Centella asiatica is notable for its wide range of biological activities beneficial to human health, particularly its cognitive enhancement and neuroprotective effects. The α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors are ionotropic glutamate receptors mediating fast excitatory neurotransmission essential in long-term potentiation widely thought to be the cellular mechanism of learning and memory. The method of whole-cell patch-clamp was used to study the effect of the acute application of Centella asiatica extract on the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor-mediated spontaneous excitatory postsynaptic currents in the entorhinal cortex of rat brain slices. The respective low dose of test compounds significantly increased the amplitude of spontaneous excitatory postsynaptic currents while having no significant effects on the frequency. The findings suggested that Centella asiatica extract increased the response of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors at the postsynaptic level, revealing the potential role of Centella asiatica in modulating the glutamatergic responses in the entorhinal cortex of rat brain slices to produce cognitive enhancement effects.
Ionotropic glutamate receptors (iGluRs) are essential in maintaining neuronal activities. The α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPARs) mediate rapid excitatory neurotransmission in the brain and are essential for cellular processes underlying synaptic plasticity. AMPARs are heterotetramers that can be activated by glutamate to allow the influx of ions to depolarise postsynaptic membranes and initiate a series of cascade signaling linked to the process of synaptic plasticity (Granger and Nicoll, 2014; Herring and Nicoll, 2016; Nicoll, 2017). AMPARs consist of four different subunits GluA1 - 4 and are widely expressed throughout the central nervous system (Henley and Wilkinson, 2016; Traynelis et al., 2010). The entorhinal cortex (EC) is one of the key structures of the medial temporal lobe memory system. Information is processed and projected from the cortical association areas through the EC to the hippocampus. The EC functions as a relay station receiving input from the hippocampus and projecting it back to widespread cortical areas (Medinilla et al., 2013; Sasaki et al., 2015; Squire and Zola-Morgan, 1991). Hence, modulation of the iGluRs in the EC may regulate synaptic efficiency essential for cognitive functions such as learning and memory. Synaptic transmission is a highly plastic and dynamic process involving a myriad of cellular components at the synapses. It can be modified by instantaneous, short, intermediate, or long-term regulatory mechanisms in the pre- and postsynaptic regions. The spontaneous excitatory postsynaptic currents (sEPSCs) of a recorded cell indicate how acute application of test compounds may modulate the current response from the pre- or postsynaptic regions of the cell. Centella asiatica (CA), also known as pegaga in Malay, is commonly consumed by local communities of Malaysia and widely used as a medicinal herb in traditional medicine such as Traditional Chinese Medicine and Ayurvedic medicine (Gray et al., 2018a). Studies showed that CA contains a high level of pentacyclic triterpenoids and the levels of phytochemicals in CA can be influenced by geographical origin, genetic, environmental and growth conditions (Gray et al., 2018a; Singh et al., 2014; Upadhyaya and Saikia, 2012; Zhang et al., 2012). CA extract and its triterpenoids asiatic acid (AA) and asiaticoside (AS) have been demonstrated to possess a wide range of biological activities beneficial to human health such as cognitive enhancing, neuroprotective, neuritogenic, increase synaptogenesis and hippocampal cell regeneration (Gray et al., 2018b; Soumyanath et al., 2012; Sun et al., 2015; Xu et al., 2012). Our previous study demonstrated that CA extract was able to improve spatial and non-spatial learning and memory in rats and enhanced the surface expression of AMPARs in the EC and hippocampus (Binti Mohd Yusuf Yeo et al., 2018; Wong et al., 2019). Various studies on the biological activities of CA have focused on the hippocampus with limited studies targeting the effects of the extract and its phytocompounds from the perspective of cellular electrophysiology involving AMPAR-mediated current responses in the EC.
The preparation of CA extract and its quantitative analysis using HPLC has been described in the previous publication (Wong et al., 2019). Briefly, the plant material was purchased from a local supplier, powdered and extracted using standard extraction protocol (60% aqueous ethanol as a solvent with plant material to the solvent ratio of 1 : 10 (w/v), 8 h extraction time with extraction temperature at 60 °C) at the extraction facility of Institute of Bioproducts Development (IBD), Universiti Teknologi Malaysia. The resulting extract was concentrated, subjected to freeze-drying, and analyzed for its photo component via HPLC analysis. The dose of CA extract was set at 100 mg/L (CA100), 300 mg/L (CA300), and 600 mg/L (CA600) and determined in accordance to previous in vivo studies and molecular analysis indicating its biological effectiveness (Doknark et al., 2014; Giribabu et al., 2014; Kumar and Gupta, 2002; Manasa and Sachin, 2016; Sari et al., 2014; Yolanda et al., 2015). An equivalent dose estimation based on the percentage of respective compounds found in the plant extract was used to estimate the doses of pure compounds (Anukunwithaya et al., 2017a,b; Haug et al., 2012; Wanasuntronwong et al., 2012). Hence, equivalent doses of AA and AS relative to the percentage of presence in the minimal effective dose of CA extract in the whole-cell patch-clamp experiments were used as a basis of dose estimation for a follow-up study of the effects of AA and AS. With the limited information acquired on the bioavailability of these pure compounds of CA and a maximum oral bioavailability at 16.25% (Yuan et al., 2015), a low dose of approximately ¼ of the equivalent dose were selected for both AA and AS for standardization of method. Three doses of AA and AS (0.004, 0.012, and 0.024 µM) were examined for their effects in modulating the AMPAR-mediated sEPSCs. Each set of data for the whole-cell patch-clamp experiments consisted of n = 4-6 good cells.
