We investigated the effects of the Chinese herb Codonopsis pilosula isolate isorhapontigenin on antioxidant factor and the PI3K/Serine/Akt signaling pathway in Parkinson's disease. This research was, therefore, carried out to explore a possible protective mechanism of isorhamnetin in Parkinson's disease. The results support that isorhapontigenin could effectively inhibit isorhapontigenin restored myeloperoxidase + induced reduction of antioxidant levels. Also, 1-Methyl-4-phenylpyridine up-regulated the expression of phosphorylated-Akt, phosphorylated-PI3K, and phosphorylated mammalian target of rapamycin, while isorhapontigenin inhibited the expression of phosphorylated-Akt, phosphorylated-PI3K, and phosphorylated- mammalian target of rapamycin. Furthermore, LY294002 improved the antioxidant effect of isorhapontigenin in PC12 cells, and insulin-like growth factor 1 inhibited the antioxidant effect of isorhapontigenin in PC12 cells. Our results support the finding that isorhamnetin enhanced the antioxidant effect induced by 1-Methyl-4-phenylpyridine in PC12 cells by suppressing the activation of the PI3K/Akt signaling pathway.
Parkinson's disease (PD) is also known as a common neurodegenerative disease, which has become the second most prevalent disease of the nervous system (Armstrong, 2017). Screening new targets for the treatment of PD, and the development of new drugs that can be used for clinical prevention of PD have enormous social and economic benefits (Zucca et al., 2017). PD has been studied for decades, but the cause and the mechanisms are still unclear due to the complexity of its pathogenesis (Athauda et al., 2017). Current medicine used for the treatment of PD is mainly based on dopamine agonists, anticholinergics, and other drugs.
Studies have shown that many extracts of traditional Chinese medicine have protective effects on neurons and can antagonize the induced cytotoxicity by inhibiting oxidative stress and cell apoptosis (Pan et al., 2011). Clinical practice has proven that traditional Chinese medicine can alleviate dyskinesia caused by levodopa drugs, relieve non-motor symptoms of PD patients, and improve patients' quality of life. Codonopsis pilosula is one of the most commonly used traditional Chinese medicines (Sun et al., 2010; Sun and Liu, 2008). Pharmacological studies have shown that Codonopsis pilosula has the function of regulating blood sugar levels, promoting hematopoietic function, reducing blood pressure, resisting hypoxia, resisting fatigue, boosting the immune system, regulating stomach contraction and anti-ulcer (Ng et al., 2004; Wang et al., 1996).
Isorhamnetin (ISO) is a flavonoid isolated from Codonopsis pilosula. Studies have shown that flavonoid plays a critical role in regulating neurological function and neuroprotection in neurodegenerative diseases (Bakhtiari et al., 2017; Magalingam et al., 2015). Recent studies have also shown that ISO has many cardiovascular effects, such as anti-myocardial hypoxia, ischemia, relieving angina pectoris, anti-arrhythmia, anti-oxygen free radicals, and reducing serum cholesterol (Boeschsaadatmandi et al., 2011; Teng et al., 2006). However, the protective effect of isorhamnetin as a monomer on PD has not been investigated. PI3K/Akt signaling transduction pathway is involved in protein synthesis in the body (Díazserrano et al., 2018). It plays a critical role in regulating cell differentiation and apoptosis (Gui et al., 2018). Specific regulation of related sites of conduction pathways can prevent neuron apoptosis and promote neuron survival (Xiao et al., 2015).
1-Methyl-4-phenylpyridine (MPP+) and isorhamnetin (ISO) were obtained from Sigma-Aldrich (Merck, Germany).
The PC12 cell line (rat pheochromocytoma tumor) was obtained from Procell company (Wuhan, P. R. China). Cells were cultivated in RPMI-1640 medium supplemented with 10% FCS (Clark Bioscience, USA). Cells were split into the group of control, ISO, MPP+, and MPP++ISO. MPP+ was diluted to 400 mmol/L with a 1 : 10 gradient in the DMEM medium. Cells were plated into a 96-wells plate, and different concentrations of MPP+ were added. ISO was diluted to 1 mg/ml with a 1 : 10 gradient in DMEM. Then it was added in a 96-wells plate at the ratio of 1 : 100, and different concentrations of ISO were added.
