Despite frequent clinical hyper- or hypothermia cases, the thermal-dependence of the endogenous pain modulation system at the spinal cord is not well understood. The spinal dorsal horn neuronal network responses during mechanical heterotopic noxious stimuli was evaluated at three different body temperatures (34, 37 or 40$^\circ $C) by measurement of lumbar cord dorsum potentials activated by electrical stimulation of the ipsilateral sural nerve in adult thiopental anesthetized rats. A noxious clamp was applied randomly to the tail, right hind paw, right forepaw, muzzle, or left forepaw. Heterotopic noxious stimuli induced a decrease of the negative wave amplitude and duration at 37$ {}^\circ $C. This effect was reduced at 40$ {}^\circ $C for both amplitude ($ - $18.2% for 37-40$ {}^\circ $C; p < 0.0005) and duration ($ - $16.4% for 37-40$ {}^\circ $C; p < 0.0001). The P wave showed neither amplitude nor duration changes at either of the three tested temperatures. Clinical range changes of temperature could modify pain sensation, while hyperthermia increased nociceptive sensory input to dorsal horn and exacerbated pain sensation in individuals with fever.
Clinical range changes in temperature of the central nervous system affect membrane and neuronal properties [1,2]. Furthermore, thermal stress increases expression of heat-sensitive molecules and induces activation of several neuronal activity modulating factors even for a physiological temperature range [3].
Neuronal thermo-dependence can affect mechanisms that modulate sensory information such as diffuse noxious inhibitory controls (DNIC) involved in counter irritation induced pain relief [4] where a noxious stimulus activates supraspinal descending inhibitory mechanisms that in turn reduce response of trigeminal and spinal dorsal horn (SDH) neurons to a new noxious stimulus [5,6].
Cord dorsum potentials (CDPs) enable the evaluation of responses from the SDH neuronal network. They reflect sequential activation of neural structures along somatosensory pathways and follow electrical stimulation of peripheral nerves within their receptive field [7]. Lumbar CDPs consist of a negative wave (N-wave) mainly generated by the integrated and synchronized response of last-order interneurons [7,8,9,10]. The N-wave is followed by a positive wave (P-wave), the result of a complex process of SDH activation induced by descending pathways from supraspinal structures which primary consist of a combination of afferent depolarization and local neuronal network interactions [7,8]. Recent experimental evidence supports the proposition that a large and heterogeneous cell population, which includes excitatory and inhibitory neurons but also different sensory neurons and primary afferents within the SDH, could be modulated by a heterotopic noxious stimulus (HNS) applied distal to a spinal segment [11].
Different temperature sensitive cationic channels that generate a transient receptor potential (TRP) are expressed at the neuroaxis, SDH neurons, sensory fibers, and non-neuronal cells of the skin. TRPV 1 to 4 are known as warm sensors that induce cell depolarization directly proportional to temperature [12,14].
Little attention has been paid to the influence of the clinical range of body temperatures on DNIC and other endogenous pain modulation systems, especially those activated by HNS, that exert their effects on the large neuronal population of the SDH where main sensory, but especially early pain processing occurs. This study aims to evaluate the effect of clinical range temperature change on cord dorsum potentials during HNS. The hypothesis is that temperature can modulate the response of N-waves i.e., spinal dorsal horn neuronal discharges after a HNS.
Animal handling and the experimental protocol were approved by the Institutional Bioethical Committee (UC-FCS-DIPI-29102012AE/ JM) following both the US National Institute of Health guide for the care and use of laboratory animals (NIH) and local regulations [15].
Adult male Sprague-Dawley strain rats weighing 300-350 g (n = 12) were housed in a low stress environment (12:12 h light: dark schedule) with food and water ad libitum. After one week of acclimatization, animals were anesthetized with thiopental (60 mg$ \cdot $Kg$ ^{-1} $; i.p.). Anesthesia was verified by absence of paw flexor and corneal reflexes during the experiment. Atropine was given subcutaneously (0.25 mg diluted in 0.5 ml NaCl 0.9%) to reduce secretions into the respiratory tract. Colorectal temperature was constant at 34; 37 or 40 $\pm$ 0.5 $^{\circ}$ C (mean $ \pm $ SD) by a feedback controlled heating blanket underneath the ventral surface of the animal. Laminectomies (T$ {}_{10} $ - L$ {}_{3} $) were performed to expose the lumbar enlargement of the spinal cord that was then covered with tempered mineral oil (body temperature $\pm$ 0.5$^{\circ}$C). The left hind paw sural nerve was dissected free and covered with tempered mineral oil. The paws were left in close contact with the heating blanket to maintain body temperature. In some experiments intracarotid blood pressure monitoring was performed to confirm that while under anesthesia, neither the electrical nor the HNS induced blood pressure increases.
