1 Institute of Brain Science, Shanxi Key Laboratory of Inflammatory Neurodegenerative Diseases, Shanxi Datong University, Datong, 037009, P. R. China
2 Research Center of Neurobiology, The Key Research Laboratory of Benefiting Qi for Acting Blood Circulation Method to Treat Multiple Sclerosis of State Administration of Traditional Chinese Medicine, Shanxi University of Traditional Chinese Medicine, Jinzhong, 030619, P. R. China
3 Department of Neurology, First Affiliated Hospital, Shanxi Medical University, Taiyuan, 030001, P. R. China
4 Department of Neurology, Datong Fifth People's Hospital, Datong, 037009, P. R. China
Abstract
Recent studies have shown that Nogo-A and the Nogo-A receptor affect
Keywords
- Fasudil
- Alzheimer's disease
- apoptosis
- Nogo-A/NgR/RhoA
- hyper-phosphorylated tau (p-tau)
Alzheimer’s disease (AD) two key pathological features are amyloid deposition or
plaques and neurofibrillary tangles. The main component of amyloid plaques is
A recent study confirmed a significant loss of hippocampus CA1 and CA3 neurons
in AD patients (Padurariu et al., 2012). The reduction of neuronal apoptosis
improves cognition and reduces anxiety-like behavior in APP/PS1 transgenic mice
(Meng et al., 2020). Previous research has demonstrated that caspase-3 is
involved in neuronal apoptosis in AD as it cleaves the adaptor protein GGA3, thus
elevating A
Nogo-A is a well-known myelin-associated protein that inhibits axonal
regeneration. Nogo-A was the earliest identified neurite outgrowth inhibitor,
which plays a significant role in developing the central nervous system (Chen et al., 2000; Pernet and Schwab, 2012; Sekine et al., 2020). Nogo-A binds to
the Nogo-66 receptor (NgR), which forms a complex with the p75 neurotrophin
receptor (p75NTR) and a BK channel regulator (LINGO1) to transduce intracellular
signals. This complex activates downstream RhoA/Rho kinase (ROCK) signaling,
resulting in inhibition of axonal regeneration and prevention of neurite/axon
outgrowth (Fournier et al., 2001; Kempf and Schwab, 2013). Nogo-A is
upregulated in the AD hippocampus and participates in synaptic plasticity (Gil et al., 2006). Activation and overexpression of Nogo-A/NgR both inhibit neurite
outgrowth by ROCK activation and promote overproduction and release of
A
Fasudil is a potent ROCK inhibitor with multiple functions in the central
nervous system (CNS). It has been shown to participate in promoting axonal
regeneration and activation of endogenous neural stem cells, inhibition of the
neuroinflammatory response, induction of the release of neurotrophic factors and
prevention of neurodegeneration caused by A
Eight-month-old male APP/PS1 transgenic mice (APPswe/PSEN1dE9) were purchased from Shanghai Research Center, and age- and sex-matched wild-type (WT) C57BL/6 mice were purchased from Vital River Laboratory Animal Technology Company Limited (Beijing, P. R. China). All animals were housed in the animal house of the Institute of Brain Science, Shanxi Datong University. The Ethics Committee approved all experiments of Shanxi Datong University, Datong, P. R. China.
APP/PS1 double transgenic mice were randomly divided into two groups: An APP/PS1 transgenic mice group (APP group, n = 8) and a Fasudil-treated APP/PS1 transgenic mice group (APP + Fa group, n = 8). Age- and sex-matched wild-type (WT) male C57BL/6J mice served as controls (WT group, n = 8). Groupings were blind during behavioral tests and analysis.
