1. Introduction
Hippocampus is a unique structure. One aspect of this uniqueness is its
special anatomy. Concealed between the mesencephalon and the medial temporal
lobe, this deep cortical structure extends through the lateral ventricle’s
inferior horn, where it lies at the posterior border of the amygdala [1].
Critical for learning and memory, the hippocampus is segmented into
several regions [2], including the hippocampus proper, CA3,
CA2, CA1 regions and the dentate gyrus (DG), a key region in hippocampal memory
formation (reviewed in [3]).
A principal cell type in the DG is the dentate gyrus granule
cells (DGGCs), characterized by unique anatomical features (reviewed in
[4]). The cone-shaped spiny dendritic arbors of the DGGCs are innervated
by different neuronal ensembles such as the input from the entorhinal cortex via
the perforant path and contralateral hippocampus via the commissural path [5, 6, 7].
Diverse GABAergic interneurons synapse on the soma,
axon initial segment, proximal and distal dendrites of DGGCs. For example,
parvalbumin-positive interneurons (PPI) synapse on the axon- initial segment and
the perisomatic domain [8]. These GABAergic interneuron inputs
to the DGGCs are involved in the synchronization of the network activities during
theta- frequency (4-10 Hz) and gamma-frequency (30-150 Hz) oscillations
[9], sharp waves-ripples (SWRs) [10], and dentate
spikes [11].
The critical network operations for neuronal synchronization require the
presynaptic terminals of the GABAergic interneurons (such as PPIs) to precisely
match their molecular counterparts at the postsynaptic sites of the DGGCs. Here,
-Aminobutyric acid type A receptors (GABARs), the GABA gated
heteropentameric chloride channels, are massively clustered in the postsynaptic
sites of the symmetric inhibitory synapses. GABARs belong to the
superfamily of ligand-gated ion channels (Cys-loop receptors)
[12], which also includes the nicotinic acetylcholine receptors
(nAChRs), the 5-hydroxytryptamine type 3 (5-HT3) receptors, the zinc-activated
ion channel (ZAC) and the glycine receptors in vertebrates [13].
Upon GABA release, the postsynaptic GABARs in the mature granule cells
become active and elicit hyperpolarizing inhibitory postsynaptic currents (IPSCs),
during which chloride and bicarbonate ions will travel through the receptor
channel depending on their electrochemical gradient. Known to be benzodiazepine
(BZ) sensitive [14, 15], these IPSCs are called phasic
inhibition. The phasic signals are typically generated rapidly (often with
sub-millisecond rise times), with the stimulus-evoked synaptic currents being in
the range of less than 10 to 200 pA at a holding potential of -50 mV, which is
known to vary in a typical quantal fashion [16].
In addition to the phasic synaptic inhibition, the DGGCs and PPIs have
some other spots where a subset of high-affinity extrasynaptic GABARs is
strategically located in the hippocampus mediate a different tone of GABAergic
inhibition than phasic inhibition. This type of GABAergic signal is called tonic
inhibition. Tonic inhibition is characterized by constant, slow currents, high
GABA affinity, slow desensitization and BZ insensitivity [17].
The tonic current is about four times larger than the total phasic current in the
DGGCs [18]. Like cerebellar granule cells [19],
where the tonic inhibition was first described, distinct subtypes of
extrasynaptic GABARs appear to mediate these relatively constant and slow
inhibitory currents [18], which have also been shown in the
neurons found almost all other major brain areas: neocortex, thalamus,
hypothalamus and brain stem [20]. This distributed fashion of
tonic inhibition, mediated by GABARs, represents distinct subunit
composition, which involves either 5 or subunits
[21]. This review will focus on the subunit containing
GABARs (-GABARs), which mediate a significant fraction of
tonic current.
2. Molecular and cellular properties of -GABARs
For GABAR research, the late 80s and 90s were exciting years.