Rats were placed in a stereotaxic frame for CDPs to be ipsilaterally recorded by a metal electrode with an uninsulated tip (250 $ \mu $m $ \varnothing $, $ < $ 50 $ \Omega $ steel filaments) orthodromically activated by electrical stimulation of the left hind paw sural nerve (hook bipolar electrode, 5 mA; 0.5 ms; 0.05 Hz; A\&M System A\&M SystemTM Mod 1700). The reference electrode was placed under the abdominal skin. The stimulation intensity was set to 10 times the threshold of N-wave induction [16] and activated both A and C fibers [17]. This was sufficient to elicit a light contraction of the hind paw. CDPs were amplified ($ \times $ 1000; A\&M SystemTM), band-pass filtered (10 Hz-500 Hz), digitized at 14.4 kHz (DATAQTM), supervised on-screen (WinDaq/XLTM), and saved to hard disk. Amplitude (from basal isoelectric line) and duration (time difference between isoelectric line crossing points) of N- and P-waves were measured off-line by means of digital cursors from a five-wave average using Data View Software [18].
HNS to elicit DNIC mechanisms consisted of applying a clamp that sequentially exerted a noxious pressure of 4.0 kg/cm2 for 5 min [18] to different body areas, i.e. tail, right hind paw, right forepaw, muzzle, and left forepaw. The clamp was previously tested on an investigator’s finger.
A total of 12 rats were studied and their results analyzed. CDPs were recorded for five minutes during the control condition (CC), i.e. sural nerve stimulation only. Thereafter, maintaining sural nerve stimulation, HNS was applied for five minutes sequentially in the tail, right hind paw, right forepaw, muzzle, and left forepaw, with five minutes of CC recording after each HNS application. This duration has been reported sufficient for recovery of basal activity in SDH neurons [4,11]. The HNS body site sequence was applied randomly clockwise or anticlockwise to every subject for each temperature (34; 37 and 40$ ^{\circ} $C), which was also randomly increased or decreased prior to each application. Wave values were analysed only after two minutes of each condition. Recordings were started after the test temperature had been changed for 15 minutes, or about 5 to 6 minutes after subjects reached a stable core temperature.
Results were obtained from the average of five N- and P-waves. Wave amplitudes ($ \mu $V) and duration (ms) were expressed as mean $ \pm $ SD. Data were normalized as a percentage of control condition values. Two rounds of HNS application were analyzed for each subject and averaged for each body site. Thermo-dependence was evaluated by Q$ {}_{10} $ values for 34-37$ ^{\circ} $C and for 37-40$ ^{\circ} $C which were linearly extrapolated for 10$ ^{\circ} $C for N- and P-wave amplitude and duration. Differences between pairs (34 to 37$ ^{\circ} $C; 37 to 40$ ^{\circ} $C and 34 to 40$ ^{\circ} $C) or groups (34, 37 and 40$ ^{\circ} $C) were analyzed by Wilcoxon sign-rank or Kruskal-Wallis tests, respectively. Data dispersion induced by HNS was evaluated by coefficient of variation Fligner-Killeen test. A p < 0.05 was considered significant for all analyses. Analysis employed the PAST statistical package [19].