Fasudil (Tianjin Chase Sun Pharmaceutical Co., Ltd.) was dissolved in a sterile
saline solution. The APP + Fa group was treated with Fasudil (25
mg/kg
Morris water maze (MWM) tests were performed as described previously (Yu et al., 2018). Briefly, the MWM was a 90 cm diameter pool, containing a
transparent platform (5
The Y-maze test was based on a previously described method (Xu et al., 2018)
to test short-term memory functions. The Y-maze consists of three symmetrical
opaque arms (30 cm long, 8 cm wide, 15 cm high), randomly designated as novel,
start or other arms. During the training period, the novel arm was blocked, and
mice freely explored the maze’s remainder for 10 min. Between tests, the maze was
wiped with 75% ethanol to eliminate olfactory cues from previous mice. All
trials were recorded, and the spontaneous alternation rate between maze arms was
calculated using the following equation: alternations (%) = (sequence of arm
choices)/(total arm choices - 2)
An in situ cell death detection kit (Beyotime Biotechnology) was used
to detect apoptotic cells with Deoxyuride-5’-triphosphate biotin nick end
labeling (TUNEL) assay, as per the manufacturer’s protocol. Half the mice were
briefly anesthetized and perfused with saline and 4% paraformaldehyde in
phosphate buffer (PBS, 0.01 M, pH 7.4). Brain tissue was collected, frozen in
liquid nitrogen and cut into 10
For double immunohistochemistry, sections were incubated with 0.3% Triton X-100
in 1% bovine serum albumin (BSA) - phosphate buffer saline (PBS) for one hour to
block unspecific binding, then incubated at 4
Half of the mice were anesthetized and perfused with saline only. Total brain
RNA was extracted (Total RNA extraction kit, Promega, USA) and reverse
transcribed (Promega, USA) for complementary DNA synthesis. The
GoTaq
Equal amounts of protein (50
All statistical analyses employed GraphPad Prism 5.0 (GraphPad Software, San
Diego, CA). Data are presented as mean
Initially, an MWM test was conducted to evaluate Fasudil’s effects on cognitive function in APP/PS1 transgenic mice. Representative swimming paths and the behavioral performance of mice in each group are displayed in Fig. 1a. These data indicate that APP/PS1 mice performed more unnecessary swimming. In the five-day initial training period, APP/PS1 Tg mice exhibited an increased latency and mean distance to the target (finding the hidden platform) compared to WT mice. Fasudil-treated mice showed reduced latency and mean distance to target compared to APP/PS1 Tg mice. However, there was no significant difference between groups in mice’s time to locate the hidden platform (Fig. 1b). The platform was removed on the sixth day, and mice were placed into the pool and allowed to swim freely for 60 seconds. APP/PS1 transgenic mice exhibited an increased latency and mean distance to target and latency to first entrance to the SW zone when compared with WT mice (Fig. 1c). This suggests that APP/PS1 transgenic mice exhibited cognitive deficits. However, this effect was partly reversed by Fasudil treatment, as evidenced by the significantly shorter time taken and distance traveled to reach the platform from the starting point on to the platform exhibited by Fasudil-treated APP/PS1 transgenic mice when compared with APP mice (Fig. 1c).
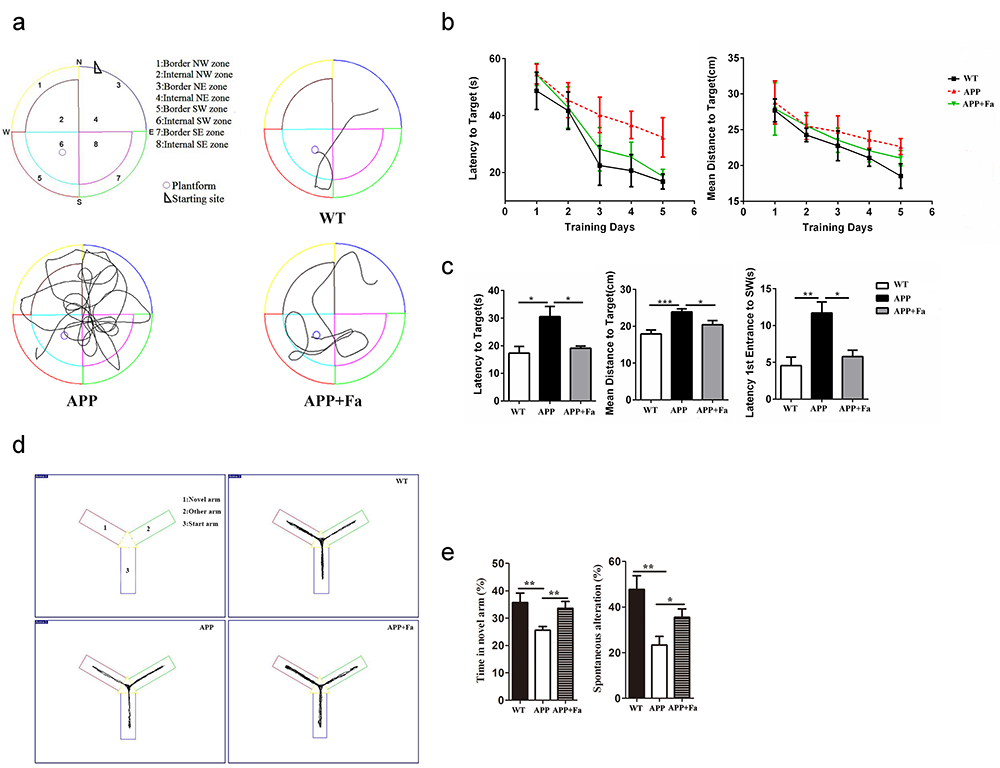 Fig. 1.
