- Academic Editor
†These authors contributed equally.
Background: Postoperative atrial fibrillation (POAF) has long been
associated with poor perioperative outcomes after coronary artery bypass grafting
(CABG). In this study, we aimed to investigate the effect of prolonged POAF
durations on perioperative outcomes of CABG. Methods: This retrospective
cohort study examined CABG patients enrolled at Beijing Anzhen Hospital from
January 2018 to September 2021. We compared patients with POAF durations
The onset of postoperative atrial fibrillation (POAF) is one of the most common complications after cardiac surgery, occurring in approximately 30% of patients who undergo coronary artery bypass grafting (CABG) [1, 2]. Although most POAF is resolved within 24 hours [3], it is still related to the occurrence of many adverse perioperative events [4, 5]. Previous studies have shown that in approximately 25% of POAF patients, sinus rhythm (SR) cannot be restored after discharge [6]. Furthermore, prolonged duration of POAF after cardiac surgery was independently associated with high long-term mortality [7], which indicates that different POAF durations could lead to differing outcomes in CABG patients. However, few studies have been conducted to fully elucidate the precise relationship between POAF duration and perioperative outcomes after CABG.
Thus, to our knowledge, this study is the first to explore, in a large cohort, the effects of prolonged POAF duration on in-hospital mortality, stroke, and other important perioperative outcomes among CABG patients.
In this retrospective study, we consecutively collected a total of 14,932
patients, who underwent isolated CABG at Beijing Anzhen Hospital between January
2018 and September 2021. The exclusion criteria were as follows: History of
atrial fibrillation (AF), non-CABG surgical procedures, missing postoperative
continuous electrocardiography (ECG) and medication records, or being
 Fig. 1.
Fig. 1.Study flowchart. CABG, coronary artery bypass grafting; ECG, electrocardiography; POAF, postoperative atrial fibrillation; PSM, propensity score matching; IPTW, inverse probability of treatment weighting.
POAF was diagnosed using a continuous ECG monitor, whereby it was defined after surgery lasting at least 5 minutes as either newly onset atrial fibrillation or atrial flutter, and treatment was required in accordance with the definition provided by the Society of Thoracic Surgeons (STS) National Database [8]. Patients with POAF were treated with amiodarone, and electrical cardioversion was administered to patients with POAF who developed hemodynamic instability. All patients were first transferred to the ICU postsurgery, where POAF occurrence was continuously monitored during their stay duration, as well as during the first 4 days after being transferred back to the general ward. If POAF was detected, patients were continually monitored until a normal SR returned or they were discharged. The POAF duration was defined as the interval between the first day and the last day that POAF occurred (continuous or intermittent POAF during the monitoring time) [7]. Ultimately, patients were first divided into 2 groups: non-POAF (n = 8244) and POAF (n = 3604). Then, the POAF group was further divided into 2 subgroups based on whether POAF had occurred for longer than 48 hours: POAF durations shorter than 48 hours (n = 2473) and longer than 48 hours (n = 1131). The 48-hour cutoff was based on AF, with that duration considered the nodal point for initiating long-term anticoagulation therapies [9].
After anesthesia, the patients who underwent CABG had access through a sternotomy. Off-pump patients were fully heparinized at the completion of harvesting the conduits, while the heart was stabilized when conducting the grafts using the Octopus tissue stabilizer system (cardiopulmonary bypass was established first in on-pump patients, and the Octopus tissue stabilizer system was not used). After all grafts were performed, proximal anastomosis was performed on the aorta using partial clamping. The heparinized state was reversed using protamine and the chest was closed (on-pump patients were firstly withdrawn from cardiopulmonary bypass, and after the heart resumed beating, the chest was closed).
Patient data were collected using an electronic medical records system,
including demographics, comorbidities, in-hospital outcomes, medications,
preoperative laboratory, echocardiography, and continuous ECG monitoring data.