Almost the entire GABAR subunit family was cloned by Seeburg and his
colleagues [22, 23, 24]. The cloning strategy was based on the classical approach:
Screening the brain cDNA libraries by synthetic DNA probes derived from purified
receptors’ peptides. Thus, eventually, it became clear that GABARs were
assembled from 19 subunit isoforms ((1-6), (1-3),
(1-3), , , , and (1-3))
which correspond to 11 structurally and functionally distinct receptor subtypes
[22, 23, 24]. In general, all these subunits share a common topological
structure: a peptide sequence which is about 450 amino acids long, made up of a
long extracellular N-terminal, a short C-terminal, four transmembrane domains,
intracellular or cytoplasmic domain located between the third and the fourth
transmembrane domains (Fig. 1). This organization was originally based on the
structural studies of acetylcholine-binding protein and nAChRs [25]. In
particular, the subunits of acetylcholine receptors and the human GABAR
3 homopentamer’s crystal structure at 3Åresolution confirmed this
prediction [25, 26]. In contrast to the subunits’ above-described properties, the
hetero-pentameric receptor structure was not fully known until recently. In
recent years, oligomerized heteropentameric receptor structure has also been
resolved in detail [27, 28, 29, 30].
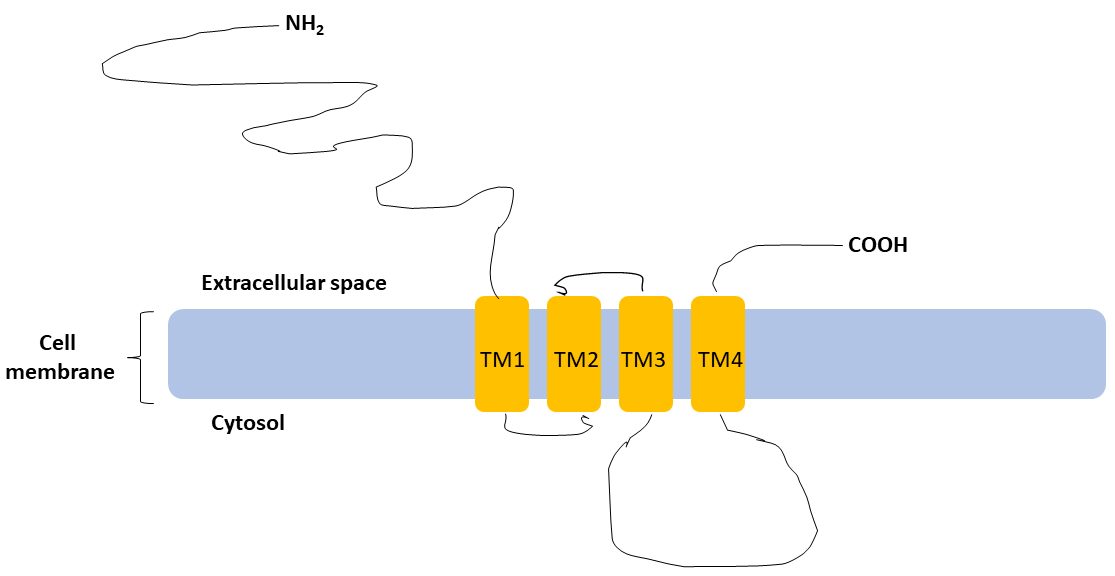 Fig. 1.
Fig. 1.
Basic structure of subunit.
subunit shares a standard topological structure with other subunits of
GABARs: a long extracellular N-terminal, a short extracellular C-terminal,
four transmembrane domains (TM1, TM2, TM3, TM4), cytoplasmic domain located
between the third (TM3) and the fourth (TM4) transmembrane domains (Figure not to
scale).
It is well known that the GABAR subunit composition determines
their differential distribution and functionality [31, 32, 33, 34, 35, 36, 37, 38]. Among the possible subunit combinations, typically, there is a combination
of 2 and 2 subunits and a single 2 or
subunit (Fig. 2), the 2, 2 and 2 combinations being
the most abundant. Indeed, about 90% of all GABARs are made up of
2-GABARs [33]. Thus, the most GABAR research is directed to
, and subunits, which are found both in the
postsynaptic and extrasynaptic locations [39]. Among the subunits, the
BZ insensitive 4 and 6 subunits form a unique partnership
with the subunit (together with subunit isoforms) in the
forebrain and cerebellum, respectively [36]. Thus, in the arrangement of
-GABARs, subunit has been hypothesized as a
replacement of the 2 subunit in the receptor heteropentamer recruited
exclusively to extrasynaptic or perisynaptic locations [40]. In the DGGCs,
4 receptors, the most common isoform of
-GABARs, are expressed. Also, 4
receptors have been identified in several other neuronal cell types (see also Table 1) [41, 42], and
like other -GABAR isoforms, localized in the extrasynaptic and
perisynaptic positions but never in the postsynaptic sites [43, 44].
