†These authors contributed equally.
Cerebral ischemia-reperfusion injury is a common complication that occurs during stroke treatment. Increasingly, microRNAs have been found to participate in the modulation of neuron function; however, the role of microRNAs in cerebral ischemia-reperfusion injury remains unclear. We developed a mechanism of cerebral ischemia-reperfusion injury using a cellular model of oxygen-glucose deprivation and reoxygenation-induced injury in human neuroblastoma SH-SY5Y cells. We found that treatment of oxygen-glucose deprivation and reoxygenation promoted the apoptosis of SH-SY5Y cells. Analysis of microRNAs sequencing revealed that the expression of microRNA-27a-5p was induced, and microRNA-29b-3p expression was inhibited in neuroblastoma cells exposed to oxygen-glucose deprivation and reoxygenation. Either inhibition of microRNA-27a-5p or overexpression of microRNA-29b-3p mitigated oxygen-glucose deprivation and reoxygenation-induced cellular apoptosis. Bach1 was authenticated as a target gene of microRNA-27a-5p. Also, microRNA-27a-5p mediated the expression of Bach 1 along with its downstream signaling. N-hydroxy-N'-(4-butyl-2-methylphenyl)-formamidine protected against oxygen-glucose deprivation and reoxygenation-induced apoptosis while decreasing miR-27a-5p expression and increasing microRNA-29b-3p expression. These results suggested that microRNA-27a-5p and microRNA-29b-3p may contribute to oxygen-glucose deprivation and reoxygenation-induced cellular injury. At the same time, N-hydroxy-N'-(4-butyl-2-methylphenyl)-formamidine protects SH-SY5Y cells against oxygen-glucose deprivation and reoxygenation-induced injury partly through the inhibition of microRNA-27-a-5p and promotion of the Bach1/HO-1 signaling pathway.
Stroke is a disease characterized by ischemic and hemorrhagic brain injury. It is the second leading cause of death for people over 60-years of age in the world and the fifth leading cause of death and disability in people aged 15 to 59 (Asakawa et al., 2017; Donnan et al., 2008; Johnston et al., 2009; Sun et al., 2013). Approximately 80% of the strokes are ischemic (Feigin et al., 2003). Recombinant tissue plasminogen activator (rtPA) is recommended for the dissolution of the occluding thrombus and can be used to relieve the ischemic symptoms in patients (Xu et al., 2013). Although this treatment allows for a particular improvement in nerve function, it can also cause more severe brain damage and dysfunction related to ischemia/reperfusion injury (Wechsler, 2011; Yellon and Hausenloy, 2007). A series of complex cellular and biochemical, molecular mechanisms are involved in ischemia/reperfusion, such as calcium overload, inflammation, apoptosis, and production of glutamate, consequently leading to brain tissue and neuronal damage (Deb et al., 2010; Wang et al., 2015). In general, the pathogenesis is poorly understood, and there is still a lack of effective treatment for ischemic stroke. Therefore, elucidation of the mechanisms underlying ischemia/reperfusion (I/R) may provide a novel therapeutic strategy for cerebral ischemia-reperfusion injury (IRI).
MicroRNAs (miRNAs) are a series of evolutionarily conserved small non-coding single-stranded RNAs with about 21-23 nucleotides that mediate gene expression of the posttranscriptional level by targeting 3’-UTR of the mRNA, thus triggering degradation or translational repression of target mRNAs (Bartel, 2004). The miRNAs could function as a crucial modulator in various pathological and physiological processes such as metabolism, inflammation, differentiation, and cell invasion (Konopka et al., 2010; Mendell and Olson, 2012; Rajasethupathy et al., 2009). An increasing number of studies have reported that cerebral ischemic injury is usually accompanied by the alternative expression of miRNA in ischemic brain tissue (Dharap et al., 2009; Rink and Khanna, 2011). Thus, miRNA has become a novel therapeutic target in IRI treatment (Pei et al., 2016; Zeng et al., 2016). However, the mechanisms of miRNAs in IRI still needs further investigation.
Toth et al. (2013) found that 20-Hydroxyeicosatetraenoic acid (20-HETE) intensely activated reactive oxygen species (ROS) and NF-κB signaling in cerebral microvascular endothelial cells, which indicated that inhibition of 20-HETE maybe prevents and protect against brain IRI. 20-Hydroxyeicosatetraenoic acid (20-HETE) is the product of arachidonic acid (AA) catalyzed by cytochrome P450 ω hydroxylase. It exerts a critical physiological and pathological function in modulating cellular proliferation, metabolism, and inflammation (Omura et al., 2006; Zhu et al., 2015).