The primary outcomes measured were in-hospital mortality, stroke, acute
respiratory failure (ARF), acute kidney injury (AKI), and significant
gastrointestinal bleeding (GIB). In-hospital mortality was defined as death from
any cause during hospitalization. Stroke was defined as a permanent neurological
deficit with imaging evidence of cerebral artery occlusion, as diagnosed by
neurologists. ARF was defined as arterial partial O
The normality distribution test for continuous variables was performed using the
Kolmogorov‒Smirnov test. The results are presented as the mean
Analyses were performed by propensity score matching (PSM) and inverse probability of treatment weighting (IPTW) to control the differences in baseline characteristics of patients between the non-POAF group and POAF group and between the group with POAF durations shorter than 48 hours and the group with POAF durations longer than 48 hours to account for selection bias and potential confounding factors in outcome comparisons between the groups. A propensity score for each patient was calculated using multivariable logistic regression. PSM was performed using the 1:1 nearest neighbor matching method and optimal matching with a caliper width of 0.25 standard deviations. Regarding IPTW, the inverse propensity score served as the weight for patients with POAF or POAF durations longer than 48 hours, and the inverse of 1 minus the propensity score served as the weight for patients without POAF or POAF durations shorter than 48 hours.
A restricted cubic spline (RCS) model with four knots (at the 5th, 35th, 65th, and 95th percentiles) and including major confounding variates associated with adverse perioperative outcomes after cardiac surgery, including age, male sex, body mass index (BMI), history of hypertension, diabetes, chronic kidney disease (CKD), stroke and cardiac surgery, operation time and preoperative level of left atrial diameter (LAD), left ventricular ejection fraction (LVEF), creatinine (Cr), creatine kinase MB (CK-MB), troponin I (TnI), and brain natriuretic peptide (BNP), was used to explore the nonlinear dose‒response relationship between POAF duration and each primary outcome. Univariable and multivariable logistic regression models were used to estimate the odds ratios (ORs) and 95% confidence intervals (CIs) of the independent association between POAF or prolonged POAF duration and the occurrence of primary outcomes. PSM, IPTW, and multivariable logistic regression analyses were performed by including the same confounding variables in the RCS. In addition, we used logistic regression analysis to analyze the risk factors that cause a prolonged POAF duration.
Statistical analyses were performed by R software (version 4.2.2, R Foundation
for Statistical Computing, Vienna, Austria). The statistical significance level
was set at two-tailed p
Out of the 11,848 patients included in this study, 75.6% were male. POAF was detected in 3604 (30.4%) patients, which was consistent with previous studies regarding the POAF prevalence among large patient cohorts who had undergone CABG [1, 2, 13]; POAF durations longer than 48 hours were observed in 1131 (31.4%) patients.