All procedures were performed on 4-6 weeks old post-weaning male Wistar Kyoto (WKY) rats weighing 100-150 g obtained from Animal Research and Service Centre (ARASC) of Universiti Sains Malaysia (USM). The protocol was in accordance with the internationally accepted principles for laboratory animal use and care and has been approved by the Animal Research and Ethics Committee, USM with the animal ethics approval code [USM/Animal Ethics Approval/2015/(98)(699)]. The animals were housed in an acrylic cage (40 cm × 25 cm × 26 cm) and maintained under standard laboratory conditions (temperature 22 ± 1°C and 12 hours light/dark cycle) with free access to water and commercial pellet diet.
CA extract solution for the acute application was prepared fresh at 100, 300, and 600 mg/mL. Pure compound AA (catalog no. 546712, Sigma Aldrich, US) and AS (catalog no. 43191, Sigma Aldrich, US) were dissolved in sterile-filtered dimethyl sulfoxide (DMSO, catalog number 3176, Tocris Bioscience, UK) to obtain stock solutions of 0.004 mM, 0.012 mM, and 0.024 mM. Stock solutions of 50 mM picrotoxin (PTX, catalog number 1128, Tocris Bioscience, UK) prepared in sterile-filtered DMSO, and 50 mM D-2-amino-5-phosphopentanoic acid (D-AP5, catalog number 0106, Tocris Bioscience, UK) were prepared in distilled water. All solutions were prepared at 1000 times more concentrated than desired concentration and to be diluted to the desired concentration in perfusing artificial cerebrospinal fluid (aCSF) during experiments.
Animals were anesthetized and decapitated using an animal guillotine. The brain was removed and placed in 4 °C cutting solution (in mM: sucrose, 110; KCl, 2.5; CaCl2, 0.5; MgCl2, 7; NaH2PO4, 1.25; NaHCO3, 25; glucose, 7; pH 7.40, bubbled with carbogen, 95% oxygen and 5% carbon dioxide). The brain tissue block was prepared for slicing using vibration microtome (Microm HM 650V, Germany) in an ice-cold cutting solution. Combined EC-hippocampal brain horizontal slices of 400 µm thick (Fig. 1) were placed into incubation chamber containing aCSF (in mM: NaCl, 126; KCl, 2.5; CaCl2, 2; MgSO4, 2; NaH2PO4, 1.25; NaHCO3, 26; glucose, 10; pH 7.40, bubbled with carbogen) at room temperature for at least one hour for recovery.
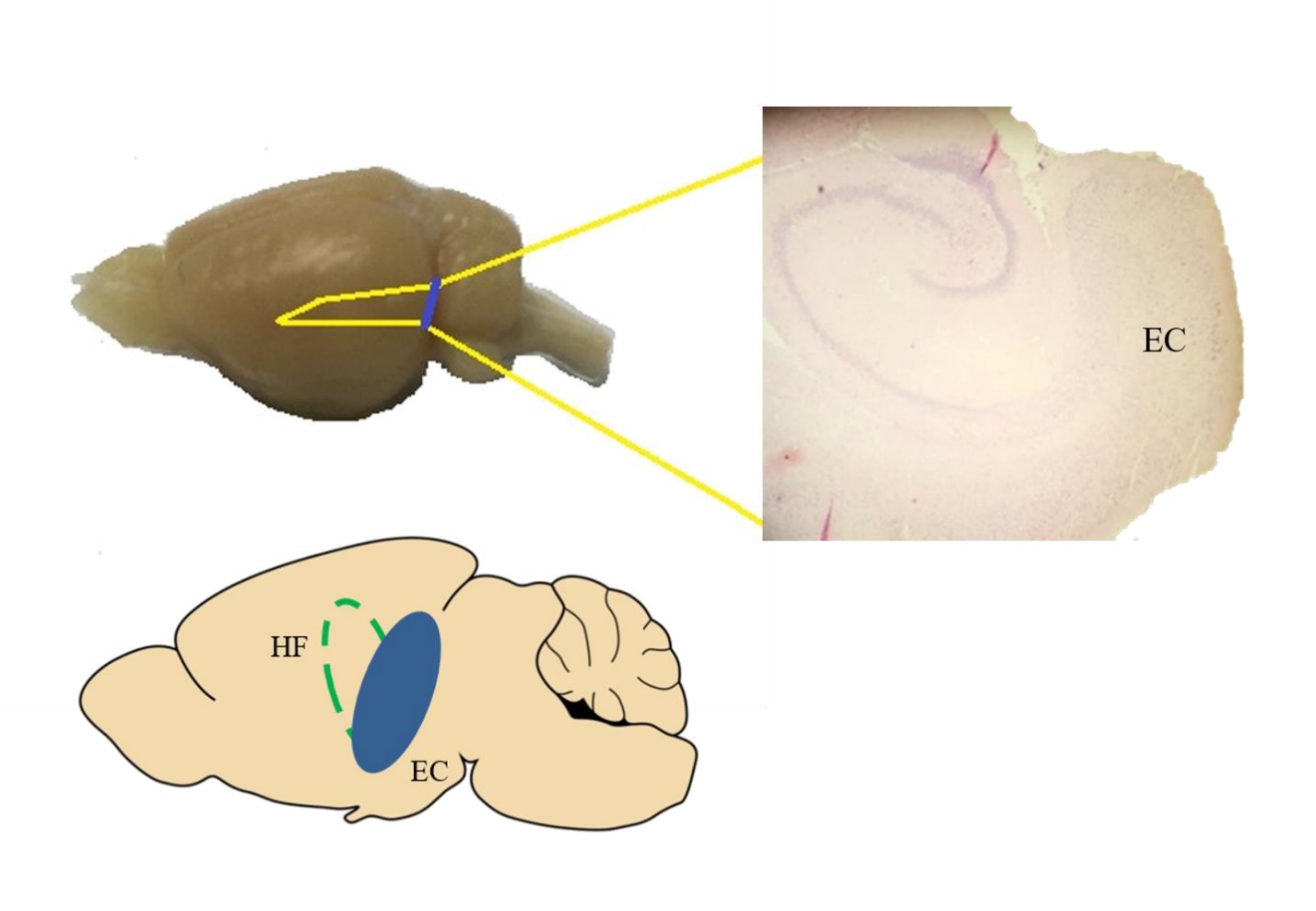 Figure 1.
Figure 1.A horizontal section containing the EC and hippocampal region. Brain slices (400 μm) from the region approximately between interaural 2.40 - 4.40 and Bregma -7.60 - -5.60 were harvested for the experiment.