Studies in cell culture and global cerebral ischemia animal showed that 20-HETE promoted apoptosis and necrosis of neuronal cells by suppressing hypoxia-induced ERK1/2 phosphorylation (Yang et al., 2012). Our previous research revealed that N-hydroxy-N'-(4-butyl-2-methylphenyl)-formamidine (HET0016), which is an inhibitor of 20-HETE, exhibits neuroprotective effects on the dysfunction of cerebral edema and the blood-brain barrier after cerebral I/R through the c-Jun N-terminal kinase (JNK) pathway (Liu et al., 2014). However, the above work had focused on the neuroprotective aspects of HET0016 against acute cerebral IRI, and the biological roles of HET0016 in hypoxia and ischemia remained poorly understood.
Here, we hypothesize that miRNA participates in ischemia/reperfusion-induced injury, and HET0016 could protect against the injury via these miRNAs. To investigate this hypothesis, oxygen-glucose deprivation, and reoxygenation (OGD/R) cellular model is employed to simulate clinical ischemia-reperfusion injury. Also, analysis of miRNA sequencing and several biological assays are carried out to illuminate the effect and mechanism of miRNAs involved and their role in HET0016 protection in ischemia and reperfusion.
The SH-SY5Y human neuroblastoma cell line obtained from American type culture collection (ATCC) was employed in the present study. SH-SY5Y cells were cultured in high-glucose dulbecco’s modified eagle medium (DMEM) (HyClone; UT, USA) supplemented with 10% FBS (Pan Biotech, Germany) in a 5% CO2 atmosphere at 37 °C.
Cells in the logarithmic phase were seeded at the density of 1 × 104 cells per well in a 96-wells culture plate, cultured for 24 hours to approximately 60-70% confluence, and then subjected to OGD/R or HET0016 with the concentrations of 0.1, 0.5, 1, 2, 5, 10, 20 μM). After treatment, the cells were administrated with 5mg/mL thiazolyl blue tetrazolium bromide (MTT, Solarbio, Beijing, P. R. China) for 3 hours. Production of formazan crystals from viable cells was dissolved using 100 μL of dimethyl sulfoxide (DMSO) per well, and optical density (OD) values were detected at 490 nm by a microplate reader (Peiqing, Shanghai, P. R. China). Each time point was repeated in four wells, and the experiment was independently performed three times. Proliferation rate was calculated following as:
Proliferation rate = (ODtreated group-ODblank)/(ODuntreated group-ODblank) × 100%.
SH-SY5Y cells were maintained in glucose-free DMEM medium and hypoxic atmosphere (1% O2, 94% N2, 5% CO2) in an anaerobic cell incubator at 37 °C for 6 hours. And then, the medium was replaced with DMEM containing glucose, and the cells were cultured for another 0-24 hours of reoxygenation under reasonable condition (95% air, 5% CO2) to perform OGD/R (Liu et al., 2019). SH-SY5Y cells cultured under normal conditions served as a control.
Total RNA was extracted from SH-SY5Y neuroblastoma cells treated with or without OGD/R. The RNA quality was tested as follows: the purity was determined using agarose gel electrophoresis and a nanodrop microspectrophotometer (OD value of 260/280), the concentration was precisely quantified using a Qubit 2.0 fluorophotometer, and the integrity of RNA was evaluated via an Agilent Bioanalyzer 2100. RNA samples of high quality were used to construct a miRNA library using a small RNA sample pre kit, with which the RNA bi-ends were connected with linkers, reverse transcribed into cDNA, and then target cDNA fragment library were obtained via PCR amplification, PAGE electrophoresis, and recovery. The constructed cDNA library was diluted to the concentration of 1 ng/μL and further tested for quality, insert size, and quantity. The library was pooled according to the expectant data size and subsequently sequenced based on Illumina HiSeq 2500 sequencing platform (Illumina, USA).
FastQC was employed for quality control of raw data from Solexa sequencing to remove linkers, as well as low-quality reads (quality value < 20) and unknown bases. The obtained tag (the clean small RNA reads) were screened and classified based on the length of the reads (16-35 bp). Four databases, including mirbase, ensemble, repeatmakser, and mirdeep2 were used to annotate the miRNA. After all miRNA expression levels were calculated and normalized with TPM, DEseq software was employed to analyze the differentially-expressed miRNA for each reoxygenation time’s point, and the two parameters of fold change (log2fold change > 1) and P-value (P < 0.05) were used to evaluate the differences. Miranda software was employed to predict the target genes of differentially expressed miRNAs, and cluster-profiler was used to perform GO and KEGG cluster analysis for the target gene and its up- and down-stream pathways.