With respect to the patient baseline parameters, significant differences were
observed between the non-POAF group and POAF groups for age, male sex, the
prevalence of hypertension, chronic obstructive pulmonary disease (COPD), stroke,
hyperlipidemia, prior cardiac surgery, the rate of on-pump CABG, postoperative
anticoagulation and the level of the operation time, LAD, LVEF, left ventricular
end-diastolic dimension (LVEDD), triglyceride (TG), total cholesterol (TC), Cr,
CK-MB, TnI, K
| Unmatched | 1:1 PSM | IPTW | |||||||
| POAF |
POAF |
p value | POAF |
POAF |
p value | POAF |
POAF |
p value | |
| n | 2473 | 1131 | 1098 | 1098 | 1093.06 | 1096.53 | |||
| Age, years | 65 (59, 70) | 66 (60, 71) | 66 (60, 71) | 66 (60, 71) | 0.724 | 66 (60, 71) | 66 (60, 71) | 0.996 | |
| Male (%) | 1930 (78) | 898 (79.4) | 0.381 | 862 (78.5) | 876 (79.8) | 0.495 | 855.3 (78.3) | 874 (79.7) | 0.336 |
| BMI, kg/m |
25.8 (23.9, 27.5) | 25.8 (24.2, 27.6) | 0.367 | 25.8 (24.1, 27.4) | 25.8 (24.2, 27.6) | 0.424 | 25.8 (23.9, 27.4) | 25.8 (24.2, 27.6) | 0.236 |
| Hypertension (%) | 1580 (63.9) | 708 (62.6) | 0.478 | 711 (64.8) | 685 (62.4) | 0.268 | 696.7 (63.7) | 685.8 (62.5) | 0.5 |
| Diabetes (%) | 954 (38.6) | 466 (41.2) | 0.144 | 405 (36.9) | 449 (40.9) | 0.06 | 424.2 (38.8) | 448.6 (40.9) | 0.246 |
| COPD (%) | 61 (2.5) | 31 (2.7) | 0.711 | 25 (2.3) | 28 (2.6) | 0.89 | 28.6 (2.6) | 28.4 (2.6) | 0.973 |
| Hyperlipidemia (%) | 1429 (57.8) | 630 (55.7) | 0.256 | 625 (56.9) | 616 (56.1) | 0.731 | 628.9 (57.5) | 612.9 (55.9) | 0.369 |
| CKD (%) | 66 (2.7) | 54 (4.8) | 0.002* | 43 (3.9) | 49 (4.5) | 0.594 | 44.4 (4.1) | 45.2 (4.1) | 0.944 |
| PCI history (%) | 247 (10) | 143 (12.6) | 0.02* | 140 (12.8) | 136 (12.4) | 0.847 | 134.6 (12.3) | 136 (12.4) | 0.946 |
| Stroke history (%) | 389 (15.7) | 184 (16.3) | 0.718 | 177 (16.1) | 179 (16.3) | 0.954 | 178.9 (16.4) | 178.9 (16.3) | 0.971 |
| Cardiac surgery history (%) | 29 (1.2) | 12 (1.1) | 0.901 | 16 (1.5) | 11 (1.0) | 0.439 | 11.8 (1.1) | 11.6 (1.1) | 0.962 |
| On-pump CABG (%) | 197 (8) | 138 (12.2) | 122 (11.1) | 121 (11.0) | 1 | 123.7 (11.3) | 123.8 (11.3) | 0.982 | |
| Operation time, h | 4 (4, 5) | 4 (4, 5) | 0.009* | 4 (4, 5) | 4 (4, 5) | 0.782 | 4 (4, 5) | 4 (4, 5) | 0.788 |
| Anticoagulation (%) | 48 (1.9) | 38 (3.4) | 0.013* | 26 (2.4) | 38 (3.5) | 0.164 | 26.1 (2.4) | 36.8 (3.4) | 0.13 |
| Antiplatelet (%) | 2400 (97.1) | 1094 (96.7) | 0.632 | 1066 (97.2) | 1068 (97.3) | 0.997 | 1054.9 (96.6) | 1064.3 (97.1) | 0.485 |
| Electrical cardioversion (%) | 135 (5.5) | 68 (6.0) | 0.504 | 61 (5.6) | 65 (5.9) | 0.714 | 60.1 (5.5) | 63.8 (5.8) | 0.727 |
| POAF duration (h) | 16 (8, 21) | 74 (60, 89) | 16 (8, 21) | 74 (60, 89) | 16 (8, 21) | 74 (60, 89) | |||
| Preoperative laboratory data | |||||||||
| TG, mmol/L | 1.6 (1.15, 1.85) | 1.52 (1.07, 1.78) | 1.56 (1.08, 1.78) | 1.52 (1.07, 1.78) | 0.333 | 1.51 (1.07, 1.76) | 1.50 (1.05, 1.77) | 0.608 | |
| TC, mmol/L | 3.96 (3.38, 4.35) | 3.96 (3.31, 4.3) | 3.96 (3.29, 4.21) | 3.94 (3.26, 4.26) | 0.975 | 3.96 (3.31, 4.28) | 3.94 (3.26, 4.26) | 0.469 | |
| Cr, µmol/L | 69 (59, 81.1) | 73 (61.8, 86.8) | 74.7 (62.3, 88.55) | 74.7 (63.5, 90.88) | 0.23 | 74.3 (62.12, 87.89) | 74.62 (63.25, 90.75) | 0.112 | |
