- Academic Editors
Background: Studies reporting the status of coronary microvascular
function in the infarct-related artery (IRA) after primary percutaneous coronary
intervention (PCI) remain limited. This study utilized the coronary
angiography-derived index of microcirculatory resistance (caIMR) to assess
coronary microvascular function in patients with ST-segment elevation myocardial
infarction (STEMI) undergoing primary PCI. Methods: We used the
FlashAngio system to measure the caIMR after primary PCI in 157 patients with
STEMI. The primary endpoint was the occurrence of a major adverse cardiovascular
event (MACE), defined as a composite endpoint encompassing cardiac mortality,
target vessel revascularization, and rehospitalization due to congestive heart
failure (CHF), myocardial infarction (MI), or angina. Results:
Approximately 30% of patients diagnosed with STEMI and who experienced
successful primary PCI during the study period had a caIMR in the IRA of
Primary percutaneous coronary intervention (PCI) remains a standard therapy for ST-segment elevation myocardial infarction (STEMI) patients. However, inadequate myocardial tissue reperfusion can still be observed, despite the success in restoring the epicardial coronary blood flow in the infarct-related artery (IRA). This suboptimal reperfusion could result from coronary microcirculatory injury or dysfunction associated with adverse cardiovascular events [1, 2, 3]. To better describe the multiple pathological mechanisms during myocardial reperfusion, the term coronary microvascular dysfunction (CMVD) has been used in patients with STEMI undergoing primary PCI.
By using a traditional pressure wire and thermodilution technique, measuring the index of microcirculatory resistance (IMR) is currently regarded as the reference standard for assessing the coronary microcirculation status [4, 5]. Pressure‒temperature wire-derived IMR demonstrates high reproducibility and specificity and is not influenced by epicardial stenosis severity or variations in hemodynamic conditions [6]. However, despite being a proven reliable method for assessing microvascular function, pressure–temperature wire-based IMR is not readily available in primary PCI cases owing to its invasive nature.
Alternatively, as an emerging technique for evaluating microvascular function, coronary angiography-derived IMR (caIMR) does not rely on pressure–temperature wires. Previous studies have demonstrated that the pressure‒temperature wire-free method is comparable to pressure‒temperature wire-based IMR, with comparable accuracy, and has been accepted as a widely adopted noninvasive physiological assessment of microvascular function [7, 8, 9]. Studies reporting the status of coronary microvascular function in the IRA after primary PCI are limited. Thus, we aimed to investigate the coronary microvascular function indicated by caIMR and its prognostic implications in patients with STEMI undergoing primary PCI.
Patients with STEMI admitted to the Beijing Hospital Catheterization Room for
primary PCI from January 2020 to December 2022 were prospectively selected. This
study was authorized by the institutional ethics committee and carried out in
accordance with the principles of the Declaration of Helsinki. STEMI was defined
as persistent chest pain lasting for at least half an hour, accompanied by
ST-segment elevation of more than 1 mm in 2 or more adjacent leads. Primary PCI
was conducted using standard procedures, and the selection of additional
interventions (such as manual thrombectomy or glycoprotein IIb/IIIa inhibitors),
while stent placement techniques were determined by the treating operator. All
patients were treated with a loading dose of aspirin 100–300 mg and clopidogrel
300 mg or ticagrelor 180 mg. Anticoagulation therapy was administered during the
primary PCI procedure with weight-adjusted unfractionated heparin or bivalirudin.
An automated injector was used during the coronary angiogram procedure. The
choice of postprocedural anticoagulation, including low molecular weight heparin
and fondaparinux, was at the discretion of the operator and according to the
thrombus burden and the risk of stent thrombosis. Multivessel disease (MVD) was
defined as stenosis
CaIMR analysis was performed using the FlashAngio system (Rainmed Ltd., Suzhou,
China, Fig. 1). First, a three-dimensional mesh was reconstructed in the target
artery using two coronary angiographic projections without overlapping and
separated by a minimum 30° angle. Second, aortic pressure was measured
using a Flash pressure transducer. Third, several parameters were estimated using
computational pressure–fluid dynamics, as previously verified [10]. Hyperemic Pa
(Pa
 Fig. 1.
Fig. 1.Representative cases of STEMI with caIMR measurement after primary PCI. PCI, percutaneous coronary intervention; LAD, left anterior descending artery; LCX, left circumflex; caIMR, coronary angiography-derived index of microcirculatory resistance; STEMI, ST-segment elevation myocardial infarction; caFFR, coronary angiography-derived index of microcirculatory resistance.
L is a nondimensional constant used to represent the distance measured from the
inlet to the distal artery. V
CaIMR was calculated after finalizing the PCI in the IRAs or reference vessels. Reference vessels were designated as nonchronic total occlusion vessels. In patients with severe coronary stenosis, Yong’s formula was used to adjust the caIMR, which accounted for the potential influence of the collateral flow-induced wedge pressure on the caIMR in the presence of substantial epicardial stenosis [13]. Two independent operators were blinded to the clinical information of the patients when performing the measurements. An agreement was reached by consensus when inconsistencies occurred.