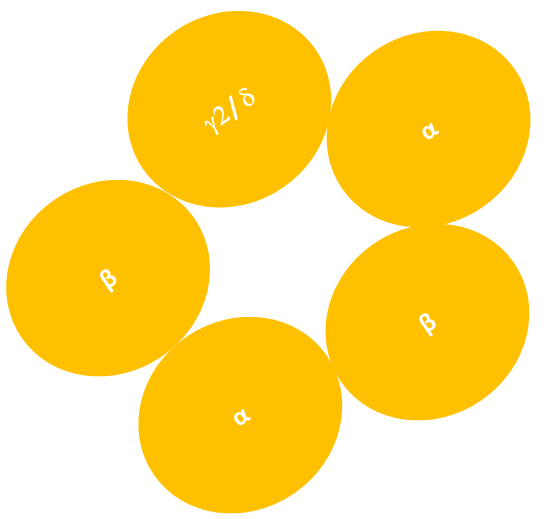 Fig. 2.
Fig. 2.
Heteropentameric structure of
-GABARs. The diagram showing the heteropentameric structure of
GABARs, which are typically composed of two , two and
one or subunit. Experimental studies suggest that the
subunit arrangement of 2-subunit containing GABARs
(2-GABARs) is counterclockwise when viewed from the extracellular
space. It is not known if this arrangement also applies to -subunit
containing GABARs (-GABARs) (Figure not to scale).
Table 1.Summary of -GABAR isoforms found in specific cells in the forebrain and cerebellum.
| Subunit composition |
Cell type |
Reference |
| 6b |
Cerebellar granule cells |
[46] |
| 1b |
Hippocampal interneurons, Neocortical interneurons |
[47, 48] |
| 42 |
Thalamic relay neurons, Striatal spiny neurons, Hippocampal dentate granule cells, Neocortical pyramidal cells |
[41, 42, 49, 50, 51, 52, 53] |
By in situ hybridization analysis, the regions of the adult rat
brain in which subunits are expressed have been studied in detail
[45]: The -subunit is expressed weakly or moderately in the regions of
the olfactory bulb (granule cells and periglomerular), neocortex (layer II/III,
layer IV, layer V/VI and pyriform cortex), hippocampus (DGGCs, stratum
pyramidalis CA1 and stratum pyramidalis CA3), basal ganglia (caudate, putamen,
nucleus accumbens, claustrum), thalamus (mediodorsal, ventral posterior nucleus,
medial-, dorso- and ventrolateral geniculate nucleus). In Table 1, a summary of
-GABAR isoforms (e.g., 4,
6, or 1) and their cell type specific distribution are shown.
This specific distribution is well reflected with the tonic inhibition. For
example, 4 receptors mediate the larger fraction
( 70%) of the tonic inhibition in the DGGCs [21].
3. Variety of -GABAR mediated inhibition
It is known that GABA mediates multiple forms of postsynaptic inhibitory
signals, such as fast and slow inhibitory postsynaptic currents [54, 55].
Additionally, -GABARs, mediating tonic
inhibition and characterized by relatively constant, slow IPSC, has been known
for the last few decades. However, tonic inhibition with these characteristics
has been considered uniform and the only inhibition associated with the
-GABARs. For example, the 4
receptors in DGGCs and thalamic relay neurons mediate such tonic currents [41, 42]. These BZ insensitive GABARs have a high affinity for the GABA diffused
from the synaptic cleft [56], besides the GABA released from GABA transporters
[57, 58]. Interestingly, the literature has started to dissect the tonic
inhibition: Depending on the subunit co-assembly, -GABRs have
different GABA sensitivity, desensitization, and kinetics [59]. For example, it
was shown that the 4 GABARs are the most
sensitive to GABA levels ranging from 100 nM to 800 nM. Whereas
12 and 532 (in addition
to 122) receptors detect GABA levels 1-10 M
range [59].
Accumulating data suggest that extrasynaptic GABARs might mediate
a significant part of tonic inhibition, independent of gating by GABA; thus,
spontaneous activity could occur [60, 61]. Such spontaneous activity also applies
to -GABARs mediated tonic inhibition [62]. This phenomenon’s
functional significance is not understood, and it is probably dependent on the
specific cell types and isoforms of -GABARs, such as
1 and 4 expressed in
these cells.