| UA, µmol/L | 334.2 (287.2, 376.1) | 334.2 (282.6, 381.2) | 0.747 | 334.2 (289.9, 382.8) | 334.2 (282.5, 380.3) | 0.37 | 334.2 (289.5, 379.7) | 334.2 (282.5, 380.8) | 0.426 |
| K |
4.08 (3.83, 4.32) | 4.1 (3.87, 4.39) | 0.002* | 4.1 (3.87, 4.38) | 4.1 (3.86, 4.38) | 0.768 | 4.09 (3.85, 4.36) | 4.1 (3.86, 4.38) | 0.605 |
| Ca |
2.21 (2.04, 2.33) | 2.2 (2.03, 2.31) | 0.01* | 2.19 (2.01, 2.31) | 2.2 (2.03, 2.31) | 0.636 | 2.19 (2.02, 2.31) | 2.2 (2.03, 2.31) | 0.934 |
| Mg |
0.88 (0.82, 0.94) | 0.89 (0.84, 0.95) | 0.001* | 0.89 (0.83, 0.95) | 0.89 (0.84, 0.95) | 0.747 | 0.89 (0.83, 0.95) | 0.89 (0.84, 0.95) | 0.827 |
| CK-MB, ng/mL | 3.1 (1.7, 6.4) | 3.1 (1.7, 6.8) | 0.469 | 3.3 (1.9, 7.2) | 3 (1.7, 6.8) | 0.115 | 3.3 (1.8, 7.1) | 3 (1.7, 6.74) | 0.159 |
| TnI, pg/mL | 0.18 (0.07, 0.51) | 0.2 (0.06, 0.64) | 0.086 | 0.19 (0.07, 0.58) | 0.19 (0.06, 0.62) | 0.978 | 0.2 (0.07, 0.61) | 0.19 (0.06, 0.61) | 0.688 |
| Mb, ng/mL | 176.9 (40.7, 303) | 175.7 (40.5, 306.9) | 0.991 | 184.9 (45.5, 312.5) | 173.9 (38.9, 302.8) | 0.222 | 181.7 (44.3, 310.1) | 175.4 (39.5, 306) | 0.436 |
| BNP, pg/mL | 205 (87, 338) | 265 (105.5, 474.5) | 242 (106.25, 410) | 257 (103, 451) | 0.361 | 248 (109, 414.26) | 255 (103, 450) | 0.697 | |
| Preoperative echocardiographic data | |||||||||
| LAD, mm | 36.66 (35, 38) | 36.66 (35, 39) | 36.66 (36, 40) | 36.66 (36, 40) | 0.379 | 36.66 (36, 40) | 36.66 (36, 40) | 0.282 | |
| LVEF, % | 59.43 (58, 65) | 59.43 (56, 63) | 59.43 (55, 63) | 59.43 (55, 63) | 0.984 | 59.43 (55, 63) | 59.43 (55, 63) | 0.569 | |
| E/A ratio | 0.84 (0.68, 0.85) | 0.85 (0.67, 0.85) | 0.454 | 0.85 (0.66, 0.85) | 0.85 (0.66, 0.85) | 0.637 | 0.83 (0.67, 0.85) | 0.85 (0.66, 0.85) | 0.661 |
| LVEDD, mm | 32.34 (29, 33) | 32.34 (30, 34) | 32.34 (30, 35) | 32.34 (30, 35) | 0.66 | 32.34 (30, 35) | 32.34 (30, 35) | 0.551 | |
Data are presented as median (25th–75th percentiles) or n (%). *, there were
significant differences between the POAF
We compared the primary outcomes between the non-POAF and POAF groups and found that after applying PSM and IPTW adjustments the in-hospital mortality, ARF, AKI, and significant GIB were all significantly higher among POAF patients than among non-POAF patients (Supplementary Table 2). These outcomes were determined to be independently associated with POAF by logistic regression analyses (Supplementary Table 3). However, even though the stroke incidence was higher among POAF compared to non-POAF, no statistically significant difference was present, nor was stroke occurrence associated with POAF in the logistic regression analyses. In terms of secondary outcomes, we also found that POAF patients had longer LOS and ICU durations.
To determine whether POAF duration affected the prognosis of postoperative CABG
patients, we compared the primary outcomes between the groups with POAF durations
longer than 48 hours and POAF durations shorter than 48 hours. For the primary
outcomes and prior to applying PSM and IPTW adjustments, we found no significant
difference in the incidence of stroke (1.9% vs. 1.3%, p = 0.147) in
patients with POAF durations longer than 48 hours compared to the controls.