Fig. 1.Fasudil improves spatial learning of APP/PS1 Tg
mice. Eight-month-old APP/PS1 Tg mice were injected with saline (n = 8)
or Fasudil (n = 8) for two months. (a) Typical diagram of the Morris
water maze test and the corresponding parameters. (b) Latency to target and mean
distance to target for five consecutive daily tests. (c) On the sixth day,
latency to target, mean distance to target, latency to first entrance to the SW
zone were recorded in a retention test session, representing the time spent and
distance traveled by animals from the starting point onto the platform or to the
SW zone. (d) Typical diagram of the Y maze tests. (e) The percentage of time
spent in the novel arm and the spontaneous alternation rate of each group in Y
maze tests. Quantitative results for several parameters are mean
Following the MWM trials, mice’s behavioral performance was assessed in the Y maze (Fig. 1d). Results showed that APP mice spent less time in the novel arm and exhibited a lower spontaneous alternation rate than the WT group (Fig. 1e). Following Fasudil treatment, the time spent in the novel arm and the spontaneous alternation rate was increased significantly (Fig. 1e). This suggests that Fasudil could rescue learning and memory deficits.
The pathogenesis of AD involves an abnormal accumulation of A
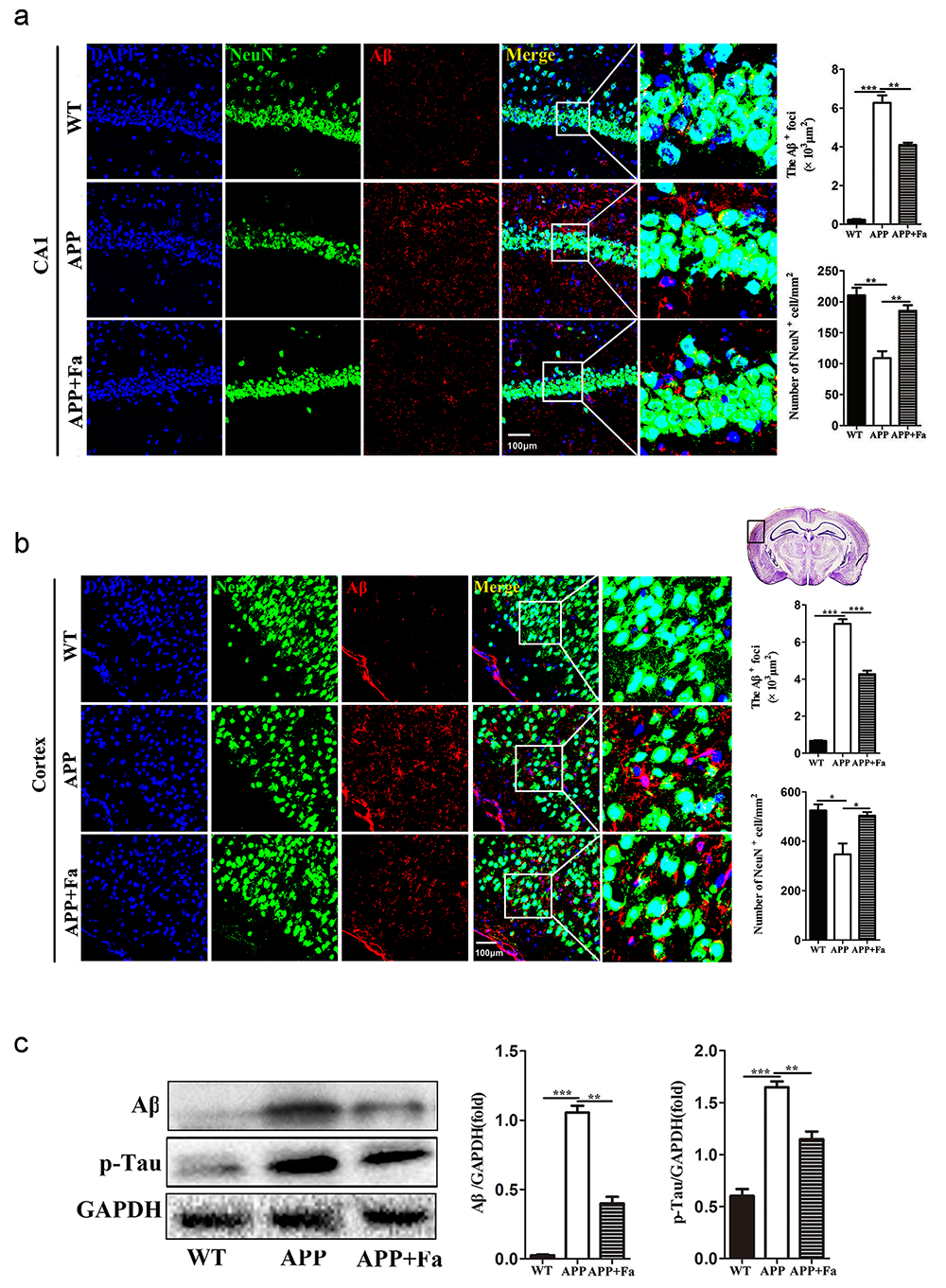 Fig. 2.