Recorded N- and P-waves showed a clear distinction of amplitude, duration, and sequence during control conditions (Fig.1). Evaluation of body temperature effect without application of HNS did not induced statistically significant changes in either N-wave (K-W = 0.28; P = 0.87) or P-wave (K-W = 1.47; p = 0.49) amplitude, or for N (K-W = 0.43; p = 0.63) or P-wave (Kruskal-Wallis: 0.58; p = 0.52) duration, for body temperatures of 34, 37 and 40$ ^{\circ} $C, respectively. HNS applied to all tested body parts, i.e. tail, contralateral hind paw, contralateral forepaw, muzzle and ipsilateral forepaw, showed a non-significant change at 34-37$ ^{\circ} $C range ($ - $5.5%; mean value from all body locations) but a statistically significant reduction of N-wave amplitude, mean value $-18.2$ % ( p < 0.0042; Fig. 2), was observed for all body sites tested in the 37-40$ ^{\circ} $C temperature range. This inhibition of N-wave amplitude was inversely proportional to body temperature (Fig. 1 and Fig. 2). HNS applied to the muzzle showed the largest reduction of N-wave amplitude for all HNS application body sites tested (mean $ - $16.1 $ \pm $ 3.2% vs. $ - $26.7 $ \pm $ 3.3%, p < 0.0027).
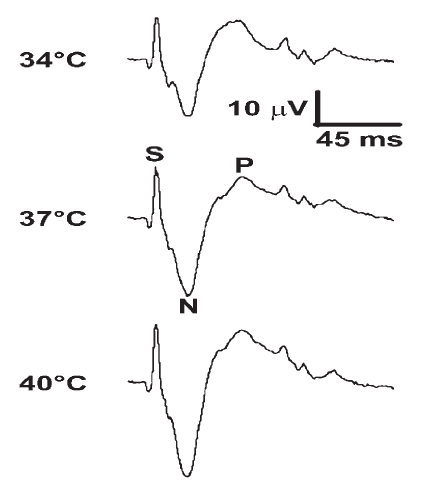 Fig. 1.
Fig. 1.Three single CDPs recorded from rat lumbar (L1) spinal cord elicited by sural nerve electrical stimulation (5 mA, 0.5 ms, 0.05 Hz) from the same animal at 34, 37 and 40$ {}^\circ $C during muzzle HNS. After stimulus artifact (S) the N-wave shows an initial sharp and high amplitude negative deflection followed by a smaller amplitude long duration positive wave (P). Note N-wave amplitude changes associated with core body temperature. Time (ms) and amplitude ($ \mu $V) are the same for all traces.
 Fig. 2.
Fig. 2.Core temperature increase percentage of N-wave amplitude when HNS was applied to tail, contralateral hind paw (CHP), contralateral forepaw (CFP), muzzle and ipsilateral forepaw (IFP). CC Control condition, i.e. recording during sural electrical stimulation without HNS. Label-place of the HNS applies always for the three temperatures (34, 37 and 40$ {}^\circ $C). Black square: tail; empty circle: CHP; dark triangle: CFP; empty square: muzzle; dark circle: IFP. Values are arithmetic mean $ \pm $ S.E.M., analyzed with Kruskal-Wallis and Dunn's tests. $ n = 12$; $ {\ast} p < 0.01$ ; $ {\ast} {\ast} p < 0.001$.
For every body area tested, HNS application reduced N-wave duration (Fig. 3). Increased body temperature significantly decreased this effect. There was a non-significant change for the low temperature range (34-37$ ^{\circ} $C; $ - $2.6%; p >0.05), but a significant reduction in HNS inhibition for the higher range of body temperature (37-40$ ^{\circ} $C; -16.4%; p <0.0039). For all body sites and temperatures tested, HNS applied to the muzzle again showed the most intense reduction of N-wave duration (mean $ - $22.3 $ \pm $ 3.3% [muzzle] vs. $ - $19.2 $ \pm $ 3.4% [all other body sites], p < 0.047). In contrast, HNS applied to the tail showed the lowest, but still significant, reduction on N-wave duration (mean $ - $13.1 $ \pm $ 3.0% vs. $ - $19.2 $ \pm $ 3.4% [all other body sites], p < 0.0081).
 Fig. 3.
Fig. 3.Core temperature increase percentage of N-wave duration when HNS was applied in tail, contralateral hind paw (CHP), contralateral forepaw (CFP), muzzle, or ipsilateral forepaw (IFP). CC Control condition, i.e. recorded during sural electrical stimulation without HNS. Label-place of the HNS always applies for the three temperatures (34, 37 and 40$ {}^\circ $C). Black square: tail; empty circle: CHP; dark triangle: CFP; empty square: muzzle; dark circle: IFP. Arithmetic mean $ \pm $ S.E.M., analyzed with Kruskal-Wallis and Dunn's tests. $ n = $ 12; $ \ast p < 0.01$; $ {\ast}{\ast} p < 0.001$.