Cell proliferation assay was conducted by a Cell Counting Kit-8 (CCK8; Lanpai, Shanghai, P. R. China). The absorbance values were measured at 450 nm using a microplate reader (Potenov; Beijing, P. R. China).
PC12 cells were washed and cultured with 5 μL of FITC Annexin V, 500 μL of binding buffer, and 5 μl of propidium iodide (PI). The apoptotic rate was determined using flow cytometry (FACS Calibur, USA). Experiments were carried out in Rieger et al. (2011).
Determination of the activity and content of glutathione peroxidase (GSH-Px), superoxide dismutase (SOD), and malondialdehyde (MDA)
The cultured cells were lysed at 4 °C, followed by centrifugation to collect the supernatant. The activity and content were determined using GSH-Px, SOD, and MDA kits.
Total proteins were extracted from the transfected cells. The protein concentration was determined using the BCA Protein Assay Kit, followed by incubation with p-PI3K (1 : 500, Youliante, Shanghai, P. R. China), PI3K (1 : 500, Youliante, Shanghai, P. R. China), p-Akt (1 : 1000, Youliante, Shanghai, P. R. China), Akt (1 : 1000, Youliante, Shanghai, P. R. China), p-mTOR (1 : 1000, Youliante, Shanghai, P. R. China), mTOR (1 : 1000, Youliante, Shanghai, P. R. China) and GAPDH (1 : 1000, Youliante, Shanghai, P. R. China) primary antibodies at 4 °C for overnight. Then anti-rabbit secondary antibody (1 : 1000, Beyotime, Shanghai, P. R. China) was then added to incubate for another 1 h. Western blot analysis was conducted as described in Finn et al. (2009).
The data were analyzed using SPSS19.0 statistical software. The results were shown as mean ± standard deviation (SD). Multi-group comparisons were conducted using one-way ANOVA. Least Significant Difference (LSD) test was used for subsequent analysis. P < 0.05 indicated significant differences.
As shown in Fig. 1A, MPP+ reduced the growth rate of PC12 tumor cells, and the cell viability gradually decreased with the increase of dose and time. At 24, 48, and 72 h, the IC50 values of PC12 cells were 1,274.56, 203.48, and 81.25 μM, respectively. Therefore, 203 μM and 48 h were selected as the working concentration and incubation period for the intervention drug of MPP+ in the following experiments. Whether MPP+ could induce apoptosis was further investigated. As shown in Fig. 1B, contrasted with the control group, the proportion of apoptotic cells treated with 1/2 MPP+, 1 MPP+, 2 MPP+ was increased to 10.5 %, 15.2 %, and 30.9 %, respectively. These data suggest that MPP+ increases the apoptotic rate of PC12 tumor cells.
 Figure 1.
Figure 1.Effect of MPP+ on growth and apoptosis. (A) Determination of cell viability in different concentrations of MPP+ in PC12 cells. (B) Determination of the apoptosis rate of PCP cells in different concentrations of MPP+. The concentration of MPP+ at 1274.56, 203.48, and 81.25 μM were shown as 1/2 MPP+, MPP+, and 2 MPP+, respectively. Scale bar = 100 μm ( × 200 times).
In Fig. 2A, there is a statistically significant difference between groups determined by one-way ANOVA (F(6, 14) = 9.321, P = 0.000). The LSD post-hoc test shows that the viability of PC12 cells pretreated with different concentrations of ISO was increased, and the concentration of 64 μM ISO had the best protective effect on PC12 cells (0.71 ± 0.04, P = 0.000), contrasted with the control group (0.45 ± 0.03). Therefore, 64 μM was selected as the intervention concentration of ISO for the following experiments. Also, compared to the control group, MPP+ increased the apoptosis rate of PC12 cells, while ISO+MPP+ decreased the apoptosis rate (Fig. 2B), indicating that ISO has a protective effect on MPP+-induced apoptosis of PC12 cells.
