- Academic Editor
Background: Coronavirus Disease 2019 (COVID-19) has risen as a global
threat to public health and can cause both respiratory and multisystemic diseases
in humans. This study aimed to describe the incidence of abnormal liver function
tests (LFTs) in post-COVID-19 pregnant women,
and to explore characteristics of pregnant women with abnormal LFTs.
Methods: This retrospective cohort study comprised 155 pregnant patients
who experienced COVID-19, alongside 76 uninfected pregnant women as a control
group. All participants were randomly selected from the Obstetrics outpatient
clinic at the Affiliated Maternity and Child Health Care Hospital of Nantong
University between December 25 2022 and January 31 2023. Demographic data and
laboratory data were collected, and results were statistically analyzed.
Results: Of the 155 pregnant women who had experienced COVID-19, 63
(40.6%) showed abnormally raised liver enzymes. In the control group, 9 (11.8%)
cases had abnormal LFTs. Differences between the two groups were statistically
significant (p
Since its outbreak in December 2019, novel coronavirus infection has quickly spread worldwide, emerging as a global health concern. The World Health Organization declared the disease a global pandemic and described it as coronavirus disease 2019 (COVID-19) [1, 2]. The virus continues to mutate in the process of transmission. Data show that to date, more than 1000 variants have been identified worldwide. Since December 2021, with the emergence of the Omicron variant, the global epidemic entered a new stage. Compared to other variants, Omicron has a lower hospitalization and mortality rate which led most countries around the world to issued control measures in early 2022 [3]. On December 7 2022, China adjusted its public health control measures in response to the widespread COVID-19 infections experienced on the mainland.
COVID-19 is considered a multisystem inflammatory disease that mainly involves the respiratory and cardiovascular systems, as well as the hepatobiliary, gastrointestinal, endocrine, and nervous systems [4]. Even after recovering from COVID-19, patients may experience medium- and long-term health effects on organs other than the lungs [5]. It has been reported that 14–76.3% of COVID-19 patients show abnormal liver function tests (LFTs), primarily characterized by elevated levels of serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) [6, 7]. During the early stages of the pandemic, there was limited data available on the impact of post-COVID-19 on pregnant women and their fetuses [8]. Accordingly, our study aimed to describe the incidence of abnormal LFTs in pregnant patients who had experienced and recovered from COVID-19. Additionally, we sought to explore the association between LFTs and the characteristics of pregnant women, and to provide insights for medical management of pregnant patients with COVID-19.
Between December 25 2022, and January 31 2023, a cohort total of 280 randomly selected charts of pregnant women who underwent an antenatal examination in Affiliated Maternity and Child Health Care Hospital of Nantong University were enrolled in this retrospective study. The randomization sequence was generated using a computer-based random number generator. The exclusion criteria were: patients with pre-existing liver disease, gestational hypertensive disorders, and other infections, as well as co-existing chronic diseases, including chronic hypertension, renal disease, collagen vascular disease, and malignant tumor. Patients with incomplete data were also excluded. Of all the eligible participants, 155 were included in the COVID-19 group, the inclusion criteria being those who had experienced COVID-19 without the above exclusion criteria. The COVID-19 positive status was confirmed by real-time polymerase chain reaction (PCR) analysis of nasal swab samples. The control group consisted of 76 patients who had anti-SARS-CoV-2 IgM/IgG antibody titers below the threshold required to indicate that they had contracted COVID-19. The study was approved by the Medical Research Ethics Committee of Affiliated Maternity and Child Health Care Hospital of Nantong University. We collected the following information from the charts of all participants: age, gestational weeks, gravidity, parity, body mass index (BMI), the number of days since COVID-19 was diagnosed, and disease duration (COVID-19 group). We judged the gestational age according to the regular ultrasound examination and confirmed it according to the last menstrual period and a first-trimester sonographic measurement. Patient data confidentiality was upheld throughout the course of the study.
Blood samples were collected at routine outpatient antenatal examination, the detection of serum liver enzymes and anti-SARS-CoV-2 IgM/IgG antibodies were included as part of regular care. Patients with abnormal LFTs had been regularly reviewed. LFTs were measured by commercially available enzyme activity tests kits (ALAT (GPT) FS and ASAT (GOT) FS, DiaSys Diagnostic System, Holzheim, Germany) on a Hitachi 7600 automatic biochemical analyzer (Hitachi 7600, Tokyo, Japan). We obtained peak values of liver enzymes and the days required for liver enzymes to return to normal from the charts of all participants.