Thermo-dependence of N-wave amplitude and duration was different for 34-37$ ^{\circ} $C range when compared with 37- 40$ ^{\circ} $C range while the P-wave did not show such differences, either in amplitude or duration (Table 1) for both temperature ranges.
| Q10 (34-37°C) | Q10 (37-40°C) | M-W U-test | |
|---|---|---|---|
| Amplitude | |||
| N-wave | 1.19 ± 0.04 | 1.58 ± 0.05 | p < 0.001 |
| P-wave | 1.26 ± 0.13 | 1.15 ± 0.15 | p > 0.05 |
| Duration | |||
| N-wave | 1.15 ± 0.04 | 1.93 ± 0.07 | p < 0.001 |
| P-wave | 1.25 ± 0.13 | 1.30 ± 0.15 | p > 0. 05 |
Body temperature changes did not induce significant changes either of amplitude or duration of P-wave (Fig. 4 and Fig. 5) at any of the tested body sites. Evaluation of dispersion (CV) of these values by the Fligner-Killeen test did not show significant changes of P-wave parameters for the three tested temperatures.
 Fig. 4.
Fig. 4.Core temperature did not modify P-wave amplitude when HNS was applied to tail, contralateral hind paw (CHP), contralateral forepaw (CFP), muzzle, or ipsilateral forepaw (IFP). CC Control condition, i.e. recorded during sural electrical stimulation without HNS. Label-place of the HNS always applies for the three temperatures (34, 37 and 40$ {}^\circ $C). Black square: tail; empty circle: CHP; dark triangle: CFP; empty square: muzzle; dark circle: IFP. Arithmetic mean $ \pm $ S.E.M., analyzed with Kruskal-Wallis and Dunn's tests. $ n = $ 12.
 Fig. 5.
Fig. 5.Core temperature did not modify P-wave duration when HNS was applied at tail, contralateral hindpaw (CHP), contralateral forepaw (CFP), muzzle, and ipsilateral forepaw (IFP). CC Control condition, i.e. recording during sural electrical stimulation without HNS. Label-place of the HNS always applies for the three temperatures (34, 37 and 40$ {}^\circ $C). Black square: tail; empty circle: contralateral hindpaw (CHP); dark triangle: contralateral forepaw (CFP); empty square: muzzle; dark circle: ipsilateral forepaw (IFP). Arithmetic mean $ \pm $ S.E.M., Kruskal-Wallis and Dunn's tests. $ n = $ 12.
A non-systematic application of an innocuous constant touch, duration five minutes, at different body parts was not able to induce changes in any CDP wave parameter (not shown).
In agreement with a recent report [11], HNS applied to different body sites activated endogenous pain modulation systems that then exerted an inhibitory effect by reducing N-wave amplitude and duration. This process showed an inverse thermo-dependence, i.e. the inhibitory effect was reduced by increased temperature for both amplitude and duration of N-wave. This is the first report of a temperature dependent reduction of descending pain modulation within a clinical range of body temperature variation (3$ ^{\circ} $C) over the normal temperature ($ \sim $37$ ^{\circ} $C).
The DNIC system has a whole body receptive field [20,21], but not every body site activates the DNIC system with the same intensity [11]. The present report goes further by describing that muzzle and tail were body sites with significantly higher and lower thermo-dependency, respectively, than hind- and fore-paws during HNS. Tissue differences of sensory receptor density at these body areas, i.e. intense trigeminal skin innervation of the muzzle [11,20], trigeminal bilateral brainstem inputs and poly-synaptic connections between trigeminal caudal nuclei and SRD, the main supraspinal structure involved in DNIC [11,20], should be considered to explain a more intense response for muzzle HNS. Reduction of the inhibition of N-wave induced by HNS in the tail was lower than that of other body sites, presumably due to its lower temperature changes, as the location is more environmentally dependent because of its greater distance from the body core. In fact, the tail shows a mean temperature of 25$ ^{\circ} $C [23]. This supports the role of tissue temperature at HNS application sites. Other areas closer to the body core, with full range temperature changes, showed reductions in HNS-induced inhibition i.e. muzzle versus paws, which further reflects the role of local tissue temperature.