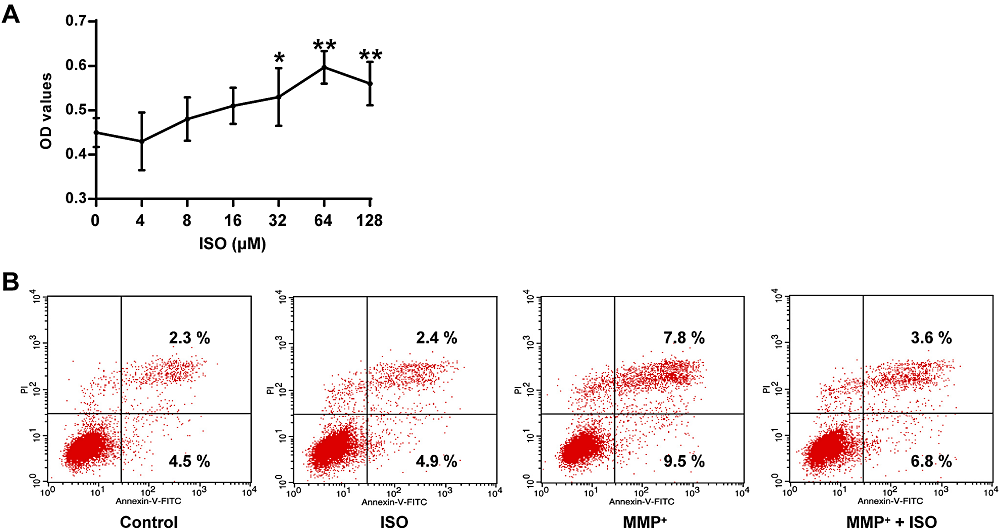 Figure 2.
Figure 2.Protective effect of ISO on MPP+ elicited damage to PC12 tumor cells. (A) Determination of cell viability in PC12 tumor cells at different concentrations of ISO. (B) Determination of apoptotic rate in PC12 cells of different treatment groups. **P < 0.01, *P < 0.05 vs. the control group.
In Fig. 3, an analysis of variance using one-way ANOVA shows significant differences among groups (GSH-Px, F(3, 8) = 6.105, P = 0.018; SOD, F(3, 8) = 7.779, P = 0.009; MDA, F(3, 8) = 18.156, P = 0.001). Post hoc analyses using the LSD post hoc criterion for significance indicate that the activity of GSH-Px (485.21 ± 40.07, P = 0.006) and SOD (455.86 ± 70.67, P = 0.003) in PC12 tumor cells induced by MPP+ was significantly reduced in the MPP+ group, and the content of MDA (2.46 ± 0.12, P = 0.000) was significantly increased. In comparison to the MPP+ group, the activity of GSH-Px and SOD in PC12 cells induced by MPP+ was significantly increased in the GSH-Px (665.13 ± 59.18, P = 0.015) and SOD (676.19 ± 50.70, P = 0.011) group and the MDA content (2.46 ± 0.12, P = 0.001) was significantly reduced. These data indicate that ISO effectively enhanced the antioxidant effect.
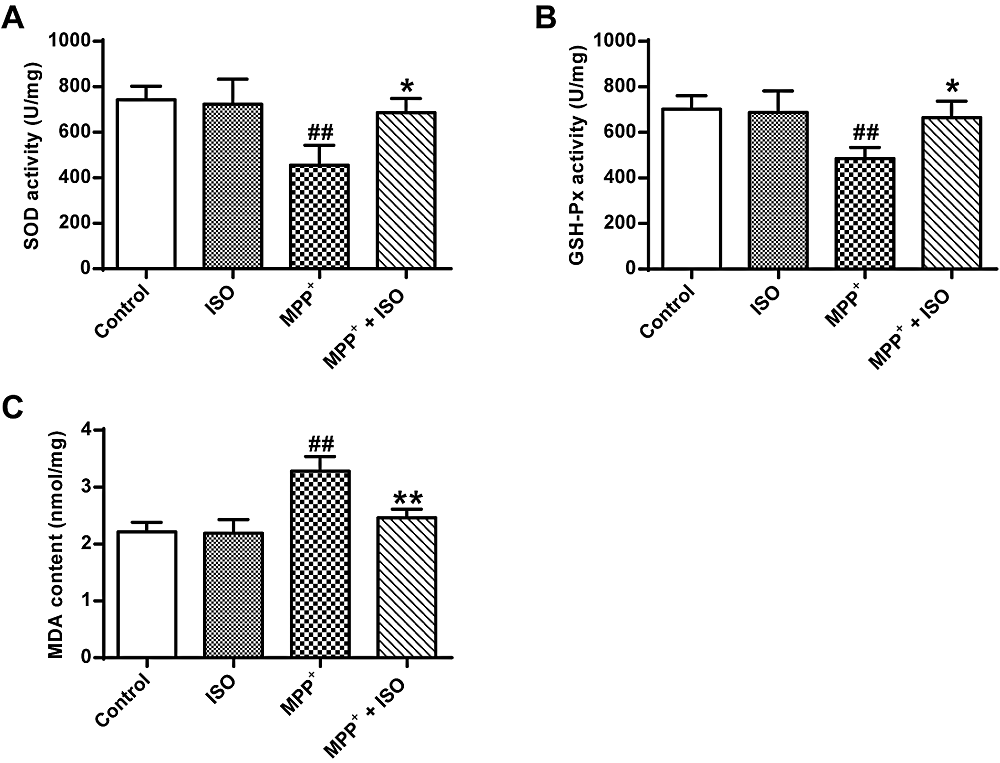 Figure 3.