Fig. 2.Fasudil reduces A
Another pathological feature of APP transgenic mice is neuronal apoptosis in the hippocampus (Obulesu and Lakshmi, 2014). TUNEL assay revealed apoptosis in APP mice’s brain tissue was significantly increased compared with the WT mice, while apoptosis was markedly reduced in APP + Fa mice (Fig. 3a). To further explore the anti-apoptotic effects of Fasudil in APP/PS1 transgenic mice, protein levels involved in apoptosis were analyzed via Western blot. Results showed that following Fasudil treatment, expression of the pro-apoptotic proteins cleaved-caspase-3 and Bax decreased significantly, while the expression of anti-apoptotic protein Bcl-2 increased significantly in APP/PS1 Tg mice (Fig. 3b).
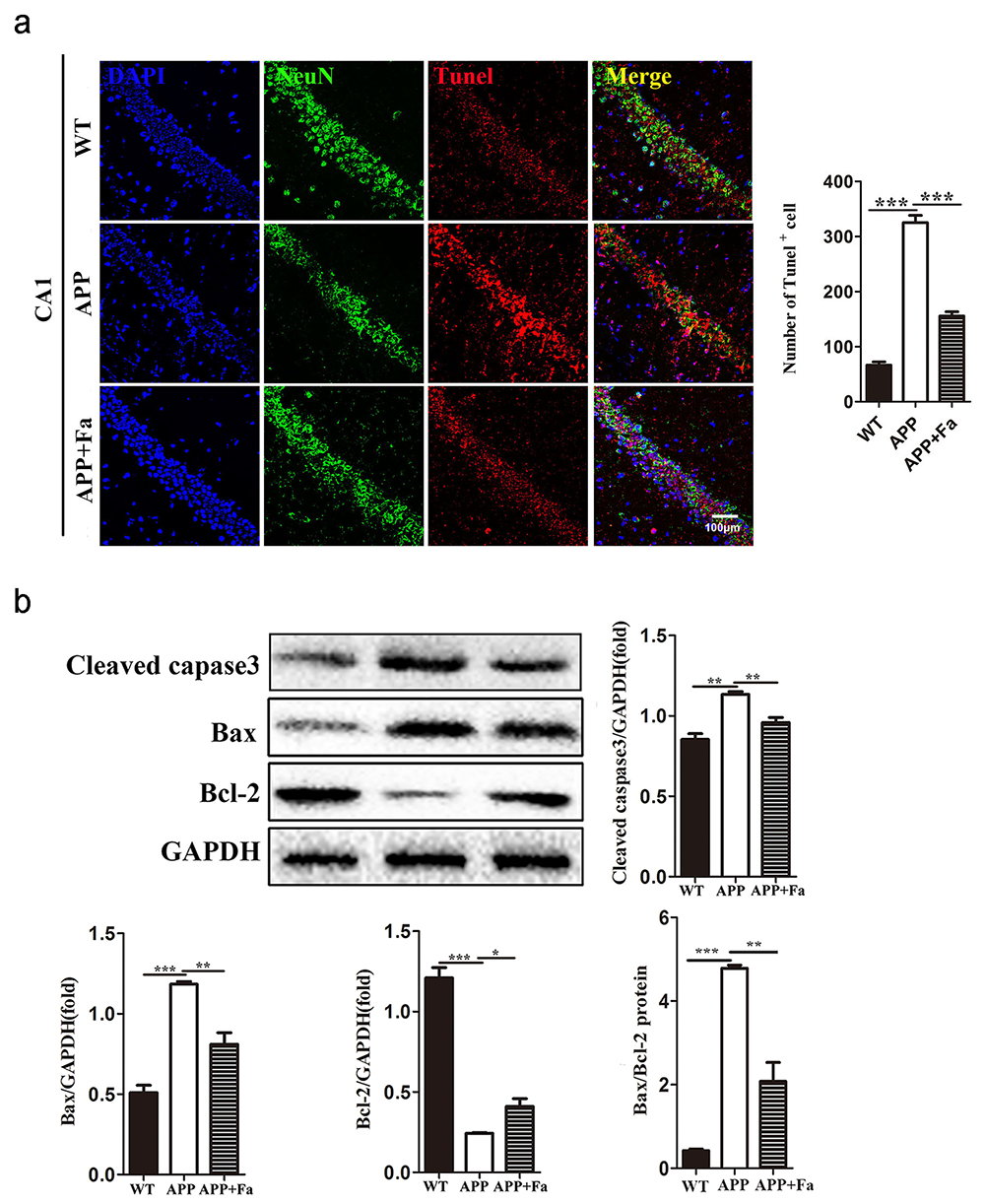 Fig. 3.