In addition to studies focusing on -GABARs, some studies
dissect the physiological roles of GABAergic inhibition without explicitly
indicating the associated subunit. So far, a few types of GABARs such as
the ones containing either the , 5 subunits or receptors
containing only subunits have been shown to mediate the tonic
inhibition [63]. Thus, it is hard to predict the role of -GABARs
in these studies. For example, in mice, in the reticular thalamic neurons, a
phasic inhibition with slowed-down kinetics is mediated by GABARs [64].
This association is linked to 4 containing GABARs, but the exact
receptor co-assembly is not clear. Possibly -GABARs might
mediate this activity because, in the thalamus, most of the 4
containing receptors involve -subunit [41]. This is supported by some
other findings, too. For example, the subunit is expressed explicitly
in the thalamus [45], including the reticular thalamic nucleus [38]. However,
this latter study represents the monkey brain, reflecting some differences
compared to the rodent brain. It turns out that, in rat and mouse brains,
-subunit is not expressed in the reticular thalamic nucleus [38, 65],
whereas in the monkey, it is [38]. Thus, it is not clear if the 4
subunit linked phasic inhibition with slowed-down kinetics [64] is mediated by
-GABARs even though the specific partnership of
subunit with 4 subunit in the forebrain, including the thalamus, is
well known [31, 41, 42, 66, 67].
Nevertheless, there is a collection of data supporting an additional
GABAergic inhibition representing an intermediate form between the classical
phasic (GABA, fast) and tonic inhibition, which is called GABA, slow [54, 55] some of which may be mediated by -GABARs as experimental
evidence supports that -GABARs contribute to postsynaptic
inhibition. Postsynaptic inhibition contributed by -GABARs was
observed in the cerebellum, thalamus and neocortex [68]; in DGGCs of the mouse
hippocampus [69, 70]. Thus, Fig. 3 shows different types of inhibition mediated
by -GABARs as a proposition. For reference, postsynaptic
2-GABARs, which mediate phasic inhibition, are also shown (Fig. 3).
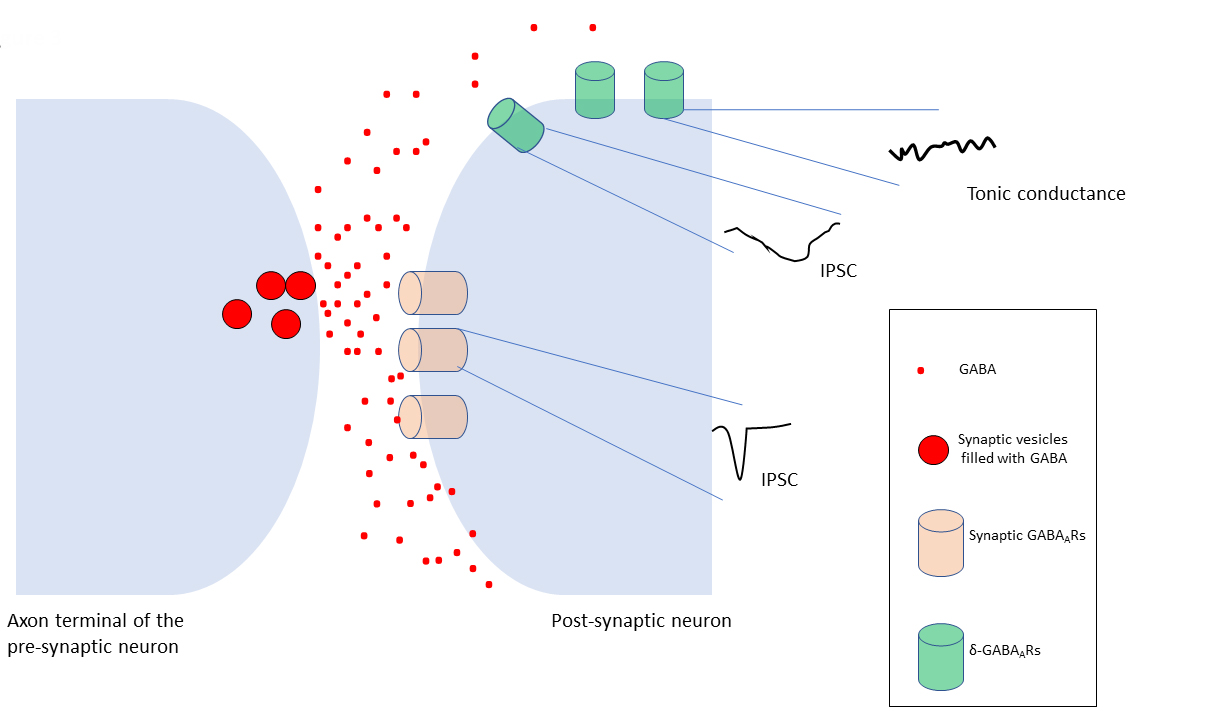 Fig. 3.