Abnormal LFTs were defined by the following standards in our hospital’s
laboratory: ALT
Non-normally distributed variables were presented as medians. The Mann-Whitney U
test was applied to compare the differences of groups. Categorical variables were
expressed as percentages. The difference between categorical variables was
examined with the
Among the 155 pregnant women who contracted COVID-19, 63 (40.6%) were observed
with abnormally raised liver enzymes. Liver injury was seen in 47 patients
(30.3%) (ALT and/or AST more than 3*ULN), and 38 (24.5%) cases had intrahepatic
cholestasis during pregnancy (ICP). In contrast, in the negative control group,
only 9 (11.8%) cases demonstrated abnormal LFTs. The comparison results
indicated that the differences between the two groups were statistically
significant (p
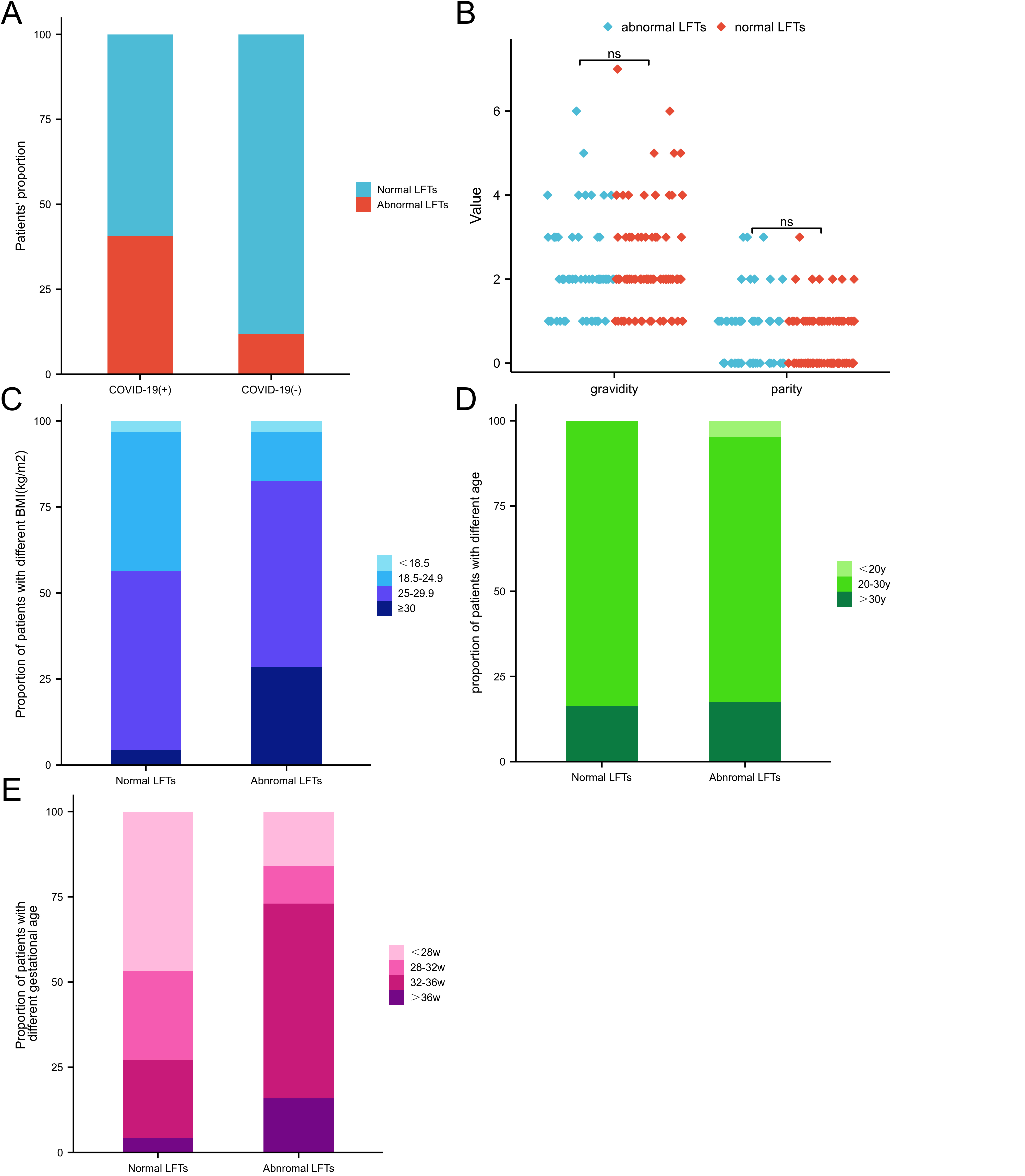 Fig. 1.