Fig. 3.Fasudil inhibits apoptosis in the brain of APP/PS1
mice. (a) Representative images of TUNEL staining in hippocampus area CA1 of
mice and the number of TUNEL immunopositive cells. (b) Detection of
cleaved-caspase-3, Bax and Bcl-2 protein levels in brains by Western blot and
quantitative results of Western blot. Quantitative results are mean
Synaptic dysfunction is another pathological characteristic of AD, contributing to cognitive function loss (Bello-Medina et al., 2019). Levels of synaptophysin and Gap43, key markers for functional synapses, are significantly reduced in AD transgenic mouse models (Bereczki et al., 2018; Goetzl et al., 2016). For these reasons, the expression of synaptophysin and Gap43 in the brain were investigated to test whether Fasudil may improve synaptic function. In the APP mouse brain, the expression of synaptophysin was significantly reduced compared to WT mice, while its expression was greatly improved after Fasudil treatment (Fig. 4a). Similarly, Gap43 mRNA and protein expression in APP + Fa mice’s brain was increased compared to untreated APP mice (Fig. 4b). This indicates that Fasudil’s positive effect on learning and memory may be related to an upregulation of synaptophysin and Gap43.
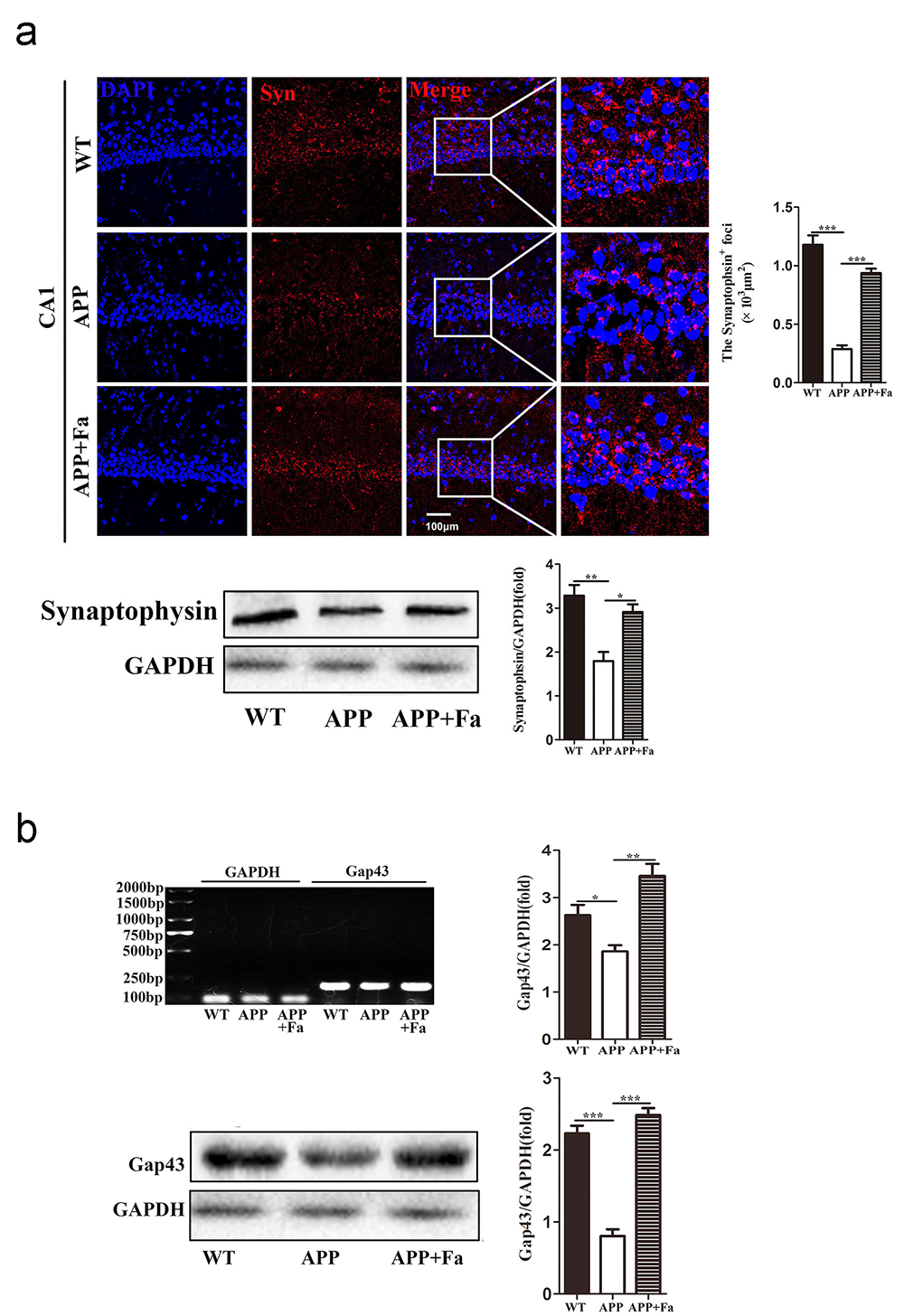 Fig. 4.