Figure 3.Effect of ISO on the activity of GSH-Px and SOD and MDA content induced by MPP+ treated PC12 cells. (A) Determination of SOD activity in different treatment groups. (B) Determination of GSH-Px activity in different treatment groups. (C) Determination of MDA content in different treatment groups vs. the control group, ## and P < 0.01; vs. the MPP+ group, **P < 0.01, *P < 0.05.
The results show significant differences between groups as determined by one-way ANOVA (p-PI3K, F(3, 8) = 31.782, P = 0.000; p-Akt, F(3, 8) = 19.921, P = 0.000; p-mTOR, F(3, 8) = 39.440, P = 0.000). The LSD post-hoc test show that compared with the control group (p-PI3K, 0.86 ± 0.07; p-Akt, 0.98 ± 0.07; p-mTOR, 0.82 ± 0.06), the expression yield of p-Akt, p-PI3K and p-mTOR induced by MPP+ in PC12 tumor cells were up-regulated in MPP+ (p-PI3K, 0.86 ± 0.07, P = 0.000; p-Akt, 0.98 ± 0.07, P = 0.000; p-mTOR, 0.82 ± 0.06, P = 0.000) and ISO groups (p-PI3K, 0.86 ± 0.07, P = 0.125; p-Akt, 0.98 ± 0.07, P = 0.022; p-mTOR, 0.82 ± 0.06, P = 0.003) (Fig. 4A-D). While compared with the MPP+ group, the expression yield of p-Akt (1.31 ± 0.06, P = 0.005), p-PI3K (1.05 ± 0.07, P = 0.000) and p-mTOR (1.29 ± 1.10, P = 0.000) in MPP+-induced PC12 cells are significantly reduced in the MPP+ + ISO group. These results demonstrate that ISO can suppress the activation of the PI3K/Akt signaling pathway.
 Figure 4.
Figure 4.The expression yields of PI3K/Akt/mTOR pathway-associated factors. (A) Representative Western blotting bands for the PI3K/Akt/mTOR signaling pathway. (B) Quantitative optical density determination of PI3K. (C) Quantitative densitometry of Akt. (D) Quantitative densitometry of mTOR. &P < 0.05, &&P < 0.01, ##P < 0.01, vs. the control group; **P < 0.01 vs. the MPP+ group.
As shown in Fig. 5A-D, there was a significant difference between groups as determined by one-way ANOVA (p-PI3K, F(2, 6) = 36.520, P = 0.000; p-Akt, F(2, 6) = 25.835, P = 0.001; p-mTOR, F(2, 6) = 19.493, P = 0.002). The LSD post-hoc test indicates that the expression yield of p-Akt (0.68 ± 0.03, P = 0.007), p-PI3K (0.79 ± 0.06, P = 0.032) and p-mTOR (0.69 ± 0.07, P = 0.036) are significantly lower in the LY294002 group. The expression yield of p-Akt, p-PI3K and p-mTOR are significantly increased in the IGF-1 group (p-PI3K, 1.45 ± 0.11, P = 0.001; p-Akt, 1.22 ± 0.10, P = 0.019; p-mTOR, 1.15 ± 0.09, P = 0.012) contrasted with that in the control group (p-PI3K, 1.01 ± 0.05; p-Akt, 0.98 ± 0.08; p-mTOR, 0.89 ± 0.05). These results suggest that LY294002 activates the PI3K/Akt/mTOR pathway, while IGF-1 activates the PI3K/Akt/mTOR pathway.
