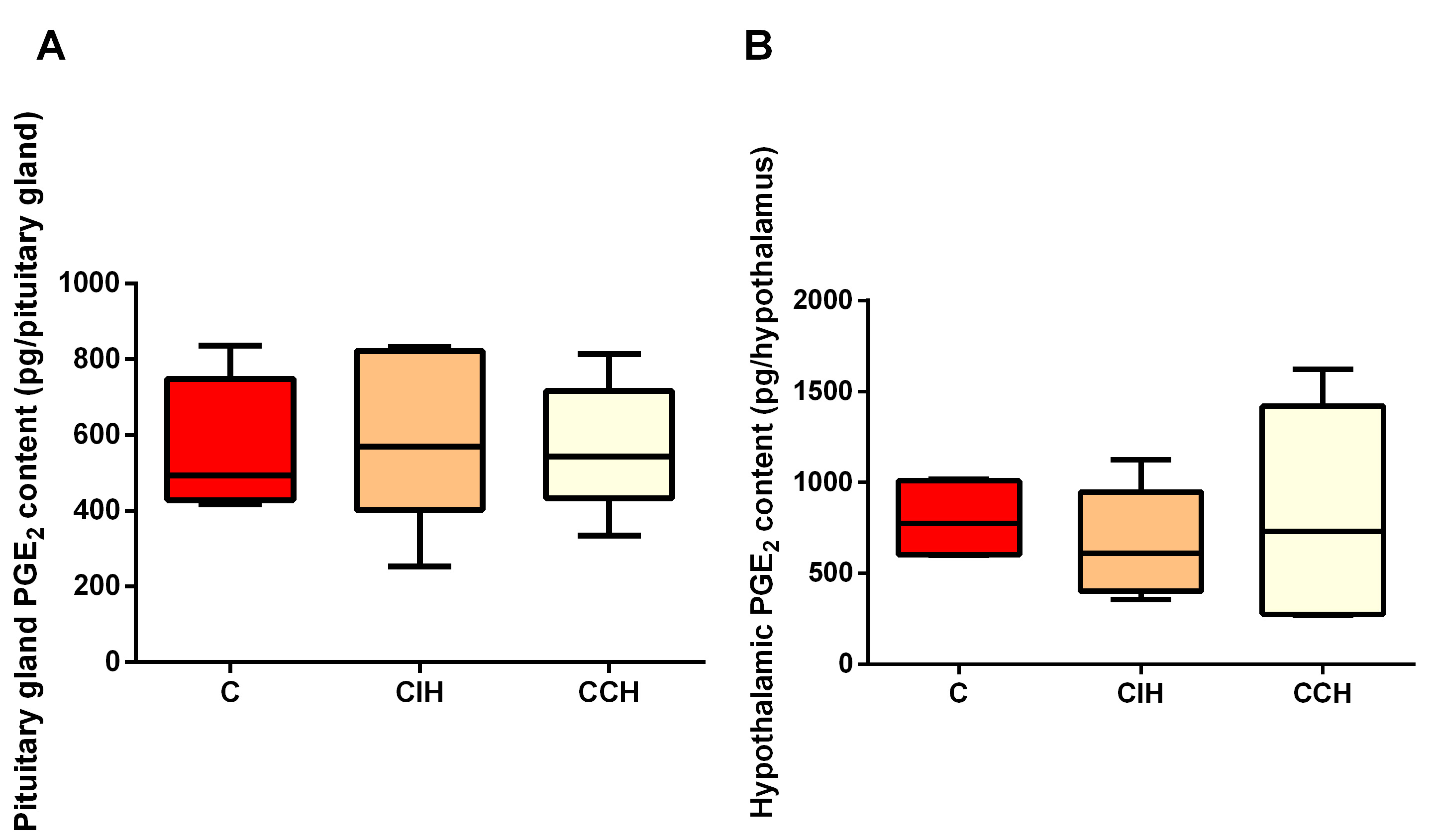 Fig. 3.
Fig. 3.Hypoxia did not seem to affect hypothalamic and
pituitary PGE
The results obtained in this study show that intermittent exposure to hypoxia increased the levels of Rfrp3 mRNA, encoding gonadotropin-inhibitory hormone synthesis, which is consistent with the lower plasma LH levels observed for this same group. The lower levels of LH would likely explain the lower T levels observed in the CIH group. To the authors knowledge, this is the first study to explore the neuropeptides that regulate HPG axis secretion under the main types of hypoxic conditions commonly affecting humans.
Findings regarding the reported effects of hypoxia on human and animal fertility are controversial. In line with studies that show no change in T levels in HX-exposed rats as compared to rats maintained under normoxic conditions [20], Nelson et al., [21] found no difference in LH or FSH levels between hypoxic animals and controls. However, human and animal studies, see Sawhaney et al., [13], Gasco et al. [16], Farias et al.[17], Richalet et al. [22], and Liu et al. [23] showed a decrease in LH, FSH and T levels. In keeping with the former reports, the present study showed intermittent HX to result in lower LH, and consequently in lower T secretion. Of note, CCH animals showed similar LH and T levels to those of controls. Based on reported studies, it could be posited that hormonal response of the HPG axis varies according to the type of hypoxic exposure (i.e., intermittent or continuous), duration of exposure (a few days or months) and the altitude to which the subjects are exposed. Previous studies have shown that intermittent exposure to hypoxia HX has a more deleterious effect on tissues than continuous exposure [17, 24]. The present results would seem to indicate that the same occurs in the case of the HPG axis. Continuous exposure would allow the body to adapt and develop mechanisms to compensate for the lower oxygen levels, i.e., enhanced differentiation of neuron non-proliferating precursors [25]. Conversely, intermittent exposure would not allow the proper cellular signals to occur, leading to a failure in the acclimation process [26].