The brain slices were placed into a recording chamber fixed with a grid and under perfusion of aCSF at 2 mL/min maintained by a peristaltic pump (ISMATEC IPC, Germany) and the bathing solution was constantly bubbled with carbogen (95% oxygen/5% carbon dioxide). The neurons were visualized and selected using an upright infrared-differential interference contrast (IR-DIC) research microscope (Nikon Eclipse E600FN) with a 10 × objective lens and 40 × water-immersion objective lens. The selected neurons had identifiable pyramidal cell morphology with prominent apical dendrite emerging from the soma (Lench et al., 2014; Medinilla et al., 2013). Images were projected to a monitor using the Retiga ELECTRO™ CCD camera (QImaging, Canada) mounted on the microscope. Electrophysiological recordings were performed at room temperature (22-24 °C) using Multiclamp 700B amplifier and pCLAMP software (Molecular Devices, Sunnyvale, CA) and digitized using Axon Digidata 1440 (Molecular Devices, Sunnyvale, USA). The glass pipettes were pulled from borosilicate glass (with filament, GB 150 EFT-10, Science Products, GmbH) using Flaming-Brown micropipette puller (model P-97, Sutter Instruments) in five-stages pulling and filled with Cs+-based intrapipette solution (in mM: Cs-gluconate, 100; NaCl, 8; MgCl2, 5; EGTA, 0.6; HEPES 40; Mg2+-ATP, 2; GTP, 0.3; pH 7.40). QX-314 (1 mM) was applied in the intrapipette solution. The pipette tips had resistance ranging from 5 to 8 MΩ. The cells were voltage-clamped at -70 mV, and PTX (50 µM) was applied to block GABAAR-mediated currents while D-AP5 (50 µM) was applied to block the NMDARs and isolate the AMPAR-mediated currents. Cell input resistance (300-500 MΩ) and series resistance (15-30 MΩ) were constantly monitored during the experiment, and cells that varied more than 20% were excluded from the analysis. Series resistance compensation was not employed to improve the signal-to-noise ratio. Signals were filtered at 2 kHz and sampled at 10 kHz under control of the pClamp 10 (Molecular Devices, Sunnyvale, USA) software program. The data were stored on disk for later offline analysis.
Data were analyzed using Clampfit 10 (Molecular Devices, Sunnyvale, USA). The template match threshold was set at 4 times for detection (de la Pena et al., 2012). For analysis, only current traces with identifiable EPSCs having a rise time shorter than decay time were chosen (Kanju et al., 2008). Mean amplitudes and frequencies for spontaneous events were analyzed and compared using statistical methods. All current responses were normalized to the control values and expressed as Mean (± SEM). Comparisons between groups are performed using Student’s t-test and one-way ANOVA. A P-value of < 0.05 is considered significant.