 Figure 5.
Figure 5.Effect of ISO on MDP+ induced SOD and GSH-Px bioactivity and MDA content. (A) Representative Western blotting bands for the PI3K/Akt/mTOR pathway. (B) Quantitative optical density determination of PI3K. (C) Quantitative densitometry of Akt. (D) Quantitative densitometry of mTOR. (E) Determination of SOD activity in different treatment groups. (F) Determination of GSH-Px activity in different treatment groups. (G) Determination of MDA content in different treatment groups. &&P < 0.01, & P < 0.05 vs. the control group, ** P < 0.01, ##P < 0.01 vs. the MPP+ group.
As shown in Fig. 5E-G, there is a significant difference between groups as determined by one-way ANOVA (GSH-Px, F(6, 14) = 42.168, P = 0.000; SOD, F(6, 14) = 34.284, P = 0.000; MDA, F(6, 14) = 60.743, P = 0.000). The LSD post hoc test indicate that the activities of SOD and GSH-Px are significantly lower in the MPP+ group (SOD, 445.08 ± 46.98, P = 0.001; GSH-Px, 458.82 ± 39.29, P = 0.004) compared to that in the control group (SOD, 693.69 ± 57.40; GSH-Px, 724.07 ± 45.15), while the MDA content (2.51 ± 0.12, P = 0.000) is significantly enhanced compared to that in the control group (1.38 ± 0.11). Compared with the MPP+ group, the bioactivities of SOD and GSH-Px in the MPP+ + ISO group (SOD, 844.58 ± 32.58, P = 0.000; GSH-Px, 884.09 ± 71.76, P = 0.000) and the MPP+ + LY294002 group (SOD, 883.38 ± 97.58, P = 0.000; GSH-Px, 923.58 ± 53.37, P = 0.000) is significantly increased, while the MDA content is significantly decreased in the MPP+ + ISO group (1.61 ± 0.56, P = 0.000) and the MPP+ + LY294002 group (1.58 ± 0.11, P = 0.000). Compared to the MPP+ group, the activity of SOD and GSH-Px in the MPP+ + IGF-1 group is significantly lower (SOD, 285.12 ± 40.80, P = 0.020; GSH-Px, 206.76 ± 31.33, P = 0.006), while the MDA content (2.86 ± 0.19, P = 0.010) is significantly higher. These results indicate that the activation of the PI3K/Akt/mTOR signal pathway improves the levels of oxidative stress in PC12 tumor cells.
The bioactivity of GSH-Px (1317.15 ± 162.00, P = 0.000) and SOD (993.65 ± 37.07, P = 0.028) in the MPP+ + ISO + LY294002 group are significantly enhanced compared to that in the MPP+ + ISO group, while the MDA content (0.99 ± 0.08, P = 0.000) is significantly decreased. The bioactivity of GSH-Px (664.29 ± 55.85, P = 0.013) and SOD (653.76 ± 81.50, P = 0.007) is significantly reduced in the MPP+ + ISO + IGF-1 group, and the MDA content (2.06 ± 0.12, P = 0.002) is significantly increased. These results indicate that ISO enhances the antioxidant effect by inhibiting the PI3K/Akt/mTOR pathway (Fig. 5E-G).