Fig. 4.Effect of Fasudil on synaptophysin and Gap43
expression. (a) Detection of synaptophysin in hippocampus area CA1 of mice by
immunofluorescence staining and Western blot. Quantitative analysis of area
(polygon) of synaptophysin
Previous studies have shown that Nogo-A/NgR is closely related to AD’s
pathogenesis by regulating the metabolism of A
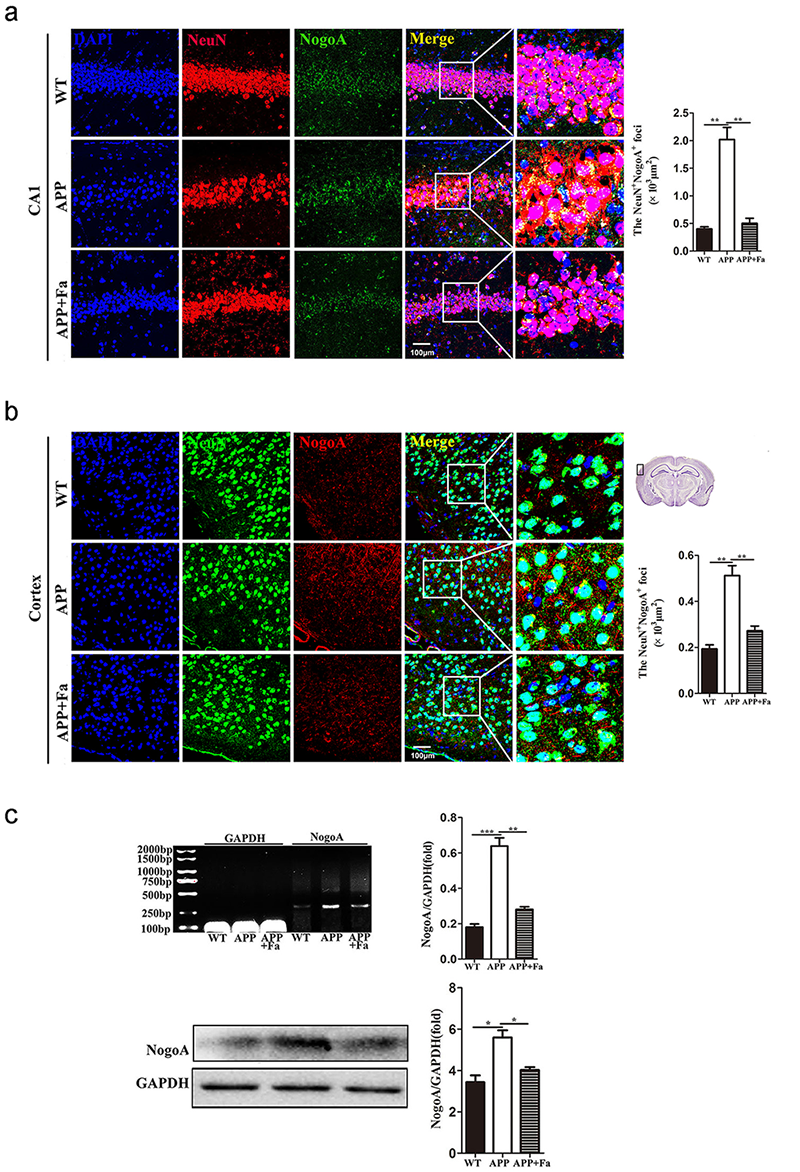 Fig. 5.
Fig. 5.Fasudil inhibits the expression of NogoA. (a)
Immunofluorescence image of neurons (NeuN, green) and NogoA (red) in
hippocampus area CA1 of mice and quantitative analysis of area (polygon) of
NogoA
The expression of the Nogo-A receptor complex, NgR/p75NTR/LINGO-1, was next investigated. Compared with the APP group, the expression of NgR in the cortex and hippocampus was reduced in APP + Fa mice (Fig. 6a and 6b). Similarly, Western blot and quantitative mRNA analysis revealed that the protein and mRNA levels of NgR in the brain were significantly decreased after Fasudil treatment (Fig. 6c). Similar results were found for other components of the Nogo-A receptor complex, including p75NTR and LINGO-1. Furthermore, the protein and mRNA levels of p75NTR and LINGO-1 were significantly increased in APP mice compared with WT mice, but this was rescued by Fasudil treatment (Fig. 6d).