Fig. 1.Comparison of the patients’ number of normal and abnormal LFTs between the two groups. Group COVID-19 (+): n = 155, normal LFTs: n = 92 (59.4%), abnormal LFTs: n = 63 (40.6%). Group COVID-19 (–): (n = 76), normal LFTs: n = 67 (88.2%), abnormal LFTs: 9 (11.8%). (A) Distribution of gravidity and parity in normal and abnormal LFTs groups post-COVID-19. (B) Composition of different BMI in normal and abnormal LFT groups post-COVID-19. (C) Composition of different age groups in normal and abnormal LFT groups post-COVID-19. (D) Composition of different gestational week groups in normal and abnormal LFT groups post-COVID-19. (E) The patients’ number in each situation (Fig. 1C,D,E) can be found in Table 3. LFTs, liver function tests; post-COVID-19, post-Coronavirus Disease 2019; BMI, body mass index; ns, no significance.
Out of the 63 patients with abnormal LFTs post-COVID-19, the median serum level of ALT was 175 U/L (range, 51–352 U/L), median AST = 113 U/L (range, 42–329 U/L), and median ALP = 123 U/L (range, 35–250 U/L). Meanwhile, the median TBA was 18.1 µmol/L (range, 1.8–33.5 µmol/L). In this study, we observed that all patients developed abnormal LFTs within the incubation period, which ranged from 7–14 days after COVID-19 infection. The median incubation period was found to be 10 days. Subsequently, the patients’ liver function returned to normal within a range of 4–26 days, with a median value of 12 days (Table 1).
| Min | Max | Median | |
| ALT (U/L) | 51 | 352 | 175 |
| AST (U/L) | 42 | 329 | 113 |
| TBA (µmol/L) | 1.8 | 33.5 | 18.1 |
| ALP (U/L) | 35 | 250 | 123 |
| Incubation period (Days) | 7 | 14 | 10 |
| Disease duration (Days) | 4 | 26 | 12 |
LFTs, liver function tests; ALT, alanine transaminase; AST, aspartate transaminase; TBA, total biliary acid; ALP, alkaline phosphatase.
We first performed univariate analysis on the factors that may affect abnormal
LFTs. The median BMI in the abnormal LFTs group (28.80 kg/m
| Characteristics | Abnormal LFTs | Normal LFTs | p value |
| min–max (median) | min–max (median) | ||
| Age (years) | 19–39 (27) | 23–34 (27) | 0.41 |
| BMI (kg/m |
18.50–39.84 (28.80) | 18.03–33.70 (26.01) | |
| Gestational age (weeks) | 12–37 (34) | 16–37 (29) | |
| Gravidity | 1–5 (2) | 1–7 (2) | 0.60 |
| Parity | 0–3 (1) | 0–3 (0) | 0.19 |
BMI, body mass index.
| Characteristics | Abnormal LFTs (n = 63) | Normal LFTs (n = 92) | p value | |
| Age groups (years) | 0.125 | |||
| 3 | 0 | |||
| 20–30 y | 49 | 77 | ||
| 11 | 15 | |||
| BMI (kg/m |
<0.001 | |||
| 2 | 3 | |||
| 18.5–24.9 | 9 | 37 | ||
| 25.0–29.9 | 34 | 48 | ||
| 18 | 4 | |||
| Gestational age (weeks) | ||||
| 10 | 43 | |||
| 28–32 w | 7 | 24 | ||
| 32–36 w | 36 | 21 | ||
| 10 | 4 | |||
Meanwhile, multivariate analysis was performed by logistic regression analysis.