Baseline recording of spontaneous PSCs of brain slices submerged in aCSF was obtained from cells voltage-clamped at -70 mV (n = 6) to measure spontaneous PSCs properties which include amplitude and frequency (Fig. 2A and 2B). One-way ANOVA analysis revealed that there was no significant change to the amplitude (F[4, 25] = 0.3422, P > 0.05) and frequency (F[4, 25] = 0.0601, P > 0.05) of PSCs recorded at -70 mV across five epochs of one minute each. A separate set of experiments was carried out to evaluate the effects of acute application of 0.1% DMSO and PTX on the pyramidal neurons of EC. Baseline spontaneous postsynaptic currents were recorded at -70 mV under perfusion of aCSF, followed by acute application of 0.1% DMSO into the perfusing aCSF (Fig. 2C and 2D). Application of 0.1% DMSO produced no significant change to all measured parameters of spontaneous postsynaptic currents of cells voltage-clamped at -70 mV (amplitude (t[10] = 0.4465, P > 0.05, 95 % CI = -0.1228 to 0.0818) and frequency (t[10] = 0.3196, P > 0.05, 95 % CI = -0.7409 to 0.5550)), indicating that all effects observed during treatment with CA extract was attributable to the treatment and it was not an effect of DMSO. On a separate set of experiments following the same protocol as previous, acute application of 50 µM PTX (Fig. 2E and 2F) did not affect all measured parameters of postsynaptic currents of cells voltage-clamped at -70 mV (amplitude (t[10] = 1.425, P > 0.05, 95 % CI = -0.0479 to 0.2178) and frequency (t[10] = 0.1681, P > 0.05, 95 % CI = -0.7918 to 0.6807)), indicating that majority of the synaptic responses of this region are glutamatergic in nature (Medinilla et al., 2013).
 Figure 2.
Figure 2.Baseline recording and verifying effects of 0.1 % DMSO and PTX on spontaneous postsynaptic currents. Recording of spontaneous postsynaptic currents at the EC produced rather stable responses (A and B). 0.1 % DMSO did not cause significant changes to the amplitude and frequency of the sEPSCs (C and D). Acute application of PTX produced no significant changes to the parameters, indicating mainly excitatory transmission at the target region (E and F).
Analysis from Student’s t-test showed that application of CA100 significantly increased the amplitude by 12.48 % (t[10] = 4.699, P = 0.0008, 95 % CI = 0.0656 to 0.1839) while no significant changes to the frequency (t[10] = 1.527, P = 0.1577, 95 % CI = -0.5924 to 0.1106) of AMPAR-mediated sEPSCs was found when compared to blocker control. The amplitude (t[10] = 0.8975, P = 0.3906, 95 % CI = -0.1578 to 0.0672) and frequency (t[10] = 1.944, P = 0.0805, 95 % CI = -0.7873 to 0.0535) did not differ significantly with the acute application of CA300 when compared to blocker control. Similarly, CA600 did not significantly alter the amplitude (t[10] = 0.5819, P = 0.5735, 95 % CI = -0.1701 to 0.0997) and frequency (t[10] = 0.7343, P = 0.4796, 95 % CI = -0.6810 to 0.3434) of sEPSCs (Fig. 3).
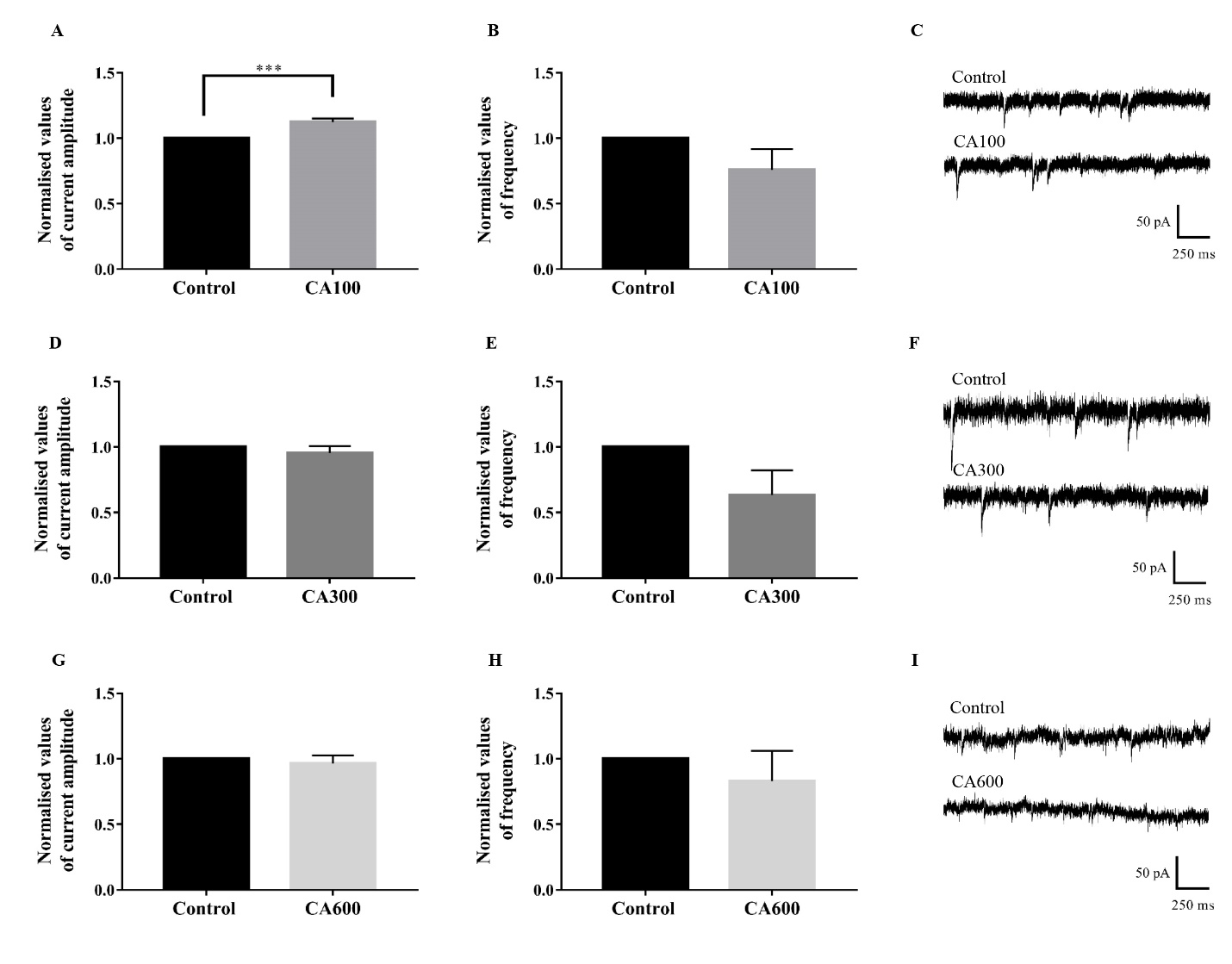 Figure 3.