Fig. 3.
Variations of GABAergic inhibition. Fast, point to
point, phasic inhibition is typically mediated by synaptic GABARs,
clustered in the postsynaptic membrane of the inhibitory synapses. These
receptors evoke inhibitory postsynaptic current (IPSC) (phasic inhibition) in a
millisecond range upon GABA binding. The GABA spillover from the synaptic region
(black arrows) results in extrasynaptic receptors, which mediate a slow
inhibitory conductance, the tonic inhibition. Phasic and tonic inhibition of
synaptic and extrasynaptic GABARs has led to a functional distinction of
these receptor subtypes. On the other hand, subsets of GABARs, including
-GABARs may have intermediate activation, desensitization, and
deactivation rates determined by the receptor subunit isoforms between these two
states. This leads to the idea that -GABARs may contribute to postsynaptic
inhibitory currents (IPSCs) (Figure not to scale).
4. Variety of functions
As mentioned above, at the neuronal level, -GABARs mediated tonic
inhibition, which is important for the threshold of action potential generation
[36, 71, 72, 73]. It is generally hypothesized that tonic inhibition decreases
neuronal excitability. Recent evidence-based computer models revealed that tonic
inhibition might also increase excitability [74].
Increasing literature shows the critical role of the nonsynaptic
GABAR and/or tonic inhibition in various functions, including network
oscillations [64, 75, 76], synaptic plasticity [77], synaptic pruning during
adolescence [78], neurogenesis [79, 80], neuronal development [81], information
processing, and cognition [81]. For example, in the dentate gyrus,
-subunit is linked to enhanced memory and neurogenesis [82].
-GABARs mediated tonic inhibition is indicated for
modulation of oscillations in the mouse hippocampal CA3 interneurons
[75]. Also, coupling presynaptic activity to postsynaptic Inhibition in the
somatosensory thalamus involved a process that influenced the
-selective allosteric modulator, DS2 [76]. These take
-GABARs from being the mediators of “shunting” inhibition
involved in controlling neuronal excitability to additional roles in the network
level activities, including but not limited to the thalamocortical system and
neurogenesis in the hippocampus.
5. -GABARs and associated pathophysiology
The subunit modulators such as sedative and hypnotic agents
[83], anxiolytic and anticonvulsive agents [84, 85] suggest that
subunit may play a role in the etiology of the relevant disorders. Alterations of
subunit or their modulation as therapeutic targets have been linked to
sex specific behavioral disruption [86], Alzheimer’s disease [87], stress induced
deficiency in learning and memory [88], fragile X syndrome [89] schizophrenia
[90], epilepsy [91], mood disorders [92, 93, 94], childhood mood disorders [95],
anxiety in methamphetamine dependence [96], major depression [97]; post-partum
depression, and post-partum psychosis [94, 98], consumption of opioids [99],
menstrual cycle related problems [100, 101], stroke [102], Fragile X Syndrome
[89, 103], traumatic brain injury [104, 105], Huntington’s disease [106], pain
[107], insomnia [83, 108, 109, 110], alcohol use disorders [111].
In animal studies, alcohol use disorders or associated behavioral
alterations have been linked to -GABARs [112, 113] and
sex-dependent [114] as well as developmental [115] factors seem to play a role in
the underlying mechanisms. At the molecular level, ethanol impacts the modulation
of the clathrin adaptor-mediated endocytosis of -GABARs [116],
and its withdrawal influences -GABARs via PKC
Activation [117]. Due to the estrous cycle-dependent plasticity of
-GABARs, which was previously shown as associated with seizure
susceptibility and anxiety [100], one study, using the model of
“Drinking-in-the-Dark binge-drinking”, showed that -GABARs are
a critical target for binge drinking in females, a phenomenon observed at higher
rates among women and girls [118]. The methylation pattern of subunit
was also suggested as a diagnostic biomarker for alcohol use disorders [111].