Cells exposed to OGD/R with or without HET0016 were harvested, total RNA was extracted, reverse transcribed into cDNA, and then subjected to real-time PCR analysis. Primer for miR-27a-5p, miR-1247-3p, miR-1-3p, miR-1275 and miR-29b-3p were synthesized by GenePharma (Shanghai, P. R. China). Real-time PCR was performed as previously described (He et al., 2016). Briefly, total RNA was extracted using Trizol (Invitrogen, USA), cDNA was generated using a reverse transcription kit (Tiangen, P. R. China), and tested using a real-time PCR amplifier (Tianlong, P. R. China). The relative RNA amounts were calculated using the “ΔΔCt method” (Schmittgen et al., 2008) and normalized with internal control of β-actin.
Cells were harvested and lysed in radio immunoprecipitation assay (RIPA) buffer. Then, cell lysates were cleared by centrifugation, and a BCA assay (Pierce, Rockford, IL, USA) was used to determine protein concentration. The 20 μg of total protein were loaded and separated by SDS-PAGE and transferred onto a PVDF membrane (Millipore, USA). The membranes were blocked with 10% fat-free milk in PBS for 1 h and incubated overnight at 4 ℃ with the following primary antibodies: GAPDH, p-AKT (Santa Cruz Biotechnology, Santa Cruz, CA, USA), Cleaved PARP, Bach1, HO-1(Cell Signaling Technology, USA). After washing three times in TBST, membranes were incubated with a peroxidase-conjugated secondary antibody for 1 h at 37 °C. Blots were visualized using the enhanced chemiluminescence (ECL) method (Hu et al., 2017).
Cells exposed to OGD/R with or without HET0016 were dissociated using trypsin lysis buffer (0.25 g trypsin in 100 mL PBS), harvested, and diluted to a concentration of 5 × 105/mL in a 2mL EP tube. The harvested cells were washed twice with precooled PBS. The cells were resuspended in 200 μL binding buffer, then 10 μL of FITC-Annexin V and 10 μL of PI were added and incubated for 15 min in the dark at room temperature, and 300 μL binding buffer was further added to resuspend and dilute the cells. The stained cells were finally detected and analyzed using a flow cytometer.
Cells exposed to OGD/R with or without HET0016 were dissociated using trypsin lysis buffer (0.25 g trypsin in 100 mL PBS), harvested, and washed twice with precooled PBS. Lysis buffer was added to cells at a ratio of 100 μL per 2 million cells and lysed for 15 minutes on ice. Lysates were added to the solutions containing substrates of DEVD-pNA, IETD-pNA, or LEHD-pNA, respectively related to caspase-3, -8, or -9, and incubated for 60-120 minutes according to caspase colorimetric assay kit (Biovision, San Francisco, USA). OD values at 405 nm were detected using a microplate reader (Peiqing, Shanghai, P. R. China).
HEK293T cells were seeded in 24-wells culture plates and cultured to 60-70% cell confluence. Constructed plasmids consisted of Bach1 3’-UTR cDNA fragment and pmirGLO dual-luciferase vector was cotransfected with miR-27a-5p mimics or miR-29b-3p into the seeded cells using Lipofectamine 2000 transfection reagent (Invitrogen, MD, USA). The transfected cells were lysed using 1 × Passive Lysis Buffer from the Dual-Luciferase Reporter Assay Kit (Promega, WI, USA), and added to the luciferase substrate. The fluorescence intensities were detected using a multifunctional microplate reader (victor 3V, PerkinElmer, USA), and the transcriptional activity of miR-27a-5p mimics or miR-29b-3p were analyzed as previously described (Schmittgen et al., 2008).
HEK293T and SH-SY5Y cells were respectively seeded in 24-wells culture plates and cultured to 60-70% cell confluence. MiRNA mimics or inhibitor were transfected into the seeded cells using Lipofectamine 2000 transfection reagent (Invitrogen, MD, USA). The transfected cells were harvested, and total RNA was extracted. Real-time PCR was employed to determine the miRNA expression level.
The data are presented as the mean ± standard deviation (SD) from three independent experiments. ANOVA with Tukey’s post-test was used to compare the differences between the two groups using SPSS software (V17.00, IBM, IL, USA).