GnRH and LH release in males is subject to a negative feedback control mechanism
that involves T [8]. The results presented here show lower T levels in
CIH-exposed animals. Hence, altered LH and GnRH levels may also be expected in
this group. However, although lower LH levels were found in CIH rats than in
controls, Gnrh mRNA levels remained unchanged. The latter finding could
indicate that CIH does not affect GnRH synthesis. Nevertheless, the potential
occurrence of posttranscriptional changes, which would ultimately affect GnRH
synthesis and secretion, cannot be ruled out. Additionally,hormonal feedback is only one of the several intricate mechanisms that control
the activity of the HPG axis. As mentioned previously, GnRH synthesis and release
are also regulated by signals from neurons and glial cells. Two of the main
neuropeptides that regulate GnRH release are Rfrp3 (negative regulator of GnRH
and kisspeptin (stimulator of GnRH neurons). The higher levels of Rfrp3
found in the hypothalamic neurons of CIH animals would explain, at least in part,
the lower levels of LH observed in this group, since Rfrp3 neurons are known to
negatively regulate GnRH and kisspeptin release at the central level and LH
release from the AH. Conversely, Kiss1 mRNA levels were higher in
continuously exposed rats, likely due to the absence of the inhibitory effect of
Rfrp3 neurons. This may account for CCH animals showing similar LH and T levels
to those of controls. Furthermore, given that PGE
There are limitations to the present study that must be noted.t. Firstly, regulation of the HPG axis involves many more factors than those analyzed in the present work. Animal body weight, for example, is one such regulator, since body fat is directly associated with HPG activity. Leptin, an adipocyte-derived protein hormone, not only conveys a signal of the amount of energy stored to the central nervous system but also plays an important role in regulating neuroendocrine function [29]. It has been demonstrated that leptin stimulates GnRH and LH release [30]. Hence, the lower body weight observed in CIH animals suggests that leptin deficiency may also dysregulate GnRH and LH release in this experimental group. Further studies are needed to corroborate the role of body weight and leptin in the regulation of the hypothalamic-pituitary-gonadal axis. The main findings of this study and the possible underlying mechanisms discussed herein can be observed in Fig. 4. Secondly, only mRNA levels of GhRH, Rfrp3 and Kiss1 were assessed in the present study. It would be of interest to analyze protein expression of these molecules. Additionally, given that GnRH and LH are regulated by multiple factors, studies should be conducted to fully characterize the mechanisms mediating hypothalamic—pituitary response under conditions of chronic hypoxia.
 Fig. 4.
Fig. 4.Main findings and possible underlying mechanisms. Hypothalamus-pituitary-gonadal axis secretion is under the influence of multiple regulatory mechanisms, both central and non-central nervous system related stimuli. Using an in vivo animal model, it is demonstrated that exposure to chronic intermittent hypoxia decreases LH and testosterone concentration, as well as increases the levels of Rfrp3 mRNA, a negative regulator for GnRH and LH secretion (principal findings are shown as full arrows). Additionally, animals exposed to CIH showed lower body weight, which may account for lower leptin (positive modulator for the hypothalamus-pituitary-gonadal axis) levels (hypothetic underlying mechanism, shown as dotted arrow). Direct effects of hypoxia on the testicles may contribute to the low testosterone concentration and should not be ruled out. Green indicates positive modulation for hormonal release, whereas red means negative modulation.
In conclusion, intermittent hypoxia would seem to be more deleterious to male fertility than continuous hypoxia. This may be explained by the negative regulation of LH release by Rfrp3, which is overexpressed in the hypothalamus of CIH rats. Continuous exposure to hypoxia would enable acclimation mechanisms to maintain kisspeptin secretion, avoiding a decrease in LH levels. The observed alteration in HPG axis function may involve other mechanisms that were not analyzed in this study, such as impaired GnRH and LH release due to leptin stimulation. Direct effects of hypoxia on the testicles may contribute to the low testosterone concentration observed in intermittently exposed animals and should not be ruled out.
AH, adenohypophysis; CCH, chronic continuous hypoxia; CIH, chronic intermittent hypoxia; FSH, follicle-stimulating hormone; GnRH, gonadotropin releasing hormone; HPG, hypothalamus-pituitary-gonadal axis; HX, hypoxia; LH, luteinizing hormone; OSA, obstructive sleep apnea; PGE2, prostaglandin E2; Rfrp-3, RFamide-related peptides; T, testosterone.
All authors participated in the conceptualization of the study, its methodological design and data analysis. ART performed the experiments, analyzed data and wrote the original draft. MPM and JFSJ were the project administrators and the responsible for grant acquisition. They also revised and edited the original draft.
Animals were purchased from the animal housing facility of the School of
Pharmacy and Biochemistry, University of Buenos Aires (Argentina). All the
animals were treated in keeping with the NIH Guide for the Care and use of
Laboratory animals (NIH 8th edition, 2011), and the protocols were approved by
the institutional Animal Care and Use Committee (IACUC, N
Authors would like to thank Julia Astrauskas for her technical assistance.
This research was funded by UBACYT 20020150100006BA and PIP2015-2017 045 CONICET grants.
The authors declare no conflict of interest.