It is important to talk about the special link between the
-subunit and epilepsy. Various mutations (missense, nonsense, and
frameshift mutations in coding DNA sequences besides mutations in the intronic,
3’ downstream, or 5’ upstream mutations) in GABA receptor subunit encoding
genes have been linked to consequences such as the distortion of protein
structure, conformation, abundance, or localization. Some of these mutations,
which are detected in 1, 3, 2, and
subunits, have been associated with idiopathic generalized epilepsies (IGEs). For
example, mutations in the 2 subunit are characterized by change of a
single amino acid (2(Q351X [119], 2(R43Q) [120], and
premature translation-termination codon (PTC)-generating mutations
2(Q351X) [121]) are associated with different IGEs. Two
subunit missense mutations, namely (E177A) and (R220H),
were reported [122, 123]. Due to the distortion in the coding sequence, missense
mutations lead to an altered amino acid sequence in the signal peptide regions of
mature peptide regions. Dibbens et al. [123] reported mutations in the
genomic region (1p36.3) of the subunit, representing susceptibility
locus for generalized epilepsies. The subunit missense mutations,
located in the subunit’s extracellular N-terminus, are associated with
generalized epilepsy with febrile seizures plus (GEFS+), a type of IGEs. These
mutations alter the channel conductance [123], gating and surface expression of
-GABARs [122]. Thus, -GABARs are considered as
targets in the treatment of epilepsy.
Neurosteroids are endogenous substances synthesized from cholesterol
into pregnenolone, which is then converted to compounds such as allopregnanolone
and allotetrahydrodeoxycorticosterone [124]. It is suggested that fluctuations in
neurosteroid interactions, such as those seen during stress or the ovarian cycle,
determine the seizure threshold, a phenomenon that is partially mediated by
-GABARs [100]. This and other evidence [125, 126] suggest that
neurosteroids are novel drug candidates for epileptic disorders [125, 128].
Consequently, due to their potent actions on -GABARs [128, 129],
-GABARs are novel therapeutic targets for the treatment of
epileptic disorders and maybe a future perspective to control epileptogenesis
[91, 130]. Ganaxolone, the synthetic analog of endogenous neurosteroid, is used
as an antiepileptic agent (catamenial epilepsy), although it is the modulator of
all GABARs, it shows a higher effect on -GABARs [131, 132, 133, 134].
Interestingly, the modulation and pharmacology of
-GABARs have become more critical recently. In addition to their
modulation by insulin [135] and oxytocin [136], recently in 2019, the
allopregnanolone brexanolone (Zulresso, the brand of Sage Therapeutics,
Inc.), one of the neurosteroids known as a potent modulator of
-GABARs has been approved by the Food and Drug Administration
(FDA) for postpartum depression1 (1Drug Approval Package, FDA (https://www.accessdata.fda.gov/drugsatfda_docs/nda/2019/211371Orig1s000TOC.cfm), accessed in
28.01.2021.) as a result of successful clinical trials [137, 138, 139].
Brexanolone seems to be effective on other mood disorders, such
as major unipolar depression and post-traumatic stress disorder [140]. A
synthetic GABAR modulator that shares a similar molecular pharmacological
profile as brexanolone, the zuranolone (SGE-217), resulted in a reduction in
depressive symptoms according to a recent phase 2 clinical trial [141].
Despite the progress, the field is dominated by many unknowns, which is
a significant bottleneck. For example, the above-mentioned preferential
modulation of -GABARs by neurosteroids is controversial and
requires further validation. Regarding this, some studies suggested that the
neurosteroid sensitivity of 4/-containing extrasynaptic
receptors may not be different than that of
//2-containing receptors [142, 143]. Along with the
other inconsistencies, which will be summarized in the section “7. The
basics of unknowns”, more research is needed for
-GABARs.
6. A circuit pharmacology for -GABARs
The variations and specificities of -GABARs in terms of
their isoforms, inhibitory action, distribution, sensitivity, modulation and
spontaneous activity, which have been described so far, lead to the question to ask whether
these properties can be utilized for circuit pharmacology. The idea of
GABAR circuit pharmacology has probably gained momentum when the diversity
of subunits and their specific pharmacology in the subunit assembly have started
to be shown [144, 145]. However, the focus was mainly on the modulators of
subunit isoforms [23, 144, 145, 146] such as 5 inverse
agonists RO4938581 [147]; S44819 [148], L-655,708 [149], Alpha5IA [150].
RO4938581 is under preclinical investigation for its potential
to cure cognitive deficits in people with Down syndrome [151], for example.