However, there were higher rates of in-hospital mortality, ARF, AKI, and
significant GIB in the patients with POAF durations longer than 48 hours (all
p
| Unadjusted | Matched | Weighted | |||||||
| POAF |
POAF |
p value | POAF |
POAF |
p value | POAF |
POAF |
p value | |
| In-hospital mortality (%) | 38 (1.5) | 41 (3.6) | 27 (2.5) | 34 (3.1) | 0.436 | 27.7 (2.5) | 35.3 (3.3) | 0.301 | |
| Stroke (%) | 31 (1.3) | 22 (1.9) | 0.147 | 14 (1.3) | 22 (2.0) | 0.239 | 15.5 (1.4) | 21.3 (1.9) | 0.275 |
| ARF (%) | 14 (0.6) | 21 (1.9) | 6 (0.5) | 20 (1.8) | 0.01* | 7.9 (0.7) | 19.6 (1.8) | 0.011* | |
| AKI (%) | 35 (1.4) | 51 (4.5) | 25 (2.3) | 42 (3.8) | 0.047* | 24.9 (2.3) | 40.8 (3.7) | 0.032* | |
| Significant GIB (%) | 17 (0.7) | 27 (2.4) | 10 (0.9) | 25 (2.3) | 0.017* | 11.2 (1.0) | 23.1 (2.1) | 0.023* | |
| Postoperative LOS, days | 7 (6, 9) | 8 (7, 12) | 7 (6, 9) | 8 (7, 12) | 7 (6, 9) | 8 (7, 12) | |||
| ICU duration, hours | 21 (17, 61) | 27 (19.8, 89.5) | 21.9 (17, 65) | 26.4 (19.8, 87.8) | 21.8 (17.8, 65.94) | 26 (19.8, 87.8) | |||
Data are presented as median (25th–75th percentiles) or n (%). *, there were
significant differences between the POAF
To further elucidate the relationship between POAF duration and the primary
outcomes, RCS analyses were conducted, whereby a linear relationship was present
between POAF duration and in-hospital mortality, stroke, and ARF (all p
for nonlinearity
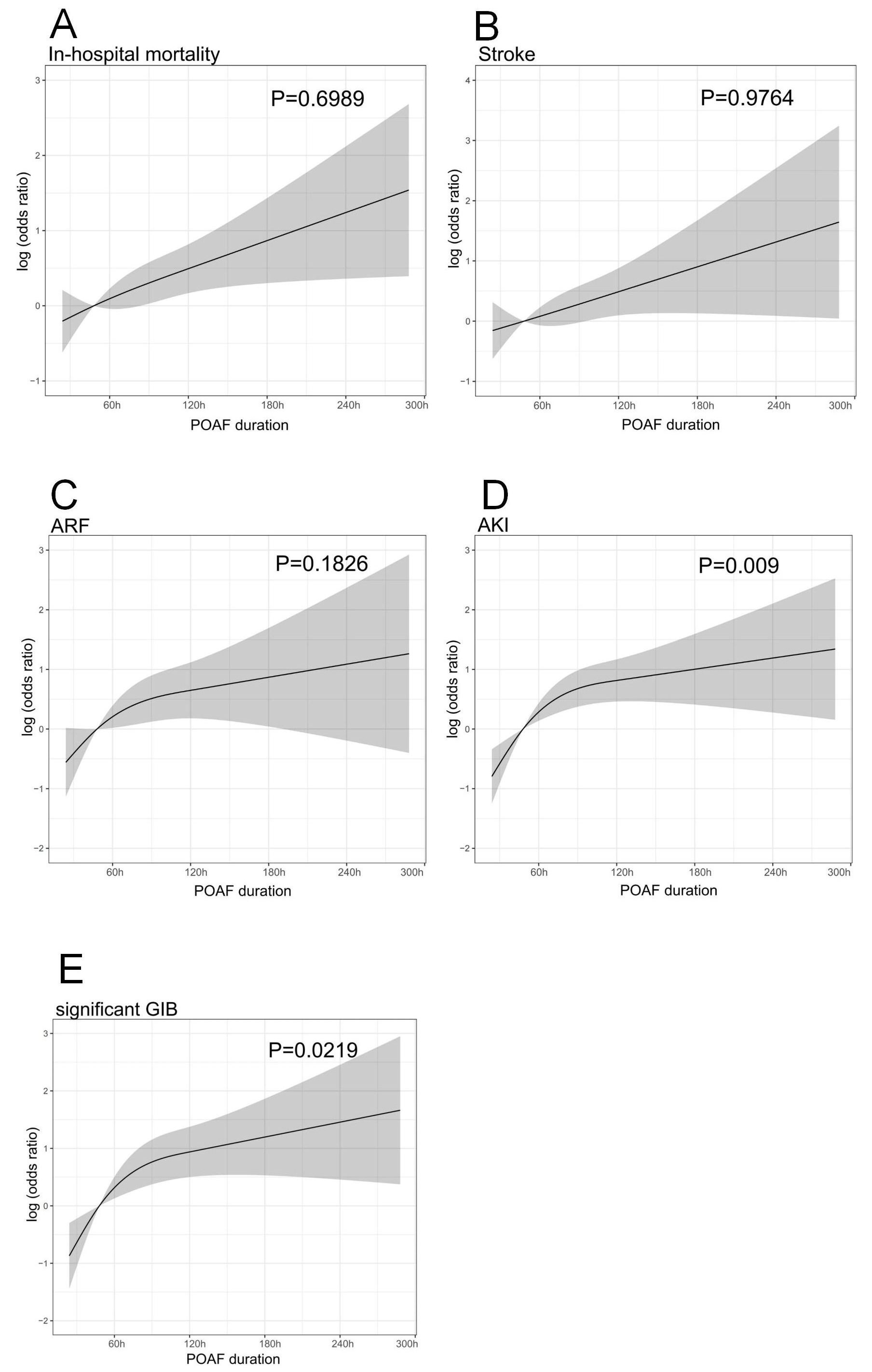 Fig. 2.
Fig. 2.Restricted cubic spline plots of associations between POAF
duration and postoperative complications. (A) In-hospital mortality, (B) stroke,
(C) acute respiratory failure (ARF), (D) acute kidney injury (AKI), (E)
significant gastrointestinal bleeding (GIB). The solid line and shaded area