Fig. 1 shows that the subjection of cells to OGD for 6 hours initiates the inhibition of cell proliferation compared to 6 hours group in the untreated curve (Fig. 1A). Meanwhile, reoxygenation for 24 hours after OGD for 6 hours caused a more obvious cell proliferation inhibition (Fig. 1B). These inhibitions indicate that neurocyte injury could be induced by OGD/R. Furthermore, flow cytometry analysis of FITC-annexin and PI dual-stains showed that the apoptosis percentage in SH-SY5Y cells exposed to OGD for 6 hours and reoxygenation for 24 hours increased from 9.5% to 32% versus the untreated group (Fig. 1C and 1D). The caspase activity test related to the apoptosis pathway showed that the activity of caspase-3, caspase-8, and caspase-9 increased compared to the untreated group (Fig. 1E). The results of western blot analysis showed that after OGD for 6 hours, cleaved-PARP protein increased as the reoxygenation time increased (Fig. 1F). These results indicate that OGD/R treatment promoted the apoptosis of SH-SY5Y cells. Therefore, the OGD/R cellular model was successfully established.
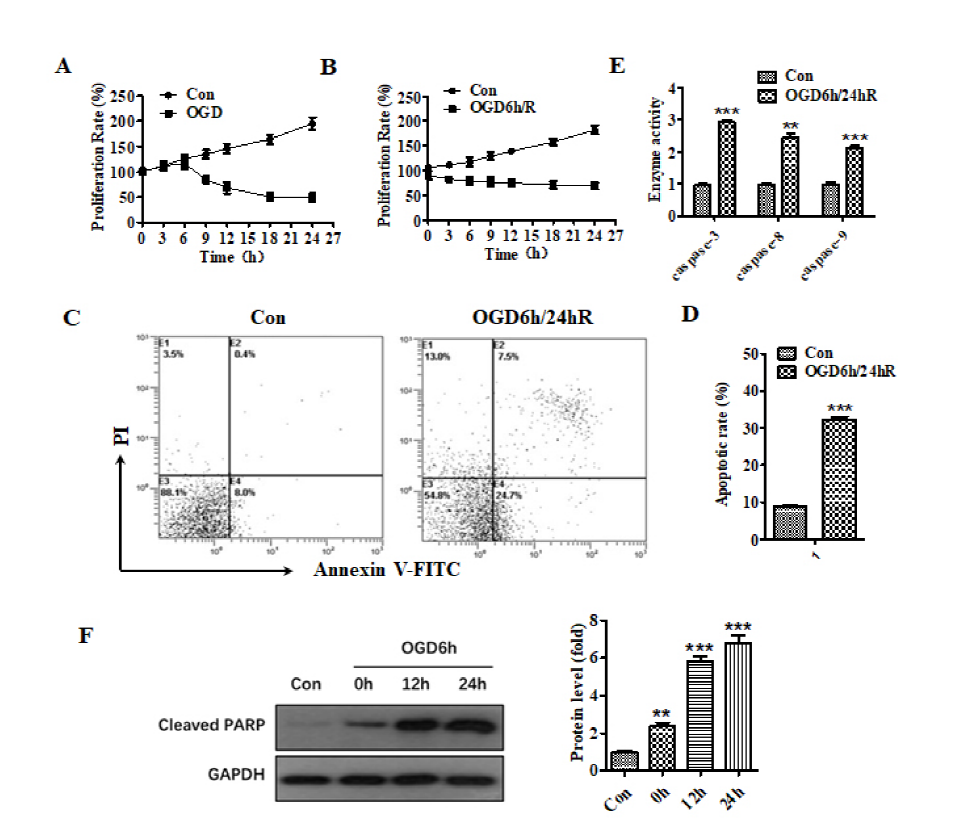 Figure 1.
Figure 1.The treatment of OGD/R promoted the apoptosis of SH-SY5Y cells. (A) Effect of different hypoxia time on the proliferation of SH-SY5Y cells. (B) Effect of different reoxygenation time on the proliferation of SH-SY5Y cells after OGD for 6 h. (C) Flow cytometry analysis of apoptosis after OGD/R treatment. (D) Histogram representation of apoptosis after OGD/R treatment. (E) The activity of caspase-3, caspase-8, and caspase-9 was increased after OGD/R treatment. (F) The expression of cleaved-PARP at different times after OGD for 6 h. ** means P < 0.01, *** means P < 0.001
The miRNA expression profiles were detected using a miRNA-seq and that there were significant differences in miRNA of OGD/R treated versus untreated cells (Fig. 2A). Results also revealed that OGD/R caused changes in the physiological regulation of cells, as well as changes in miRNA-related signaling pathways. Further, differential miRNA expression was screened by DESeq (Anders and Huber, 2010) software analysis. As seen in Fig. 2B, there were significant changes in the miRNAs of cells treated with OGD for 6 hours and reoxygenated for 24 hours, compared with the untreated control group, among which expression of hsa-miR-27a-5p, hsa-miR-1247-3p, hsa-miR-1-3p and hsa-miR-1275 were increased, and hsa-miR-29b-3p expression was decreased. We performed qPCR to verify the screened miRNAs. As shown in Fig. 2C, the expression of miRNA-27a-5p significantly increased (P < 0.01), and the expression of miRNA-29b-3p decreased (P < 0.05) after OGD/R, which confirmed the data from miRNA-seq.