Given that at body temperatures lower than 37$ ^{\circ} $C non-significant changes of N-waves during HNS were observed, but that significant N-wave changes were observed over 37$ ^{\circ} $C, could reflect a bias effect of the body temperature reaching the activation threshold of TRP channels, so that he same magnitude of temperature change (3$ ^{\circ} $C) might be subthrehold under 37$ ^{\circ} $C but suprathreshold over 37$ ^{\circ} $C.
Acute pain and inflammatory soup enzymatic activity i.e. cyclooxygenases, phospholipases C and A, and lipoxygenase, among many others, display similar or even greater thermo-dependence [3, 24]. These enzymes increase their activity with temperature as well as the concentration of their products which are components of the inflammatory soup, inducing neuronal excitation. Noxious mechanical stimulation similar to that used in the present study was able to induce increased pain-related enzymatic activation which increased with temperature not only at the stimulus application body sites but also in the spinal dorsal horn [24]. However, this increased activity should be expressed as increased nociception resulting in a higher activity of the descending pain modulation system. In contrast, results support the notion of an attenuated efficiency of this modulation at temperatures over 37$ ^{\circ} $C, hence, providing evidence for the participation of additional mechanisms to those of temperature-dependent enzymatic activation(see discussion below).
Hyperthermia activates the immune system, and immune cells in turn sensitize primary afferent neurons, increasing pain sensation. In fact, the cytokine IL6-COX2-PGE2 axis that drives fever [25] also plays a major role in nociception [20]. It is probable that the acute increase in temperature seen in these experiments, activates fast innate immune responses via phagocytic or cytotoxic effectors [25], but as discussed in the previous section, a locally increased response should increase pain modulation, but rather, results strongly suggest additional mechanisms which in turn attenuate modulation at higher temperatures.
At low temperature, deactivation of the voltage-gated sodium channel is less extreme than the activation process. This results in longer channel opening times and increased membrane depolarization and excitability. At temperatures around 42$ ^{\circ} $C, activation and deactivation processes accelerate, thus reducing both channel opening time and neuronal excitability. These effects tend to reduce activation signals coming into the SDH [26,27,28], but the inhibitory effect is probably less intense for lower temperature increases [28].
The dorsolateral funiculus is the main descending pathway for modulation of SDH. It exerts inhibitory and/or excitatory pain modulation through a balance between serotonergic (5-HT) and noradrenergic ($ \alpha _{2} $) receptor effects, respectively. This results in an algebraic effect [20,29,30], but descending modulation could not evoke a generally antinociceptive tone, instead excitatory and inhibitory systems may simultaneously be active and modulate SDH neuronal responses [31]. During resting conditions, HNS application moves this balance toward noradrenergic-mediated inhibition and away from serotonin-mediated excitation [21,32]. These two descending brain stem systems are interconnected and influence each other [21], resulting in a final balance at the SDH with tonic input from the rostral ventral medulla (RVM) system and phasic input from the subnucleous reticularis dorsalis (SRD) system [20,29]. Results reported here might indicate the expression of a differential thermo-dependence in the descending modulation to the SDH in which, maintaining the same stimulation conditions, increased temperature unbalances the descending effect toward serotonergic, over adrenergic, pathways and resulting in decreased HNS inhibition.
DNIC exerts also a differential control over A$ \beta $-fibers, and A$ \delta $- and C-fibers [33]. In this study, despite the fact that A$ \beta $-fibers were probably more activated at HNS sites due to the use of a noxious clamp than at the test site where the sural nerve was electrically stimulated, the N-wave was reduced at 40$ ^{\circ} $C, suggesting a weakening of DNIC inhibition. Additionally, small diameter dorsal root ganglia neurons show heat-induced depolarization at two different temperatures, above 42$ ^{\circ} $C with a Q$ {}_{10}$=$ 18$ to 27 due to activation of TRPV1 channels, and above 52$ ^{\circ} $C, due to TRPV2 channels. It should be noted that temperatures evaluated in the present study (34, 37 or 40$ ^{\circ} $C) certainly correspond to the animal body core, are far below the noxious threshold, and below the lowest activation threshold for both TRPV1 and TRPV2 channels.