It was found that gestational age (OR: 1.095 [1.021–1.174]) and BMI (OR: 1.169
[1.059–1.289]) remained a significant independent risk factors for liver injury
(p
| Factor | Outcome | ||||
| Standard Error (SE) | Wald | OR (95% CI) | p value | ||
| Age | –0.001 | 0.056 | 0.000 | 0.999 (0.896–1.115) | 0.993 |
| Gravidity | –0.212 | 0.209 | 1.033 | 0.809 (0.538–1.218) | 0.310 |
| Parity | 0.237 | 0.336 | 0.496 | 1.267 (0.656–2.449) | 0.481 |
| Gestational age | 0.090 | 0.036 | 6.413 | 1.095 (1.021–1.174) | 0.011 |
| BMI | 0.156 | 0.050 | 9.667 | 1.169 (1.059–1.289) | 0.002 |
OR, odds ratio; 95% CI, 95% confidence interval.
In addition to the respiratory system, COVID-19 causes injuries to other organs and systems, such as the gastroenteric system, cardiovascular system, kidneys, and livers [12]. It is now recognized that some patients who have recovered from COVID-19 can experience a variety of persistent symptoms, commonly referred to as ‘long COVID’. Liver injury is indeed one of the most common symptoms observed in patients who have experienced or even recovered from COVID-19. Several studies have reported the incidence of liver injury [13, 14]. The liver plays a vital role in host defense against microbes and is involved in most systemic infections as it receives a dual blood supply from the systemic and portal circulation [15]. The early COVID-19 strain was accompanied by severe symptoms, high mortality, and high probability of abnormal liver function up to 76.3% [16]. The Omicron variant was relatively mild; accordingly, the probability of liver injury decreased. As a special group, there is a scarcity of clinical data on pregnant women with COVID-19.
At present, there are no large sample studies showing that COVID-19 in pregnant
women will lead to a significant increase in liver function, total bile acid and
other indicators, nor have there been clear studies suggesting a causal
relationship between COVID-19 in pregnant women and abnormal liver function.
After China’s epidemic prevention policy was adjusted at the end of 2022, many
pregnant women infected with COVID-19 were admitted to our hospital; a
significant increase in abnormal LFTs was observed. In this study, we excluded
pregnant women with pre-existing liver disease, pregnancy-induced hypertension,
other infections, and co-existing diseases (including chronic hypertension, renal
disease, collagen vascular disease, and malignant tumor). We found that 40.6% of
the COVID-19 group had abnormal liver function, a significantly higher rate than
that of ordinary pregnant women, and the difference was statistically significant
(
In the early period of pandemic, the proportion of liver injuries was high, with
the potential for severe liver damage and even liver failure and death. With the
variation of the virus strain and the popularization of vaccination, the
pathogenicity of the virus decreased significantly. In our study, abnormal liver
function was characterized by mild to moderate elevation of transaminase (ALT,
AST
The underlying mechanism of liver injury in patients with COVID-19 is not fully understood. Current studies have shown that possible mechanisms include direct viral action on hepatocytes or biliary ducts, systemic inflammatory response and cytokine storm, hypoxia state, hypercoagulable state, and drug-induced liver damage [17, 18, 19]. Generally, the result is attributable to multiple factors. First of all, angiotensin converting enzyme 2 (ACE2) has been identified as a functional host receptor for severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2), which is responsible for COVID-19 [20]. The liver is one of the first-line targets of cell injury in COVID-19 through its high expression of ACE2 [21]. However, some researchers contend that liver function abnormality caused by COVID-19 is due to bile duct cell dysfunction rather than hepatocyte injury. Their research shows that the main target of SARS-CoV-2 in the liver is bile duct cells. They found that the expression of ACE2 in hepatocytes was 20 times less than that in bile duct cells [7].