represent the log-transformed odds ratios and corresponding 95% confidence
intervals. A linear relationship was present between POAF duration with
in-hospital mortality, stroke, and ARF (all p for non-linearity
We also conducted logistic regression analyses, in which a longer POAF duration was not independently associated with in-hospital death (adjusted OR: 1.60, 95% CI: 0.97–2.65, p = 0.068) and stroke (adjusted OR: 1.28, 95% CI: 0.71–2.34, p = 0.414) but was independently associated with ARF (adjusted OR: 2.96, 95% CI: 1.47–6.09, p = 0.003), AKI (adjusted OR: 2.37, 95% CI: 1.42–3.99, p = 0.001), and significant GIB (adjusted OR: 2.60, 95% CI: 1.38–5.03, p = 0.004). Moreover, the results were still valid even after adjusting for PSM and IPTW (Table 3). Furthermore, the POAF group with a duration longer than 48 hours had a longer postoperative LOS and ICU duration (Table 2).
| Methods | In-hospital mortality | Stroke | ARF | AKI | Significant GIB | |||||
| OR (95%CI) | p value | OR (95%CI) | p value | OR (95%CI) | p value | OR (95%CI) | p value | OR (95%CI) | p value | |
| Unadjusted | 2.29 (1.46–3.58) | 1.56 (0.89–2.70) | 0.112 | 3.32 (1.70–6.70) | 0.001 | 3.29 (2.14–5.12) | 3.53 (1.94–6.63) | |||
| Matched | 1.27 (0.76–2.13) | 0.364 | 1.58 (0.81–3.18) | 0.182 | 3.38 (1.43–9.26) | 0.009 | 1.71 (1.04–2.86) | 0.037 | 2.53 (1.25–5.56) | 0.014 |
| Weighted | 1.28 (0.87–1.91) | 0.218 | 1.38 (0.83–2.33) | 0.220 | 2.49 (1.34–4.93) | 0.006 | 1.66 (1.12–2.48) | 0.012 | 2.08 (1.20–3.72) | 0.011 |
| Multivariable | 1.60 (0.97–2.65) | 0.068 | 1.28 (0.71–2.34) | 0.414 | 2.96 (1.47–6.09) | 0.003 | 2.37 (1.42–3.99) | 0.001 | 2.60 (1.38–5.03) | 0.004 |
ARF, acute respiratory failure; AKI, acute kidney failure; GIB, gastrointestinal bleeding; POAF, postoperative atrial fibrillation; OR. odds ratio.