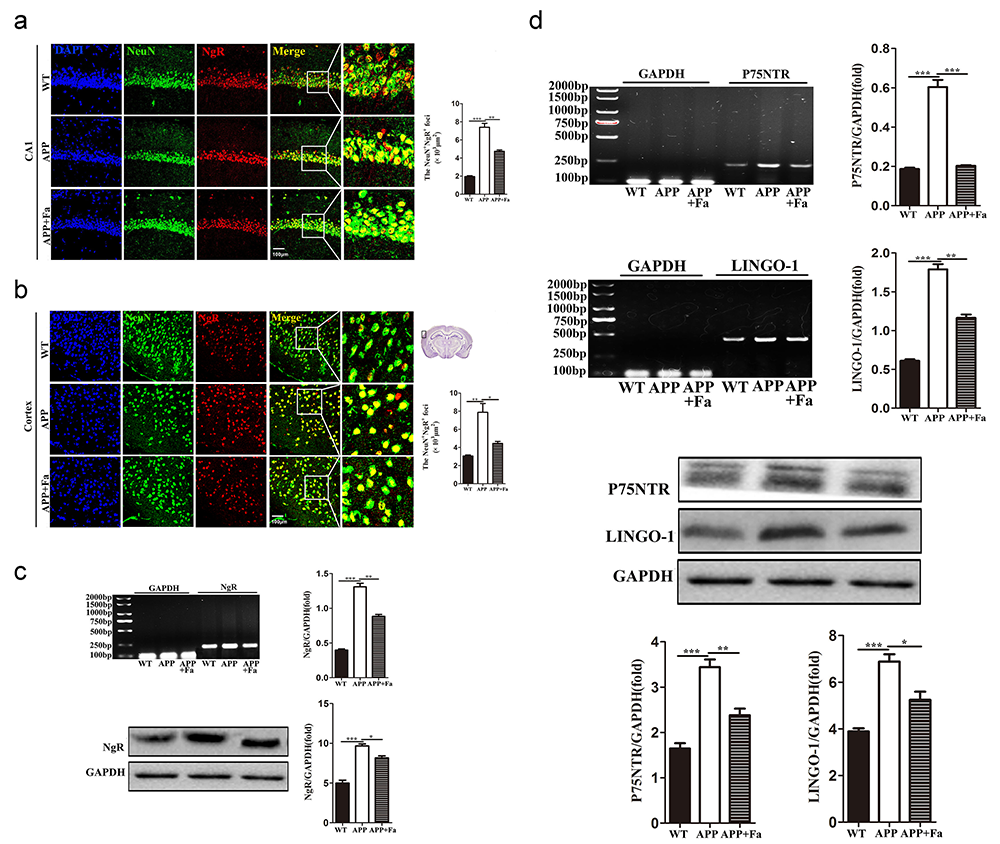 Fig. 6.
Fig. 6.Fasudil inhibits the expression of the
NgR/p75NTR/LINGO-1 receptor complex. (a) Immunofluorescence image of neurons
(NeuN, green) and NgR (red) in hippocampus area CA1 of mice and quantitative
analysis of area (polygon) of NgR
Previous studies have provided substantial evidence that Fasudil inhibits the ROCK pathway and promotes neuroregeneration and remyelination (Li et al., 2017; Wang et al., 2020). Here, ROCK2 and phospho (p)-ROCK2 protein was quantified in the brain of Fasudil-treated and untreated APP mice. Expression of ROCK2 and p-ROCK2 was found to be significantly reduced after Fasudil treatment. Additionally, the activity of ROCK2, measured as the proportion of p-ROCK2 and total ROCK2, was found to be decreased after Fasudil treatment (Fig. 7).
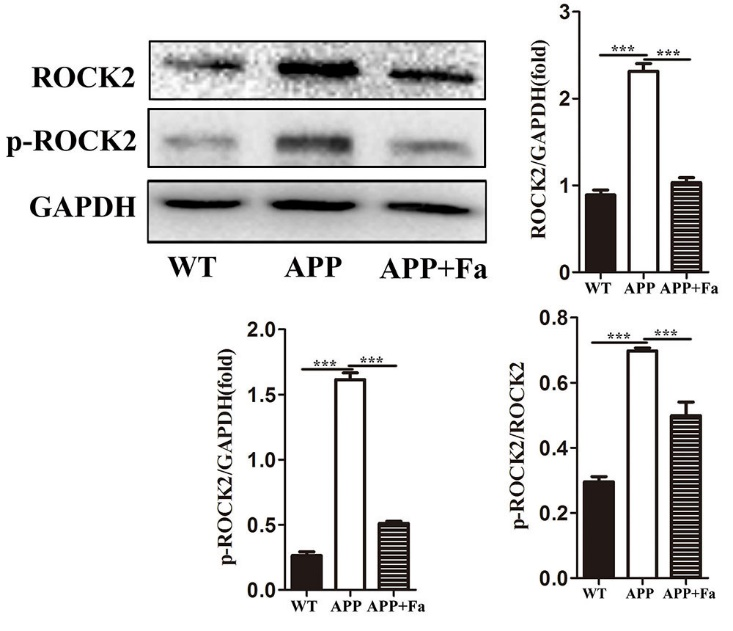 Fig. 7.