Figure 3.Effects of CA extract on AMPAR-mediated sEPSCs. The AMPAR-mediated currents were isolated with PTX and D-AP5 and recorded as control. At a low dose, acute application of CA100 extract increased the amplitude (A) of sEPSCs but had no significant effect on the frequency (B). Trace example (C) of control and CA100 were shown (Student’s t-test, n = 6). Acute application of CA300 (D-F) and CA600 (G-I) did not produce significant changes to the amplitude and frequency of the AMPAR-mediated currents.
Further experiments were performed using AA. Acute application of AA (0.004 µM) significantly increased the amplitude by 9.4 % (t[8] = 2.615, P = 0.0309, 95 % CI = 0.0112 to 0.1775) while showing no significant change to the frequency (t[8] = 0.4567, P = 0.66, 95 % CI = -0.5375 to 0.8029). Analysis also showed that application of AA at 0.012 µM did not significantly alter the amplitude (t[8] = 1.131, P = 0.2908, 95 % CI = -0.0184 to 0.0538) and frequency (t[8] = 0.4984, P = 0.6316, 95 % CI = -0.4092 to 0.6349). Similar trend was observed with the application of AA at 0.024 µM, producing no significant effects on the amplitude (t[8] = 0.3331, P = 0.7476, 95 % CI = -0.0598 to 0.0447) and frequency (t[8] = 0.5495, P= 0.5977, 95 % CI = -0.3073 to 0.4995) of sEPSCs (Fig. 4).
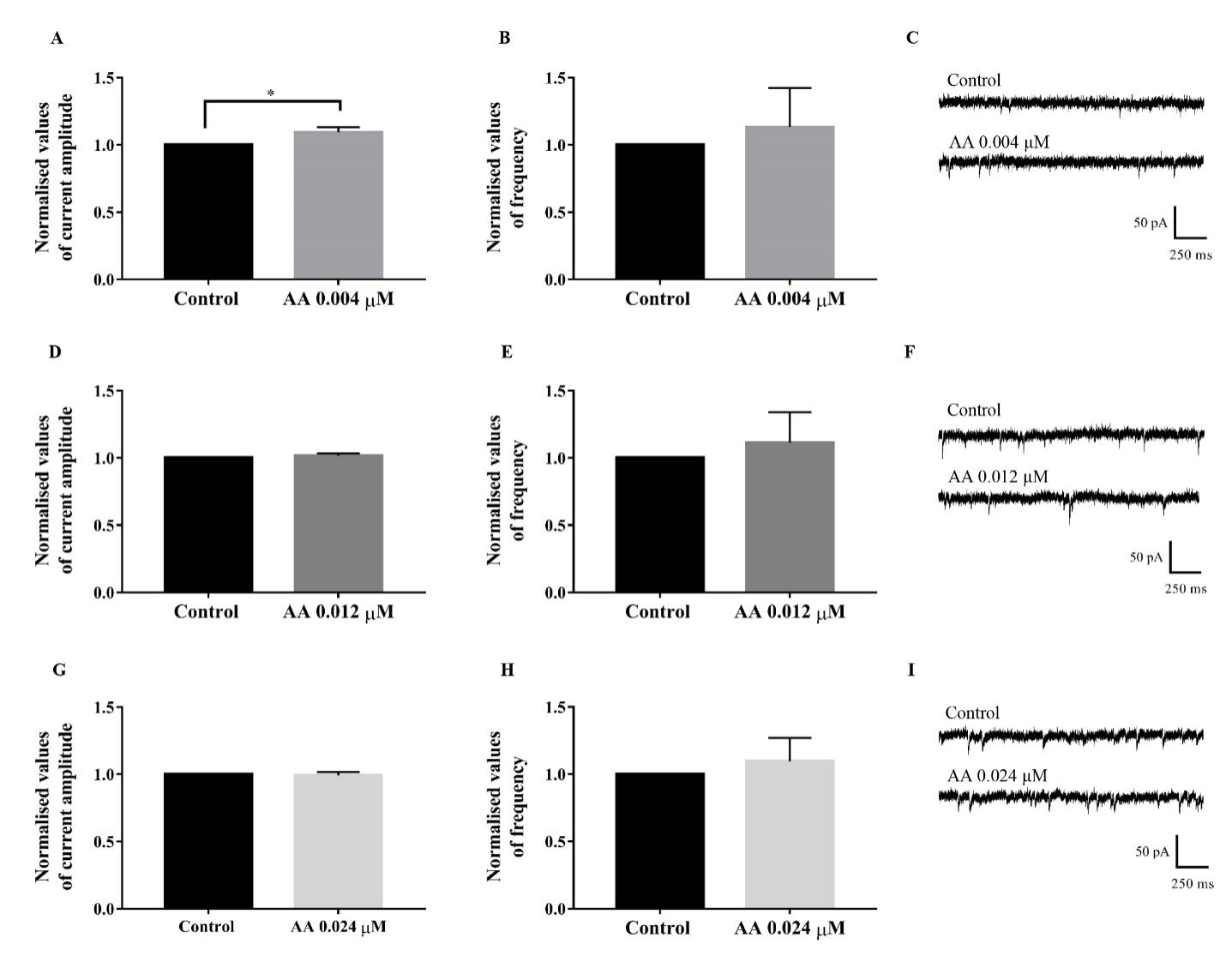 Figure 4.