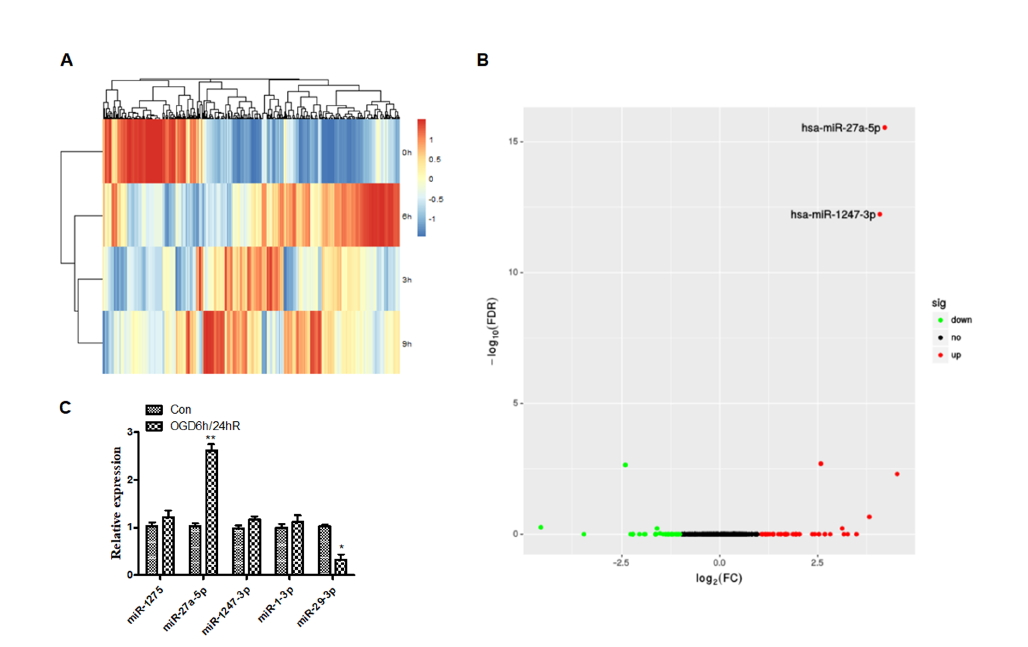 Figure 2.
Figure 2.Differential expression of micro-RNA in SH-SY5Y cells after oxygen-glucose deprivation. (A) Clustered heat map of the differentially expressed miRNAs after OGD treatment. Rows represent miRNAs, while columns represent the time of OGD. (B) Differential expression of micro-RNAs. The red and green points in the plot represent the differentially up or down-regulated miRNAs with statistical significance. (C) Performance of qPCR to verify the screened miRNAs after OGD/R treatment.
Fig. 3 shows that miR-27a-5p is significantly upregulated in SH-SY5Y cells subjected to OGD/R compared to the untreated control group (Fig. 3A), and in contrast, miRNA-29b-3p is downregulated in contrast (Fig. 3C). These results indicate a crucial effect of miR-27a-5p and miRNA-29b-3p in SH-SY5Y cells subjected to OGD/R. To further elucidate the exact biological role of miR-27a-5p and miRNA-29b-3p in SH-SY5Y cells subjected to OGD/R, experiments analyzing the loss or gain of function were carried out by transfecting mimic or inhibitor miRNA, and the activity of caspase-3, -8 and -9 were examined. The results show that inhibition of miRNA-27a-5p or overexpression of miR-29b-3p improved cell viability (Fig. 3F) and inhibited the activity of caspase-3, -8, and-9 (Fig. 3B and 3D). AKT protein is an important indicator of the PI3K/AKT signaling pathway reflecting cellular proliferation. The phosphorylation level of AKT decreased during the inhibition of neurocyte proliferation and the induction of neurocyte injury. Western blotting in Fig. 3G showed that miR-27a-5b inhibitor and miR-29b-3p mimic rescue the downregulation of p-AKT subjecting to OGD/R. Briefly, these data demonstrate that overexpression of miR-29b-3p or inhibition of miRNA-27a-5p alleviates OGD/R-mediated cell apoptosis and proliferation.