Fig. 7.Fasudil inhibits the rhoA/ROCK pathway. Detection
of ROCK2 and phospho-ROCK2 by Western blot. Quantitative results of Western blot
are expressed as fold changes relative to GAPDH bands and ratio of gray values
between p-ROCK and ROCK. Quantitative results are mean
Although the pathogenesis of AD is now partially understood, effective treatment
strategies for delay or prevention of AD remain to be developed. Therefore, there
is an urgent requirement to understand AD’s pathogenesis more clearly to identify
effective intervention targets for clinical management. The ROCK signaling
pathway is involved in a series of pathological AD processes (Aguilar et al., 2017). Fasudil has been shown to protect against A
APP/PS1 double transgenic mice are known to develop AD-like pathology and are
widely used to study AD (Ferguson et al., 2013; Yu et al., 2020). Here, the
efficacy of a two-month course of Fasudil treatment (intraperitoneal (i.p.)
injection of 25 mg/kg/day) was investigated in the eight-month-old APP/PS1
transgenic mice. It was found that Fasudil treatment improves learning and
cognitive abilities and ameliorates both A
Synaptic dysfunction is closely related to AD’s cognitive deficits, and studies
have suggested that abnormally elevated A
Another pathological feature of AD is neuronal apoptosis, and apoptosis-related proteins are involved in pathologically neuronal death in AD (Engidawork et al., 2001). In this study, a reduced number of neurons were observed in the hippocampus area CA1 in APP/PS1 transgenic mice; however, Fasudil treatment significantly reduced neuronal apoptosis in this mouse model. Proteins of the caspase family play crucial roles in the process of apoptosis, with caspase-3 acting as a direct effector of apoptosis (Porter and Jänicke, 1999). The results reported here show that cleaved-caspase-3 expression in the brain tissue of APP/PS1 transgenic mice was significantly increased, while Fasudil treatment significantly inhibited caspase-3 expression. The ratio of Bax and Bcl-2 determines the cell (Sun et al., 2012); the data reported here indicate that the ratio of Bax/Bcl-2 expression is altered in APP/PS1 transgenic mice. Fasudil induced resistance to cellular apoptosis by reducing the Bax/Bcl-2 ratio. This demonstrates that Fasudil has an anti-apoptotic effect in APP/PS1 transgenic mice.
The Rho/ROCK pathway is downstream of NogoA/NgR. It is involved in the
progression of AD via regulation of APP metabolism, A
Nogo-A is a membrane protein abundantly expressed in both neurons and
oligodendrocytes that limits synaptic plasticity and neurite outgrowth (Pernet and Schwab, 2012). Nogo-A transduces intracellular signals by binding to a
receptor complex consisting of NgR, p75NTR and LINGO-1 and activates the
downstream Rho/Rho kinase signaling pathway preventing further axonal growth and
inhibiting myelin formation (Fournier et al., 2001; Kempf and Schwab, 2013).
Many studies have proposed that Nogo-A promotes the development of AD. For
example, one study found that the deletion of the Nogo gene improves learning and
memory deficits in APP transgenic mice and restores the levels of some synaptic
markers, including synaptophysin and Gap43 (Masliah et al., 2010).
Furthermore, it has been suggested that Nogo-A may trigger the occurrence and
development of AD by affecting A
Similarly, NgR plays an essential role in axonal and synaptic plasticity and may
participate in the pathological process of AD by affecting the metabolism of
amyloid precursor protein (Park and Strittmatter, 2008). Further study has
demonstrated that NgR knockdown in the perforant path rescues cognitive deficits
and synaptic function by decreasing the level of amyloid precursor protein and
A
From the present study, it can be concluded that Fasudil effectively rescues
cognitive deficits, reduces A
Min-Fang Guo participated in the study’s design, conducted most of the experiments, analyzed the results, and wrote most of the manuscript. Hui-Yu Zhang and Pei-Jun Zhang carried out the mouse behavioral tests, immunoassays and proofread the article. Xiao-Qin Liu, Wen-Yue Wei, Yu-Yin Wang and Bing-Tao Mu performed the RT-PCR and Western blot experiments. Li-Juan Song and Zhi Chai helped with data analysis. Cun-Gen Ma and Jie-Zhong Yu designed the experiments. All datasets generated for this study are included in the article.
All animals were housed in the animal house of the Institute of Brain Science, Shanxi Datong University. The Ethics Committee approved all experiments of Shanxi Datong University, Datong, P. R. China.
This work was supported by grants from the National Natural Science Foundation of P. R. China (No. 81473577 and 81471412), and the Scientific and technological innovation team of integrated Chinese and Western medicine for the prevention and treatment of nervous system diseases, Shanxi University of Chinese Medicine (2018TD-012), Shanxi Applied Basic Research Project (201901D211538), Natural Fund Project of Shanxi Province (201901D111334) and Research Project Supported by Shanxi Scholarship Council of P. R. China (2014-7), Project of Shanxi Province Platform Base (201805D131005 and 201805D111009), Shanxi Province Key R & D Plan (2106ZD0505), Science and Technology Innovation Projects of Universities in Shanxi Province (2020L0484) and Platform Base Plan Project of Datong (2019198).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.