Figure 4.Effects of AA on AMPAR-mediated sEPSCs. The AMPAR-mediated currents were isolated with PTX and D-AP5 and recorded as control. AA 0.004 µM significantly increased the amplitude (A) of sEPSCs while having no significant effect on the frequency (B). Trace example (C) of control and AA 0.004 µM were shown (Student’s t-test, n = 5). There were no significant changes to the amplitude and frequency of the AMPAR-mediated currents in the acute application of AA 0.012 µM (D-F) and AA 0.024 µM (G-I).
Acute application of AS (0.004 µM) significantly increased the amplitude by 12.5 % (t[6] = 4.590, P = 0.0037, 95 % CI = 0.0584 to 0.1916) while showing no significant change to the frequency (t[6] = 0.1689, P = 0.8714, 95 % CI = -0.5288 to 0.6072) (Fig. 5). Application of AS at 0.012 µM did not significantly alter the amplitude (t[6] = 0.883, P = 0.4112, 95 % CI = -0.3057 to 0.6509) and frequency (t[6] = 1.035, P = 0.3404, 95 % CI = -0.9099 to 0.3688). Similarly, acute application of AS at 0.024 µM did not significantly alter the amplitude (t[6] = 1.885, P = 0.1084, 95 % CI = -0.1625 to 0.0211) and frequency (t[6] = 0.2894, P = 0.782, 95 % CI = -0.9089 to 0.7167) of sEPSCs.
 Figure 5.
Figure 5.Effects of AS on AMPAR-mediated sEPSCs. The AMPAR-mediated currents were isolated with PTX and D-AP5 and recorded as control. AS significantly increased the amplitude (A) of sEPSCs only at a low dose while had no significant effect on the frequency (B). Trace example (C) of control and AS 0.004 µM were shown (Student’s t-test, n = 4). There were no significant changes to the amplitude and frequency of the AMPAR-mediated currents in the acute application of AS 0.012 µM (D-F) and AS 0.024 µM (G-I).
The EC is a crucial region within the medial temporal lobe system interacting with hippocampal regions through glutamatergic inputs with extensive synaptic transmission and plasticity properties. It acts as a processor of information projecting to and from the hippocampus, reflecting its essential role in learning and memory and various cognitive processes such as spatial cognition, representation and navigation, attention, and conditioning (Coutureau and Di Scala, 2009; Igarashi et al., 2014; Lipton and Eichenbaum, 2008; Squire and Zola-Morgan, 1991; Witter and Moser, 2006). Besides extending direct projections with the cortical regions, the EC maintains reciprocal projections with the hippocampal regions and is a major source of projections to the hippocampus (Medinilla et al., 2013; Sasaki et al., 2015). The enhanced efficacy of the neuronal networks within the medial temporal lobe system is thought to improve cognitive functions. Furthermore, many pathological conditions were shown to be attributable to dysfunctional EC and EC-hippocampal network, highlighting the important role of EC in cognition and the potential effects of a strengthened synaptic transmission on the neural network (Booth et al., 2016; Scharfman and Chao, 2013). Several studies have put forward evidence for the potential therapeutic effects of Centella asiatica (CA) in modulating cognitive function. CA has been widely used as medicinal herbs by the local communities as well as the Ayurvedic and Traditional Chinese Medicine to treat illnesses and maintain general good health (Gohil et al., 2010; Gray et al., 2018a; Lokanathan et al., 2016). Various studies showed that CA and its phytochemicals display numerous biological activities such as enhancing cognitive functions, promoting synaptogenesis and cell regeneration in the hippocampus, as well as conferring neurogenic and neuroprotective properties (Gadahad et al., 2008; Gray et al., 2016; Kumar et al., 2009, 2011; Soumyanath et al., 2012). Our previous study showed that oral administration of CA extract was able to enhance spatial and non-spatial learning and memory in rats and significantly enhanced surface expression of AMPAR subunits GluA1 and GluA2 in the CA1, CA3 and EC (Wong et al., 2019), suggesting the potential link between enhanced glutamatergic excitatory transmission and cognitive enhancement mediated by CA.