Since the subunit-specific function and specific modulation are key to the strategy of
circuit pharmacology, -GABAR seem to fit into this strategy.
Among the isoforms of -GABARs, two population receptors are
expressed in the hippocampus. 1 receptors are
expressed predominantly in hippocampal interneurons, whereas
4 receptors are expressed predominantly in granule
cells of the dentate gyrus (DGGCs) (Table 1). One study selectively silenced one
population of these isoforms: 1 expressed in the
Parvalbumin positive interneurons [152]. Thus, using the “PV/Cre-Gabrd/floxed
system”, it was reported that in vitro oscillations in the
CA3 region were altered in both PV-Gabrd(+/-) and PV-Gabrd(-/-) mice in these
interneurons. Interestingly, the increased oscillations were lowered
to control PV-Gabrd(+/-) levels when 100 nM allopregnanolone
(3,5-tetrahydroprogesterone) was used. But when 10 M
synthetic -GABAR positive allosteric modulator
4-Chloro-N-[2-(2-thienyl)imidazo[1,2-a]pyridin-3-yl] benzamide (DS-2) was used,
this was not observed. DS-2 selectively targets 4
receptors but not the 1 receptors, which are
expressed in the interneurons. These suggest the specific role of
1 isoform in the hippocampus’s integrative network
operations, in a way that can be modulated by selective agents. In line with
this, another study examined the paired whole-cell recordings from synaptically
coupled reticular thalamus and thalamocortical neurons of the ventrobasal complex
in brain slices of 4 knock-out (4(0/0)) mice. Results suggest
a dynamic and activity-dependent engagement of -GABARs receptors
for the coupling of presynaptic activity to postsynaptic excitability, a process
sensitive to DS2, the specific modulator 4//
receptors [76]. The resolution of the three-dimensional structure of GABAR
subtypes in recent years will trigger the design of novel drugs targeting
specific -GABAR isoforms, which will likely aid the treatment of
network disorders by circuit pharmacology approach.
7. The remaining unknowns
GABAR research has been challenged by the receptors’ unusual molecular and cellular diversity and thus a huge effort is required to fully understand the properties of GABARs. Here, we will briefly mention the unknowns related to molecular and modulatory features of -GABARs, only. The nonsynaptic localization -GABARs is well established
by electron microscopy studies [43, 44]. However, it is unknown if a passive or
an active mechanism mediates this specific nonsynaptic localization pattern.
Previously, it was suggested that the subunit’s intracellular domain might play a
role in this process [153]. The intracellular domain, which is found in between
the third and the fourth transmembrane domains, is a large cytoplasmic domain,
highly conserved across the whole span of vertebrate evolution [153].
Despite new studies [154, 155, 156], the current knowledge about the
assembly and stoichiometry -GABARs is limited. Several studies
have shown the stoichiometry of -GABARs as 2,
2 and [157, 158]. For example, one recent study suggested
that recombinant 13 receptors have the same
stoichiometry and subunit arrangement with 132
receptors. However, these results are not entirely conclusive [155]. Thus, the
basics such as assembly rules, stoichiometry, and arrangement of
-GABARs, and their membrane trafficking, maintenance and
modulation are not precisely known. For instance, in the in vitro live
neuroblastoma cells, our group reported that recombinant subunits
require both and subunits for membrane targeting [159],
confirming the previously hypothesized analogy (2 subunit is replaced
by in the -GABAR arrangement) between
subunit and 2 subunit: it is known that 2 cannot assemble
into receptors inserted in the cell membrane without and/or
subunits [160, 161]. In contrast to our findings [159], some other previous
studies suggest that and containing
receptors exist and show functionality in Xenopus oocytes [162, 163]. So, there is no consensus.
This may arise from the methodological variations used during in vitro
studies: use of different vectors, cell types, or subunit isoforms, experimental
strategy (such as fluorescent protein tagging location) may impact on these
results. For example, in HEK-293T cells, quantification of fluorescent
alpha-bungarotoxin bound subunits on Western blots of surface immunopurified
tagged GABARs led to the conclusion that the cell surface expression of
2- GABARs was regulated by the
ratio of subunit cDNAs transfected [164].
The distribution of subunit has been shown in different
species, which shows species-specific variations. For instance, in the reticular
thalamus, caudate, putamen and globus pallidus, there is an expression of
subunit in the monkey, while this expression is absent in the rat
[38]. The human brain distribution of subunit is not known fully. At
the same time, some studies reported the distribution of 1-3,
2/3, and 2 subunits in the human striatum [165] and
thalamus [37].