Second, COVID-19 triggers a systemic inflammatory response that progressively reduces peripheral blood lymphocytes and raises inflammatory factors, leading to cytokine storm. Immune-mediated cytokine storm causes the release of a large number of pro-inflammatory cytokines, which can aggravate the non-specific immune inflammatory response of the liver and lead to secondary liver injury [22]. Moreover, COVID-19 can lead to hypoxemia. In turn, ischemia and hypoxia can lead to cell injury. With the reoxygenation of hepatocytes, the rapid recovery of blood flow will lead to metabolic abnormalities, production of reactive oxygen species, inflammation, and cell death [23]. Furthermore, SARS-CoV-2 induces hypercoagulation. Copious data indicate a relationship between hypercoagulation and liver injury in COVID-19 patients [24].
Finally, fever and cough are the main clinical manifestations of COVID-19 patients. Many patients may use antipyretic drugs, such as paracetamol, acetaminophen (APAP), and azithromycin, which are commonly used drugs that may cause liver cell damage. Some patients may experience elevated serum transaminase levels after administration [25]. In light of these facts, pregnancy, as a special period, is characterized by increased pro-inflammatory response, hypercoagulability, and decreased liver blood flow. Therefore, liver damage is more likely to occur under the action of COVID-19.
In the general population, it has been reported that male sex, older age, and higher BMI, are associated with higher risk of abnormal liver function following COVID-19 infection [22]. In our study population of pregnant women, we observed that higher gestational age and higher BMI were associated with a higher risk of developing post-COVID-19 liver injury. However, such risk did not correlate with gravidity, parity, and maternal age. In our study, the median gestational week for liver damage was 34 weeks, which was consistent with the high incidence of ICP during pregnancy. The possible reasons for the above results are as follows. During pregnancy, the estrogen production is significantly increased, which is a well-known risk factor for ICP and liver injury [26]. Moreover, the excretion, detoxification and metabolism of the fetus increase burden of mother’s liver [27]. With the increase of gestational week, the risk of liver injury increases gradually. In addition, obesity impairs metabolism, induces inflammation and eventually lead to liver injury [28].
This study has certain limitations that need to be acknowledged. Firstly, the study design is retrospective, which inherently comes with limitations associated with such observational studies. Relying on the information reported in patient records, we were unable to identify risk factors for all pregnant women with COVID-19. Our study has excluded pregnant women with hypertension or pre-existing liver disease, who might be more prone to suffer abnormal LFTs and may show more severe symptoms [29]. We consider that these excluded criteria could explain why we have a lower incidence of severe liver injury than previous studies. In addition, our follow-up time was relatively short; in the future, we will follow up for a longer time to observe the effects of Long-COVID on liver function.
Pregnant women are at increased risk of liver injury after contracting COVID-19. With the increase of gestational age and BMI, the risk of liver injury increases. For post-COVID-19 pregnant women with greater gestational age or higher BMI, we should consider closely monitoring liver function and be on guard against the occurrence of serious complications. Fortunately, as far as the current prevalent COVID-19 variants are concerned, most of the liver injury is reversible and the liver function damage is mild to moderate. Transient abnormal LFTs do not require excessive medication. Post-COVID-19 liver injury may be the result of virus infection and invasion, inflammatory reaction, hypoxia, hypercoagulable state, and the use of hepatotoxic drugs during treatment. Pregnant women may also terminate pregnancy early because of liver damage or ICP.
Accordingly, appropriate treatment should be taken after the occurrence of liver injury. At the same time, liver function should be closely monitored to prevent more serious liver injury, reduce the incidence of preterm delivery, and improve maternal and fetal outcome. In addition, the treatment process should appropriately simplify the use of drugs, reduce the risk of drug-induced liver injury, and discourage the use of suspicious drugs that cause liver injury in time. Our results should be beneficial for treatment selection and clinical research.
All data generated or analyzed during this study are included in this published article.
XH conceived and designed the study. JZ collected cases and inputted the data. YX performed the literature search and data extraction. LZ analyzed and interpreted the data. YG designed the research drafted the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
All subjects gave their informed consent for inclusion before they participated in the study. The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of Affiliated Maternity and Child Health Care Hospital of Nantong University (approval number: Y2013021).
We would like to express gratitude to all patients who participated in our work. We wish to thank our colleagues who organized the work, especially our leader of department, Yiqian Ding.
This work was supported by the Scientific research project of Nantong Health Committee (QA2021046).
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