Essential phytocompounds of CA generally have low oral bioavailability, and administration of CA extract in rat models produced more prolonged exposure of its phytocompounds compared with separate administration of the individual compounds (Hengjumrut et al., 2018; Khemawoot et al., 2018). The low oral bioavailability of bioactive components in natural products is not an unusual phenomenon due to factors such as poor solubility, slow absorption, breakdown and dissolution within the gastrointestinal tract, and gastrointestinal transit time variations of the biocompounds (Anukunwithaya et al., 2017a,b; Yuan et al., 2015). In the current study, CA100 was the lowest dose of extract found to be effective in modulating AMPAR-mediated sEPSCs. Hence, for a follow-up study of the effects of AA and AS, equivalent doses were estimated from the minimal effective dose of CA extract at CA100. With the limited information on the bioavailability of these pure compounds of CA and a maximum oral bioavailability at 16.25% (Yuan et al., 2015), a low dose of approximately ¼ of the equivalent dose was selected for both AA and AS for standardization of method.
Interestingly, the first study assessing the effects of CA extract through electrophysiology recording was recently published, and CA extract was found to have a half-maximal effective concentration of 0.25 µg/ml, which was ¼ of the maximum effective dose used in the study (Wanasuntronwong et al., 2018). This reflects the relevance of the application of low dose AA and AS at ¼ of the equivalent dose of CA100. In vitro studies on neuroprotective effects of AA and AS revealed that these compounds have an effective dose ranging from 0.01 to 100 nM (Sun et al., 2015; Xiong et al., 2009; Xu et al., 2012) but the positive effects on cells were reduced with higher concentrations of compounds (Xiong et al., 2009; Xu et al., 2012). Different low-dose and high-dose effects of compounds have been observed in ligand-gated ion channels (Hinton et al., 2017; Mihic et al., 1994; Valle-Mojica et al., 2011) and despite the relatively low concentration used in the current study, AA and AS exerted significant effects on AMPAR-mediated sEPSCs. AMPARs are a subtype of iGluRs that play a crucial role in mediating fast excitatory neurotransmission in the CNS. Changes to the numbers and properties of AMPARs at the postsynaptic membrane are developmentally and activity regulated. These changes are essential for synaptic plasticity, formation, and stabilization of excitatory synapses, and formation of the neural circuit (Henley and Wilkinson, 2016; Herring and Nicoll, 2016). CA extract, AA, and AS increased the amplitude of AMPAR-mediated sEPSCs at their respective low dose without having significant changes to the frequency of the current response. This indicated a postsynaptic effect on AMPAR-mediated currents with postsynaptic alterations in transmitter-receptor interactions (Luo et al., 2014; Zhaowei et al., 2014) that led to an increase of synaptic strength between presynaptic and postsynaptic neurons. An increase of synaptic strength with the postsynaptic effect is potentially due to higher expression of AMPARs at the postsynaptic membrane, and this increase of synaptic strength has a role in cognitive enhancement (Barre et al., 2016; Morita et al., 2014).
Our previous study showed that 14-days oral administration of CA extract enhanced the surface expression of GluA1 and GluA2 subunits in the EC, supporting the notion that the higher number of functional receptors at synapses leads to stronger synaptic strength as reflected from the increase of AMPAR-mediated sEPSCs amplitude (Binti Mohd Yusuf Yeo et al., 2018; Wong et al., 2019). Synaptic transmission is strengthened with the rapid trafficking of AMPARs to the postsynaptic density, with the majority of the synaptic AMPARs being GluA1-A2 heteromers (Henley and Wilkinson, 2016; Sachser et al., 2017; Traynelis et al., 2010). The extrasynaptic pool of AMPARs are highly mobile and readily exchanged between synaptic and extrasynaptic membranes through lateral diffusion. LTP induction involves rapid incorporation of calcium permeable-AMPARs allowing the influx of Ca2+ that leads to transient increase of conductance and is subsequently replaced by calcium impermeable-AMPARs (Hanley, 2014; Jaafari et al., 2012). The dynamic recycling and trafficking of AMPARs are regulated through posttranslational modification of the receptor subunits and require interactions with accessory and auxiliary proteins through signaling cascades that involve kinases and phosphatases. Phenolic compounds are secondary metabolites produced by plants and are widely distributed in all foods of plant origin, including CA. These compounds interact with kinases to modulate signal transduction pathways that regulate cell survival and gene expression, activate pathways in LTP, enhance cognitive functions and confer neuroprotective effects (Kyselova, 2011; Rendeiro et al., 2012; Spencer, 2007; Vauzour et al., 2008). Phosphorylation of receptor subunits at multiple sites by several kinases can produce different effects on interactions with accessory and auxiliary proteins at the synapses and affecting channel conductance (Hussain et al., 2015; Shepherd and Huganir, 2007).