The evaluated SDH response involves three main components: a) Primary afferents and SDH where the main noxious stimulus was applied and processed, e.g. sural nerve and segmental lumbar SDH, b) Distant body site with its primary afferents and corresponding segmental SDH, where HNS was applied and processed, and c) Supraspinal modulation centers and their descending pathways separated into two different functional subsystems. The first exhibits a phasic response at the SRD (DNIC), the second shows a tonic response exerting noradrenergic- and opioid-mediated inhibition and serotonin-mediated excitation of SDH neurons [21,32]. In these results, reduced inhibitory effects by temperature increases may be the result of two possible conditions, the first, increased activity at the noxious stimulation test site, i.e. sural nerve pathway or at the lumbar SDH neuronal population to a constant intensity stimulus. This option seems less probable as the same increased activity should occur at the HNS application sites with the same temperature changes i.e. fore and hind paws. The second situation concerns HNS application sites, especially the tail, where lesser skin temperature changes with the lowest changes in inhibitory effect were observed and enhanced the role of temperature in peripheral tissue for nocifensive responses.
SDH excitability of A$ \delta $ and C-fibers activated by an intense sural nerve electrical stimulation could be enhanced at 40$ ^{\circ} $C due to their expression of TRPV3 and TRPV4 channels. This results in an increased incoming signal from the test site (sural) overcoming a smaller increase in HNS-induced descending inhibition, which at its site was mostly activated through A$ \beta $ fibers with a low expression of TRP channels [12,13]. During increased temperature, the HNS signal to brainstem modulation centers remains relatively the same or shows a small increase, but the signal at the test noxious site shows relatively increased activity, therefore, the balance of descending modulation shifts towards reduction of the inhibitory effect. A shift in the balance of descending modulation towards a reduction of adrenergicopiod-mediated inhibition and/or increased 5HT excitation is also possible. Excluding HNS applied to the tail (vide supra), this misbalance could not be generated by a reduced activity of the sensory process from the HNS site because it shares the same temperature as the test site. As a result of this misbalance at the SDH, increased neuronal excitability, number of activated interneurons, and discharge synchronization to incoming sensory input, are expressed by increased amplitude and duration of the N-wave [7,10,11,16].
In contrast with the N-wave, the P-wave did not show thermal dependence through amplitude and duration changes. It is ruled by various mechanisms and processes more complicated than those governing the N-wave [7, 11, 16, 20]. The P-wave could show changes such as increased coefficient of variation of amplitude or duration [11], but the temperatures tested did not induce such P-wave responses. Complexity of the P-wave origin and its different response as compared with the N-wave requires further investigation. However, one of the reported results could be related to this point when in absence of HNS, changes in body temperature did not modify either N- or P-wave amplitude and duration. This suggests that thermo-dependent effects are mainly exerted through the phasic part of the descending pain modulation system i.e. from the subnucleus reticularis dorsalis system [29,34].
Results of the present study strongly suggest that increased body temperature within the clinical range of about 3$ ^{\circ} $C i.e. fever, could increase pain sensation by reduction of HNS-induced inhibition. This conclusion contributes to understanding of the behavior of the clinically ill and may explain the low pain threshold and hyperalgesia shown by subjects during fever [35, 36]. Additionally, experimental evidence is provided for weakened pain regulation during increased body temperature, which is present in the majority of inflammation cases [36]. Hypothermia could induce the opposite response for fever, decreasing pain sensation by enhancement of HNS-induced inhibition at SDH additional to its enzymatic slow-down effect.
Clinical range changes of temperature could modify pain sensation, moreover, hyperthermia increases nociceptive sensory input to the dorsal horn, and could exacerbate pain sensation in individuals with fever.
The authors thank Dr.Horacio Vanegas and Dr.Enrique Vázquez for their valuable comments and criticism and Dr. Virginia Vivas-O'Connor for her critical reading of the manuscript.
This study was supported by Dirección de investigación y producción intelectual, Facultad de Ciencias de la Salud, Universidad de Carabobo, Valencia, Venezuela (FI-2014-6 to AEZ), and Centro de Estudios Avanzados, Instituto Venezolano de Investigaciones Científicas, Caracas, Venezuela (CEA-2014 to JMG).
All authors declare no conflicts of interest.