 Figure 3.
Figure 3.Inhibition of miRNA-27a-5p or overexpression of miRNA-29b-3p attenuated OGD/R-induced cell apoptosis, and miRNA-27a-5p targeted Bach1-3’-UTR. Cells were transfected with inhibitor-miR-27a-5p or mimic-miR-29b-3p for 24 h prior to OGD/R treatment. (A and C) Expression of miR-27a-5p and miR-29b-3p was detected by qPCR after OGD/R. (B and D) The activity of caspase-3, caspase-8, and caspase-9 was decreased after transfected with inhibitor-miR-27a-5p or mimic-miR-29b-3p in cells subjected to OGD/R. (E) Dual-luciferase reporter assay revealed that miR-27a-5p targeted the 3’-UTR of Bach1. (F) Cellular proliferation was tested by MTT assay. (G)Expression of cleaved-PARP, p-AKT, Bach1, and HO-1 was detected by western blotting. * means P < 0.05 vs. blank control, ** P < 0.01 vs. blank control, *** P < 0.001 vs blank control, # P < 0.05 vs. inhibitor/mimic NC.
To clarify the potential mechanism of miR-27a-5p and miR-29b-3p in neurocellular injury in response to OGD/R, we employed bioinformatics to analyze the target gene of miRNA. We found that Bach1, characterized by a transcription factor, could bind miR-27a-5p via the highly conserved binding domain at the 3'- UTR. Dual-luciferase reporter assay revealed that transcriptional regulation of Bach1 was inhibited by miR-27a-5p overexpression, but there was no obvious change with miR-29b-3p overexpression in SH-SY5Y cells subjected to OGD/R (Fig. 3E). Moreover, Bach1 expression was induced by inhibition of miR-27a-5p (Fig. 3G). Briefly, these data confirm that Bach1 is a miR-27a-5p target gene. To further investigate the effect of miR-27a-5p on the Bach1-regulated signaling pathway in neurocellular injury in response to OGD/R, we performed a western blot assay, which revealed that expression of HO-1 characterized by a downstream target gene was also suppressed in response to miR-27a-5p inhibition. Still, there was no effect in response to miR-29b-3p. These results indicate that miR-27a-5p can bind to the Bach1 gene and can mediate its transcriptional activity and downstream signaling to contribute to cellular injury in response to OGD/R.
The results of the MTT assay displaying HET0016 protection against OGD/R-induced neurocyte injury are shown in Fig. 4A. Compared to the OGD/R model group, 0.1 μM of HET0016 had no protective effect, while 0.5 μM and 2 μM had limited and maximum protective effect, respectively. We further analyzed apoptosis of SH-SY5Y cells subjected to OGD/R with the administration of 2 μM HET0016. The apoptotic rate of the OGD/R model group significantly increased compared to the untreated group (P < 0.001). In contrast, the apoptotic rate of cells pretreated with HET0016 for 24 h was significantly reduced compared to the OGD/R group (Fig. 4B). The results indicate that 2 μM HET0016 can inhibit the apoptosis of human neuroblastomas caused by OGD/R.
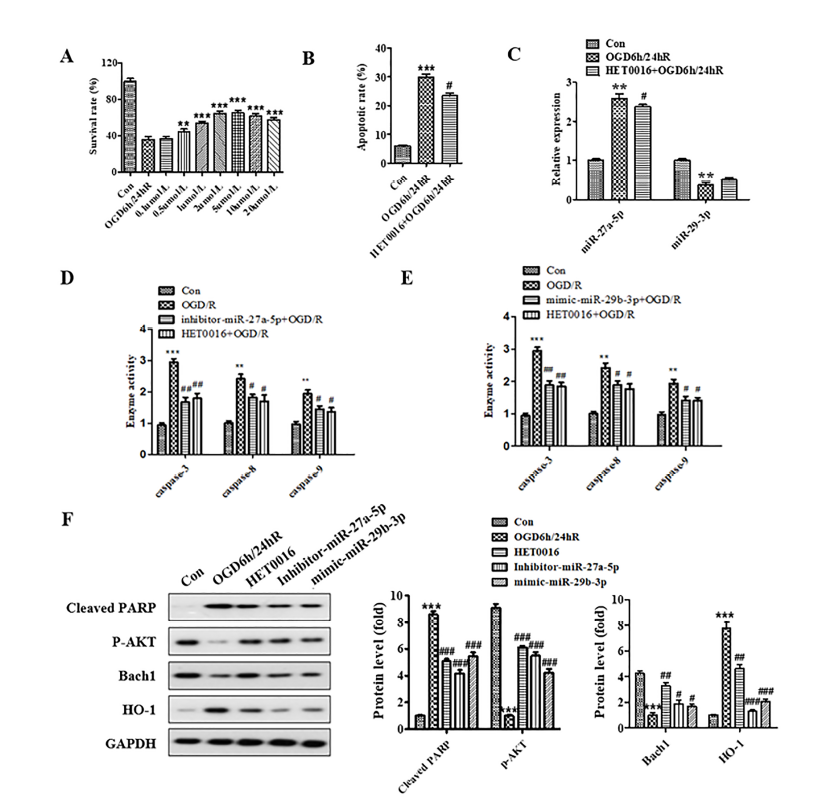 Figure 4.