Previous in vitro study using SH-SY5Y human neuroblastoma cells showed that CA extract significantly increased the neurite outgrowth and elongation (Xu et al., 2012). This observation was also found in cells treated with AA, and it was shown to be ERK-dependent (Soumyanath et al., 2005). Neurite elongation properties of CA were mediated by different signaling pathways induced by AA and AS (Nalinratana et al., 2018). The triterpenoids were found to promote neurite outgrowth via activation of ERK and CREB, and they showed distinct preference on signaling pathways of neurite outgrowth. AA was found to confer neuroprotective effects on dopaminergic neurons of chronic Parkinson’s disease mouse model by increasing phosphorylation of PI3K, AKt, mTOR, and GSK-3β that leads to activation of signaling pathways (Nataraj et al., 2017). AA appeared to exert its effects synergistically with other triterpenoids to increase neuronal differentiation through the activation of MEK signaling pathway (Jiang et al., 2016; Lin et al., 2017) and the MEK/ERK signaling pathway has been indicated to be important in cognitive functions mediated by glutamatergic receptors (Jiang et al., 2015; Ramis et al., 2013; Spencer, 2007). With the multiple potential mechanisms of action and interaction with various molecular components, CA extract and the pure compounds AA and AS may have acted directly or indirectly on these signaling pathways, which led to an increase of the current amplitude of AMPAR-mediated sEPSCs.
It is interesting to note that CA extract and its phytochemicals were only effective towards AMPAR-mediated current responses at their respective low dose, suggesting potential synergistic and/or antagonistic effects of individual or several components in the extract. Plant compounds have the potential of exerting dual mode of action, and it can be influenced by an effective range of concentration for the compounds tested (Kong et al., 2000; Spencer et al., 2003; Xiong et al., 2009; Xu et al., 2012). The observations from the current study suggested that there exists an effective window whereby the components act together to produce desired effects of which beyond that window, the interaction of the components nullify or mask the desired effects (Li and Zhang, 2008; Yang et al., 2014). It was proposed that an optimal drug combination of components in CA could be an analog of nerve growth factor due to higher potency compared to a single component, further indicating the complexity of interactions between the effective components in CA (Lin et al., 2017; Long et al., 2015).
This study revealed the postsynaptic effects of the acute application of CA extract and its pure components on AMPAR-mediated responses in rat brain slices, potentially via the increase of functional AMPARs at the postsynaptic density. Based on the present study, further research such as LTP studies of animals treated with CA extract and pure compounds are warranted to provide strong support from the aspect of cellular mechanism for the enhancement effects of CA on learning and memory. The translation of results between in vivo and in vitro study of CA extract remains the most significant challenge and the limitation of the study. Acute application of CA extract may produce different effects on receptor responses and surface expression due to immediate contact between effective components and receptors. For consecutive oral administration, components in the CA extract are subjected to further physiological processes in the body before the affective component(s) reaches the CNS and crosses the blood-brain-barrier to exert effects (Banks, 2016; Tajes et al., 2014; Warren, 2018). This will require further studies on the pharmacokinetics of CA extract and transport of effective components in CA extract across the blood-brain-barrier to establish a more targeted dose of CA extract and pure compounds. Molecular docking studies would be useful to establish binding feasibility of these molecules to produce synergistic and/or antagonistic effects.
In conclusion, the present study demonstrated that CA extract, AA, and AS could act directly on the EC to modulate AMPAR-mediated sEPSCs at the postsynaptic level, in line with findings supporting the potential role of CA in cognitive enhancement modulated through increased AMPAR-mediated neurotransmission.
aCSF: Artificial cerebrospinal fluid; AMPA: α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid; AMPARs: α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors; AA: Asiatic acid; AS: Asiaticoside; CA: Centella asiatica; EC: Entorhinal cortex; iGluRs: Ionotropic glutamate receptors; PSCs: Postsynaptic currents; sEPSCs: Spontaneous excitatory postsynaptic currents.
The protocol was in accordance with the internationally accepted principles for laboratory animal use and care and has been approved by the Animal Research and Ethics Committee, USM with the animal ethics approval code [USM/Animal Ethics Approval/2015/(98)(699)].
We would like to extend our appreciation to Dr. Muthuraju Sangu and Ms. Nor Aqilah Binti Mohd Yusuf Yeo for their work in identifying the effects of CA extract on learning and memory and AMPAR GluA1 of rats as part of the research efforts in the Department of Neurosciences, USM; Prof. Dr. Mohd Ilham Adenan from UiTM for providing the CA powdered extract; Prof. Dr. Nor Hadiani Ismail and Ms. Siti Sarwana Binti Husin for their work performed in Herbal Quality Analysis Laboratory of AuRIns (UiTM) to identify and quantify the marker compounds in the CA extract used for this study. This work was supported by NKEA Research Grant Scheme (NRGS Grant, NH1014D049) from the Herbal Development Office (HDO) of the Ministry of Agriculture and Agro-based Industry, Malaysia, awarded to Prof. Dr. Tengku Sifzizul Tengku Muhammad and his team in collaborative work between Universiti Malaysia Terengganu, Universiti Sains Malaysia and Universiti Teknologi MARA (Grant no: 304/CNEURO/6150137/K123).
The authors state no conflict of interest.