Sensitivity to neuroactive steroids has also been questioned.
Neuroactive steroids such as allopregnanolone (35P) and
allotetrahydrodeoxycorticosterone (THDOC) are considered to selectively affect
-GABARs over 2-GABARs. -GABARs
sensitivity to neurosteroids in specific brain regions [166] is hypothesized to
be very specific such that the endogenous neurosteroid THDOC at physiologically
relevant concentrations (10-100 nM) selectively increases the
tonic current, with almost no effect on the phasic current in mouse dentate gyrus
granule cells and cortical granule cells [128, 167]. Thus, selective interaction
of -GABARs with neurosteroids has been hypothesized to have
clinical significance due to tonic inhibition’s modulation, impacting
excitability, seizure susceptibility, and behavior [100]. On the other hand, the
neurosteroid binding site has been identified in the transmembrane domain of the
-subunit [168]. Moreover, a recent study suggests that neurosteroids
act through both -containing and non--containing receptors
[143]. Thus, the degree of neurosteroid selectivity of -GABARs is
questionable [142, 143].
Similarly, the mechanism by which ethanol potentiates GABARs is
still not fully understood, and several publications have reported contradicting
results. In general, 2-GABAR subtypes are sensitive to ethanol
at amounts required for high intoxication, whereas the extra-synaptic
-GABARs are hypothesized to be most sensitive to ethanol at
levels of social drinking, that is less than 30 mM [47, 70, 113, 169, 170].
However, this has been challanged by some publications [171, 172].
8. Conclusions
Increasing studies open new horizons on the -GABAR’s
neurobiology; however, the complexity continues to be a challenge. On the
one hand, it could turn out that -GABARs function may be broader
than previously hypothesized. This is well reflected with studies showing the
possible contribution of -GABAR mediated inhibition to the
control of major thalamocortical oscillations. Also, the possibility of some
other forms of phasic inhibition, with roles in the integrative function and
network oscillations, may underlie an even broader spectrum of physiological
functions of -GABAR during health and disease.
On the other hand, knowledge is deficient in the level of “basics”.
There is uncertainty regarding the knowledge about the assembly [155], membrane
targeting [159], clustering [153] and modulation of -GABARs
[142], for example. Without elucidation of the mechanisms involved in these basic
receptor mechanisms, precisely, it will be challenging to unravel the
-GABA receptor physiological significance and plasticity during
health and disease. Thus, there is a need for a focused establishment of these
“basics” in a subtype-specific fashion. Such an effort requires novel
methodologies and careful consideration of experimental subject design.
Experimental parameters appear to have a critical impact on the GABAR
research illustrated by the lack of convergent findings obtained by the
experimentation on the same subject by different methods.
Abbreviations
BZs, Benzodiazepines; CNS, Central nervous system; DGGC, Dentate gyrus
granule cell; DS-2, 4-Chloro-N-[2-(2-thienyl)imidazo[1,2-a]pyridin-3-yl]
benzamide; FDA, Food and Drug Administration; GABA, -Aminobutyric acid;
GABARs, -Aminobutyric acid type A receptors; GABA(A) receptor,
-Aminobutyric acid type A receptor; GABA-d, GABA receptor subunit; -GABARs, subunit containing GABARs;
2-GABARs, 2 subunit containing GABARs; HEK293T
cells, Human embryonic kidney 293T cells; IPSC, Inhibitory postsynaptic currents;
M, Micro molar; N, Asparagine; nM, Nano molar; nAChRs, Nicotinic
acetylcholine receptors; NMDA, N-methyl-D-aspartate receptor; pA, pico Amper;
PPI, Parvalbumin positive interneurons;
Q, Glutamine; THDOC, Allotetrahydrodeoxycorticosterone; W, Tryptophan.
Author contributions
AA conceptualized the study, identified the purpose and the scope,
analyzed the literature, synthesized the knowledge and wrote the paper.
Acknowledgment
The author thanks three anonymous reviewers for excellent
criticism of the article.
Conflict of interest
The authors declare no conflict of interest. Given her role as the Editorial Board Member of JIN, Prof. Ayla Arslan had no involvement in the peer-review of this article and has no access to information regarding its peer-review.
 Fig. 1.
Fig. 1. Fig. 2.
Fig. 2. Fig. 3.
Fig. 3.