To control the bias in the effect of anticoagulation and antiplatelet therapy on bleeding/ischemic events, we further included postoperative anticoagulation and antiplatelet therapy (only records of postoperative anticoagulation and antiplatelet regimens prior to stroke or significant GIB occurrences), applying the same confounding variables in RCS into the multivariable logistic regression of stroke and significant GIB. The association between prolonged POAF duration and stroke (adjusted OR: 1.23, 95% CI: 0.67–2.25, p = 0.497) and significant GIB (adjusted OR: 2.61, 95% CI: 1.38–5.05, p = 0.003) remained consistent with the previous multivariable logistic regression analyses (stroke: adjusted OR: 1.28, 95% CI: 0.71–2.34, p = 0.414; significant GIB: adjusted OR: 2.60, 95% CI: 1.38–5.03, p = 0.004), after including postoperative anticoagulation and antiplatelet therapy as confounding variables in the logistic regression analyses.
After including POAF risk factors, such as age, male sex, BMI, hypertension,
diabetes, LAD, LVEF, CK-MB, K
POAF is a common complication after CABG; however, the impact of POAF duration
on the occurrence of other postsurgical complications has remained largely
unexamined. To the best of our knowledge, this is the first study to focus on the
impact of prolonged POAF duration on perioperative outcomes among CABG patients.
Our findings suggest that POAF is associated with increased in-hospital
mortality, ARF, AKI, and significant GIB compared to non-POAF. Additionally, POAF
durations longer than 48 hours were independently associated with postoperative
ARF, AKI, and significant GIB but not with in-hospital mortality or stroke.
Patients with POAF durations
Many studies have confirmed the correlation between POAF and in-hospital mortality and many adverse perioperative outcomes, such as perioperative stroke and perioperative acute renal failure [4, 5], which is consistent with our observation. The long-term follow-up results showed that POAF is significantly associated with the development of subsequent AF [14, 15] and an adverse long-term prognosis [4, 16, 17, 18]. It is generally recognized that POAF possesses a brief and self-limited time course [19]. However, some studies have shown that not all POAF will disappear in a short time [6, 20]. Although limited studies have focused on the impact of the course of POAF on prognosis, which has led to clinicians having blind spots in the treatment of patients with POAF with delayed reversion to SR. Previous studies have shown that postoperative POAF durations longer than 48 hours or POAF recurrent events more than twice after cardiac surgery are independently associated with reduced long-term survival [7, 21]. Rezk et al. [22] showed that POAF patients with electrical cardioversion or sustained AF were independently associated with an increased long-term risk of heart failure but not with an increased long-term risk of death, thromboembolic complications, or bleeding. These results of poor prognosis show that we should not only focus on the occurrence or absence of POAF but also regard them as a ”continuous variable” to project further analysis.
However, these studies mostly focused on long-term prognoses, which may have the disadvantage of the underlying reasons behind their differences being obscured by the accumulation of confounding factors over time. Although this disadvantage is mitigated in our study by focusing on short-term perioperative prognoses, the greater capacity to identify underlying risk factors for adverse events is more critical for perioperative cardiac surgical management. This is due to this stage being associated with the highest postoperative mortality and complication occurrence rates. Thus, minimizing perioperative adverse event risk is a crucial concern. Although Sigurdsson et al. [7] mentioned that prolonged POAF durations increased postoperative LOS and ICU duration in patients undergoing cardiac surgery, the study did not further analyze other perioperative endpoints. In the analysis of in-hospital outcomes by patients with prolonged POAF durations, a study of ICU patients, excluding cardiac surgery, showed that patients with new-onset AF had no significant difference or in-hospital death or stroke compared to the control, although there was a time-dependent association between AF duration and hospital mortality [23]. One study also highlighted that new-onset AF lasting more than 6 hours in ICU patients is associated with in-hospital death and stroke [24].