The effects of isorhamnetin on antioxidant factors and the PI3K/Akt signaling pathway in Parkinson's cell model was investigated. MPP+ was applied to PC12 cells for 48 hrs to establish a model of PD cell injury in vitro. The cell viability and apoptosis were measured, and it showed that ISO pretreatment increased the survival rate of MPP+-induced damage in PC12 cells, and decreased the apoptotic rate of PC12 cells
Codonopsis pilosula is a tonic Chinese traditional medicine (Castiglioni et al., 2011; Caudle et al., 2006; Lees, 1993; Rektorova et al., 2011; Scally et al., 2011). Codonopsis pilosula has anti-ischemic, hypolipidemic, anti-oxidation, and immunity enhancement effects, and therefore high medicinal values (Xin et al., 2012; Zhang et al., 2010). Isorhamnetin (ISO), a type of flavonoid, is an essential active ingredient in Codonopsis pilosula. In the PD model, flavonoid shows a strong antioxidant enzyme activation, which can effectively reduce neuron injury (Angeline et al., 2013).
Free radical scavenging systems in the body include SOD, catalase, MDA, and GSH-Px (Wang et al., 2011). The level of SOD activity indirectly reflects the body's ability to scavenge oxygen free radicals. GSH-Px is an important peroxide-degrading enzyme that catalyzes the reaction of reduced GSH to hydroperoxides and protects cell membrane structure and mitochondrial function (Bao et al., 2013). The content of MDA can reflect the rate of lipid peroxidation (Laguna et al., 2015). Studies have shown that lipid peroxidation selectively increases in the nigrostriatal cells of a PD patient (Ahlskog, 2005), in which the levels of GSH and GSH-Px were decreased, while the levels of MDA were raised.
Oxidative stress is well documented in PD (Li et al., 2014). Oxidative stress has long been considered to play a critical role in the pathogenesis of AD. Some antioxidants rich natural extracts are believed to be neuroprotective, and therefore may have cognitive benefits in aging and neurodegenerative diseases (Rabadiya et al., 2012). For example, Ingale and Kasture (2017) found that the flower butanol extract improved PD by improving antioxidant capacity.
Our research revealed that ISO raised the activity of SOD and GSH-Px in PC12 cells, and reduced the content of MDA, which was consistent with previous studies (Dong et al., 2015). It showed that ISO could increase the activity of the antioxidant system of the PD cell model to different degrees, reduce lipid peroxidation, improve the antioxidant effect, and have a neuroprotective role.
Studies have shown close relation to Chinese medicine action with the regulation of the PI3K/AKT-mTOR signaling pathway (Wu et al., 2016). Many pharmacological compounds have proved to have a neuroprotective role in oxidative stress by activating the PI3K/Akt pathway (Zhang et al., 2016). For example, Zhang et al. (2016) found that salidroside (SAL) could protect cell apoptosis induced by 1-methyl-4-phenylpyridine by regulating PI3K/Akt/GSK3β pathway, and Baicalein can significantly inhibit the damage of 6-O-HDA to MES23.5 neurons. Its mechanism might involve the activation of the PI3K/Akt pathway (Zhang et al., 2012).
We found that ISO down-regulated the expression of p-PI3K, p-Akt, and p-mTOR in MPP+-induced PC12 cells. Also, LY294002 inhibited the PI3K/Akt/mTOR signaling pathway, while IGF-1 activated the PI3K/Akt/mTOR signaling pathway. It was also found that activation of the PI3K/Akt/mTOR signaling pathway increased oxidative stress levels. The antioxidant factors were significantly increased after adding LY294002. Hence, combining our present findings with previous studies (Xu et al., 2013), it is demonstrated that the anti-apoptotic protective mechanism of ISO on MPP+ induced PC12 cells is through PI3K/Akt signaling pathway.
The anti-apoptotic protective mechanism of ISO on MPP+ induced PC12 cells by reducing oxidative stress and inhibiting the PI3K/Akt signaling pathway. This would provide an experimental basis for the clinical application of ISO in the treatment of PD, and a theoretical basis for the future development of ISO. This finding will also provide insight into the pathogenesis of PD and provide an experimental basis of its clinical prevention.
Youquan Gu is responsible for experimental findings, data analysis & collection, statistical analysis; Tianhong Wang and Jun Chen are accountable for the literature research, data acquisition, and manuscript preparation; Zhe Zhou, Ying Wang, Jiangjun Chen, Ning Liu, and Zhenxiu Jiang are responsible for experimental findings, data analysis, statistical analysis, manuscript preparation & editing.
This research was funded through 1506RJZA259/Gansu natural science foundation.
The authors declare no conflict of interest.
