- Academic Editor
-
-
-
Background: Coronavirus Disease 2019 (COVID-19) has risen as a global
threat to public health and can cause both respiratory and multisystemic diseases
in humans. This study aimed to describe the incidence of abnormal liver function
tests (LFTs) in post-COVID-19 pregnant women,
and to explore characteristics of pregnant women with abnormal LFTs.
Methods: This retrospective cohort study comprised 155 pregnant patients
who experienced COVID-19, alongside 76 uninfected pregnant women as a control
group. All participants were randomly selected from the Obstetrics outpatient
clinic at the Affiliated Maternity and Child Health Care Hospital of Nantong
University between December 25 2022 and January 31 2023. Demographic data and
laboratory data were collected, and results were statistically analyzed.
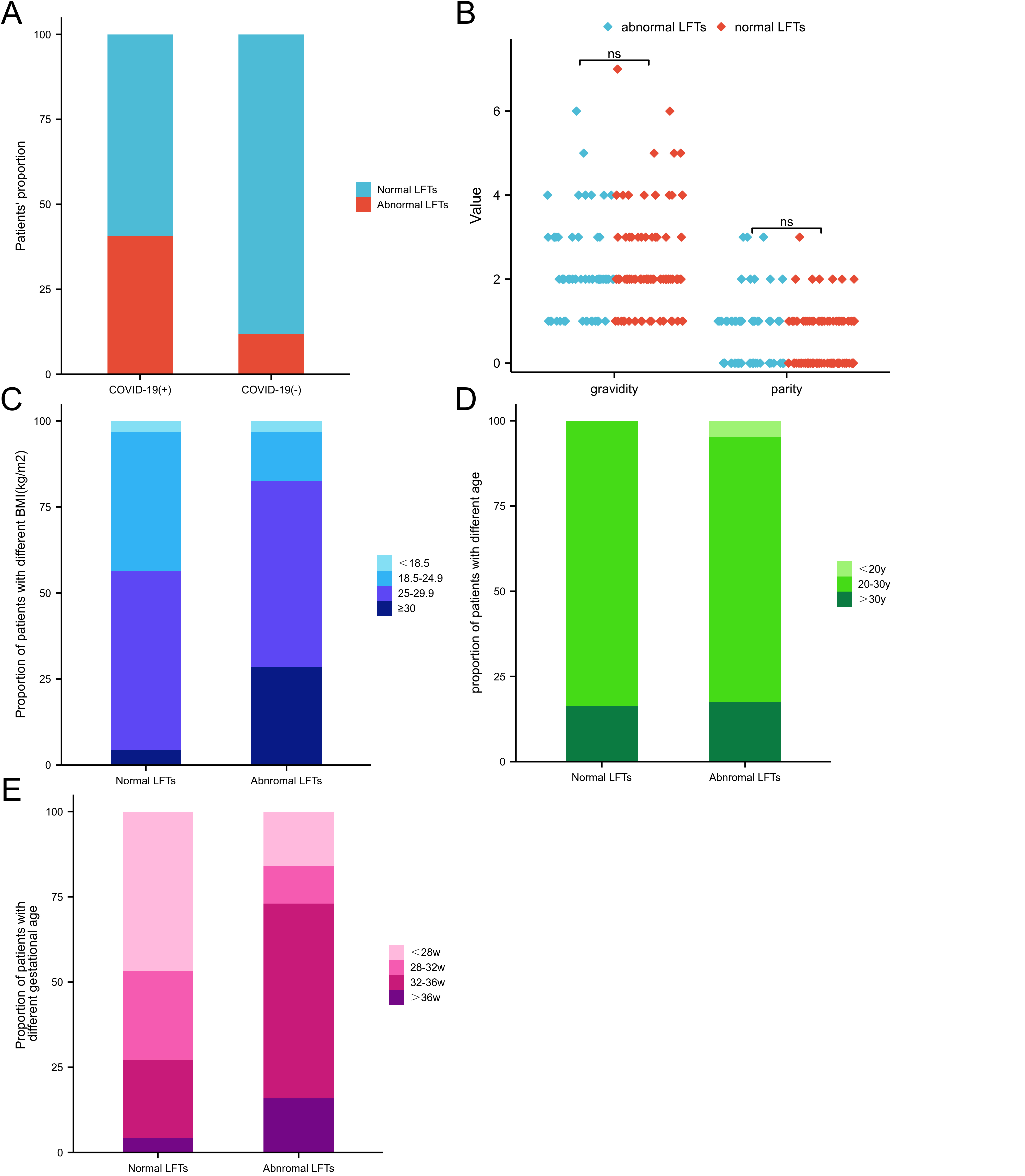
Results: Of the 155 pregnant women who had experienced COVID-19, 63
(40.6%) showed abnormally raised liver enzymes. In the control group, 9 (11.8%)
cases had abnormal LFTs. Differences between the two groups were statistically
significant (p