Figure 4.HET0016 protected SH-SY5Y cell against OGD/R-induced injury through regulation of miR-27a-5p and miR-29b-3p and Bach1/HO-1 signal pathway. (A) Cells were treated with HET0016 in different concentrations for 24 h before OGD/R treatment. (B) Administration of 2 μM HET0016 attenuated the apoptosis induced by OGD/R. (C) Administration of 2 μM HET0016 decreased the expression of miR-27a-5p and increased the expression of miR-29b-3p in SH-SY5Y cells subjected to OGD/R. (D and E) Administration of 2 μM HET0016 suppressed the activity of caspase-3, caspase-8, and caspase-9 in SH-SY5Y cells subjected to OGD/R. (F) Expression of cleaved-PARP, p-AKT, Bach1, and HO-1 was detected by western blotting. * means P < 0.05 vs. blank control, ** P < 0.01 vs. blank control, *** P < 0.001 vs. blank control, # P < 0.05 vs. OGD/R.
RT-PCR analysis of the HET0016 regulation of miR-27a-5p and miR-29b-3p is shown in Fig. 4C. The results show the expression of miR-27a-5p was significantly increased (P < 0.01), while the expression of miR-29b-3p was decreased in OGD/R model group compared to the untreated group. Also, the expression of miR-27a-5p decreased, and the expression of miR-29b-3p weakly increased after the HET0016 administration. These results show that HET0016 can inhibit the expression of miR-27a-5p and upregulate the expression of miR-29b-3p. Caspase activity analysis showed that HET0016 could suppress the caspase-3, -8, and -9 activity induced by OGD/R (Fig. 4D and 4E). We further detected the expression of related proteins by western blot, and the result showed that HET0016 upregulated the expression of p-AKT and Bach1 and downregulated the expression of cleaved-PARP and HO-1 (Fig. 4F). These results indicated that HET0016 could protect SH-SY5Y cells against ischemia/reperfusion through regulation of miR-27a-5p and miR-29b-3p and the Bach1/HO-1 signaling pathway.
To investigate the mechanism mediating neuronal survival during IRI, we designed an in vitro study to simulate clinical ischemia-reperfusion injury using an oxygen/glucose deprivation and reoxygenation (OGD/R) cellular model. We found that OGD/R promoted apoptosis in SH-SY5Y cells. MiRNA-seq analysis demonstrated that miRNA-27a-5p expression was greatly induced, while miRNA-29b-3p was attenuated in OGD/R. These findings suggest that miR-27a-5p and miR-29b-3p can contribute to OGD/R-induced apoptosis.
MiRNA sequencing analysis detects all miRNAs expressed by a species under specific functional states and is a rapid and effective technique for exploring disease pathogenesis, as well as drug targets and pathways. To further clarify the potential mechanism of neuronal injury in response to OGD/R, we used miRNA sequence analysis to detect changes in miRNAs in human neuroblastoma cells before and after OGD treatment to analyze the relationship between differentially expressed miRNAs and OGD-induced neurological damage. We screened several miRNAs that were affected by the OGD/R treatment. We found that miR-27a-5p, miR-1247-3p, miR-1-3p, and miR-1275 were enhanced, while miR-29b-3p was decreased. Furthermore, we used qPCR to verify the expression of five miRNAs after hypoxia for 6 hours and reoxygenation for 24 hours. We found that miR-27a-5p expression was increased, and miRNA-29b-3p was decreased, while no change was found in miR-1247-3p, miR-1-3p, and miR-1275 (compared to the untreated group).