Anticoagulation regimens lasting for at least 3 weeks prior to and 4 weeks after cardioversion have long been recommended for AF durations longer than 48 hours to reduce stroke and systemic embolism risks [9]. Although anticoagulation of POAF is recommended in all guidelines, the class of recommendation varies. The American Heart Association/American College of Cardiology/Heart Rhythm Society (AHA/ACC/HRS) and European Society of Cardiology (ESC) guidelines do not specify a threshold duration time for initiating treatment [25, 26], whereas the Canadian Cardiovascular Society (CCS) recommends anticoagulation for POAF lasting more than 72 hours [27], whereas the same time point is 48 hours in the European Association for Cardio-Thoracic Surgery (EACTS) guidelines [28]. Furthermore, there is a lack of convincing evidence to support anticoagulation at these time points in POAF. This is not helped by the presence of polarized results regarding the usage of anticoagulation regimens for POAF patients, in which multiple studies have indicated that such patients do not benefit from these regimens due to increased bleeding risk [29, 30]. This makes the anticoagulation regimens of POAF controversial in clinical practice, in part because the effect of a prolonged duration of POAF on prognosis is unknown. In this study, we evaluated the effect of POAF lasting more than 48 hours on in-hospital stroke. Although the stroke incidence was higher in patients with POAF durations longer than 48 hours, the difference was not statistically significant. Considering the high risk of anticoagulation-related bleeding, we recommend that the benefits of routinely starting anticoagulation after POAF should be carefully weighed, particularly among those patients with low CHA2DS2-VASc scores.
Despite the current focus on the risk of thromboembolism and the indication of anticoagulation therapy for prolonged POAF, other perioperative problems caused by prolonged POAF leave much room for further exploration. We found that prolonged POAF duration is independently associated with perioperative ARF, AKI, and significant GIB, thereby suggesting that it was a noteworthy factor behind poorer perioperative outcomes among CABG patients. We speculate that persistent POAF may cause a prolonged period of reduced cardiac ejection, which causes a continuous worsening of cardiac function that causes the blood pressure to lower, making the perfusion of peripheral organs insufficient. Prolonged POAF may be associated with higher sympathetic excitation, which causes oxygen depletion and metabolic abnormalities in the body. In addition, prolonged POAF may increase the level of inflammatory response in the body, and the release of large amounts of harmful inflammatory factors may increase tissue damage and cause organ dysfunction. However, the above speculations need to be further verified by experiments.
Based on these findings and explorations, we suggest that the detection of POAF should be intensified and that once POAF is detected, proactive electrical cardioversion may be beneficial in improving the patient’s prognosis. In addition, several recent studies focusing on improved surgical approaches and intraoperative management to reduce the incidence of POAF have shown promising results, such as a reduction in retained blood into the pericardial sac and the use of Del Nido cardioplegia, which may represent a new direction in the treatment of POAF [31, 32, 33].
We found that POAF occurrence among CABG patients was associated with increased in-hospital mortality, ARF, AKI, significant GIB, and longer postoperative LOS and ICU durations. All of these patient outcomes, except for increased in-hospital mortality and stroke rate, were also linked to a POAF duration longer than 48 hours, compared to a duration shorter than 48 hours. Overall, the occurrence of poorer patient outcomes was higher among patients with longer POAF; therefore, postoperative monitoring of POAF and positive intervention after detection may be more helpful in optimizing post-CABG patient outcomes.
In terms of monitoring POAF, not every patient was monitored until discharge, which leaves a proportion of patients with delayed onset of POAF who may not have been identified. The STS database definition of POAF has now been updated, and due to the early design of this study, the new criteria were not used to diagnose patients, which may have caused an overestimation in the number of patients with POAF. Given the intermittent nature of some POAF cases and its reoccurrence after conversion of the sinus rate with antiarrhythmic drugs, the exact duration of POAF could not be clearly recorded; therefore, the study design referring to previous studies used the time of last occurrence minus the time of first occurrence as the duration of POAF, which may lead to an overestimation of the duration. Limited by the characteristics of retrospective studies, some important variables, such as the use of postoperative vasoactive drugs and intraoperative variables were not counted in this study; however, studying the above indicators may provide new insights to explain the relationship between POAF and clinical outcomes.
The datasets generated and/or analyzed during the current study are not publicly available due to the nature of this research, participants of this study did not agree for their data to be shared publicly but are available from the corresponding author on reasonable request.
HQ and XY conception designed the research study. HQ and ZP performed the research. XY and KH provided study data. HQ and EX analyzed the data. HQ wrote the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
This study was approved by the Ethics Committee of Beijing Anzhen Hospital, Capital Medical University (ethics approval number: 2023131X). Written informed consent was obtained from all patients. All patients included agreed to participate in the study.
We thank Beijing Anzhen Hospital for data support.
This research was funded by the Beijing Natural Science Foundation, grant number No.7222049.
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