Studies have investigated miRNA expression patterns, as well as miRNA target genes and related signaling pathways. For instance, Kulshreshtha et al. (2007) first reported the change in expression levels of various miRNAs in cancer cell lines with induced hypoxia (Kulshreshtha et al., 2007). More recently, Sabirzhanov et al. (2014) have shown that overexpression of miR-27a in cortical neurons could inhibit the activity of the proapoptotic gene Caspase-3, while Chhabra et al. (2009) showed that overexpression of miR-23a, miR-27a, and miR-24-2 in HEK293T cells promoted apoptosis.
By querying targetscan7.0 data, we found that miR-27a-5p had two possible binding sites on the Bach1 gene. To determine whether miR-27a-5p could target Bach1, we performed a dual-luciferase reporter gene activity assay. We constructed a Bach1 3'-UTR reporter gene vector and co-transfected the reporter gene vector with miR-27a-5p inhibitor and mimic-miR-29b-3p. Luciferase activity was significantly decreased after the transfection of miR-27a-5p inhibitor, while the activity of luciferase was unchanged after the transfection of mimic-miR-29b-3p. The results suggest that there is a functional binding site on the 3'-UTR of Bach1 for miR-27a-5p.
HO-1 (Heme Oxygenase-1) is an important member of the microsomal enzyme system initiating the heme degradation, which plays a key role in the physiological and pathological processes such as growth and differentiation (Loboda et al., 2008). The transcriptional expression of HO-1 is usually mediated by a transcriptional factor of Bach1. Recently, several lines of evidence have suggested that the increased expression of HO-1 participated in the various agents’ beneficial effects against ischemic damage (Pei et al., 2016). However, the mechanism that Bach1/HO-1 was initiated and exerted a protective effect against ischemic damage remains obscure. Our results indicate that OGD/R can increase the miR-27a-5p level and mediate the Bach1/HO-1 signaling pathway. Furthermore, we confirmed that miR-27a-5p could target Bach1 and miR-27a-5p inhibitor to alleviate the induction of the Bach1/HO-1 pathway response to OGD/R. These results imply that the Bach1/HO-1 pathway can exert a response to miR-27a-5p, resulting in OGD/R-induced cellular injury.
HET0016 is an important molecular inhibitor of 20-HETE (20-Hydroxyeicosatetra-enoic acid), which exerts a critical physiological and pathological function in modulating cerebral microvascular endothelial cells and brain ischemia injury (Yang et al., 2012). Our previous findings indicate that HET0016 exerted a protecting effect on the blood-brain barrier subjecting to cerebral ischemia-reperfusion (Liu et al., 2014). In this paper, we further report that HET0016 can protect SH-SY5Y cells against OGD/R through the mediation of miR-27a-5p and miR-29b-3p and Bach1/HO-1 signaling pathway. Our data, therefore, delineates a new mechanism of HET0016’s protective effect against OGD/R-induced cellular injury.
In summary, oxygen-glucose deprivation/reperfusion can induce apoptosis of SH-SY5Y cells and upregulates miR-27a-5p, while downregulating the expression of miR-29b-3p. Also, HET0016 has a protective effect against oxygen-glucose deprivation/reperfusion-induced injury, which may be achieved by downregulating miR-27a-5p and upregulating the expression of miR-29b-3p. Further, miR-27a-5p is involved in the pathophysiological process of oxygen-glucose deprivation/reperfusion-induced injury and participates in the anti-apoptotic effect of oxygen-glucose deprivation/reperfusion-induced injury by regulating Bach1/HO-1 signaling. Therefore, these miRNAs are potential therapeutic targets that require further verification.
ATCC: American type culture collection; DMEM: dulbecco’s modified eagle medium; DMSO: dimethyl sulfoxide; I/R: ischemia/reperfusion; IRI: ischemia-reperfusion injury; JNK: c-Jun N-terminal kinase; RIPA: radio immunoprecipitation assay; ROS: reactive oxygen species.
The present study was supported by grants obtained from the National Natural Science Foundation (81704170, 81873378, 81503669), Administration of Traditional Chinese Medicine of Heilongjiang Province (ZHY16-003), Heilongjiang University of Chinese Medicine (2014bs01), Heilongjiang Natural Science Foundation (H2015031), Postdoctoral Initiation Fund of Heilongjiang Province (LBH-Q18117), Training Project of Key Young Researcher from Shenzhen People’s Hospital (SYKYPY201925). Thanks to the anonymous reviewers for suggestions on improving the first version of the manuscript.
The authors declare no conflict of interest.