The prespecified primary endpoint was the occurrence of major adverse
cardiovascular events (MACEs) at 3 months and 1 year. MACEs were defined as a
composite of cardiac death, target vessel revascularization, rehospitalization
due to congestive heart failure (CHF), myocardial infarction (MI), or angina.
Follow-up was performed through clinic visits, medical record reviews, and phone
contact. Survival free of MACEs = (total amount of patients – number of patients
with MACEs/total amount of patients)
Continuous variables and categorical variables were expressed as mean
A total of 194 patients with STEMI who underwent primary PCI were enrolled
during the study period. A total of 37 patients were excluded: 2 presented with
cardiogenic shock, 5 underwent failed primary PCI, and 30 had poor coronary
angiography images and an insufficient angiography view for measurement. Thus,
157 patients were included in the final analysis (Fig. 2). The mean age of the
study population was 62.8
 Fig. 2.
Fig. 2.Study flowchart. PCI, percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction; IRA, infarct-related artery; caIMR, coronary angiography-derived index of microcirculatory resistance.
| Overall | caIMR |
caIMR |
p value | ||
| n = 157 | n = 110 | n = 47 | |||
| Age (years) | 62.8 |
62.9 |
62.6 |
0.93 | |
| Male | 120 (76.4) | 80 (72.7) | 40 (85.1) | 0.14 | |
| BMI (kg/m |
25.4 |
25.1 |
26.1 |
0.16 | |
| Current smoker | 73 (46.5) | 51 (46.4) | 22 (46.8) | 1.00 | |
| Hypertension | 95 (60.5) | 63 (57.3) | 32 (68.1) | 0.28 | |
| Diabetes mellitus | 55 (35.0) | 40 (36.4) | 15 (31.9) | 0.72 | |
| Dyslipidemia | 64 (40.8) | 44 (40.0) | 20 (42.6) | 0.90 | |
| WBC (×10 |
10.1 |
10.1 |
10.1 |
0.95 | |
| Troponin I peak (ng/mL) | 20.9 |
19.4 |
24.4 |
0.002 | |
| CK-MB peak (ng/mL) | 180.1 |
157.0 |
233.7 |
||
| LDL-C (mmol/L) | 2.7 |
2.8 |
2.6 |
0.42 | |
| LVEF before discharge (%) | 47.9 |
48.5 |
46.4 |
0.32 | |
| eGFR (mL/min/1.73 m |
85.0 |
85.9 |
82.9 |
0.43 | |
| Medication | |||||
| Aspirin | 154 (98.1) | 108 (98.2) | 46 (97.9) | 1.00 | |
| Clopidogrel | 97 (61.8) | 70 (63.6) | 27 (57.4) | 0.58 | |
| Ticagrelor | 59 (37.6) | 39 (35.5) | 20 (42.6) | 0.51 | |
| Statins | 148 (94.3) | 104 (94.5) | 44 (93.6) | 1.00 | |
| ACEIs/ARBs | 79 (50.3) | 57 (51.8) | 22 (46.8) | 0.69 | |
| Beta blocker | 114 (72.6) | 78 (70.9) | 36 (76.6) | 0.59 | |
Values are mean
caIMR, coronary angiography-derived index of microcirculatory resistance; BMI,
body mass index; WBC, white blood cells; CK-MB, creatine kinase-myocardial band;
LDL-C, low-density lipoprotein cholesterol; LVEF, left ventricular ejection
fraction; eGFR, estimated glomerular filtration rate; ACEI,
angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor antagonist.
The angiographic and procedural characteristics are presented in Table 2. The
door-to-balloon time was comparable between the groups. The left anterior
descending artery (54.1%) was the primary culprit vessel, followed by the right
coronary artery (30.6%). The proportion of patients with MVD or LM disease was
similar in both groups and presented a similar distribution in the IRA. More
patients received thrombus aspiration (31.8% vs. 57.4%, p = 0.005) and
tirofiban administration (8.2% vs. 25.5%, p = 0.008) in the caIMR
| Overall | caIMR |
caIMR |
p value | |||
| n = 157 | n = 110 | n = 47 | ||||
| Angiographic and procedural characteristics | ||||||
| SBP (mmHg) | 122 |
120 |
126 |
0.13 | ||
| DBP (mmHg) | 72 |
70 |
77 |
0.01 | ||
| Door-to-balloon time (min) | 125 (89, 175) | 122 (88, 177) | 130 (90, 167) | 0.83 | ||
| Culprit vessel | 0.11 | |||||
| LAD | 85 (54.1) | 58 (52.7) | 27 (57.4) | |||
| LCX | 21 (13.4) | 11 (10.0) | 10 (21.3) | |||
| RCA | 48 (30.6) | 39 (35.5) | 9 (19.1) | |||
| Multivessel disease | 84 (53.5) | 58 (52.7) | 26 (55.3) | 0.77 | ||
| LM disease | 10(6.4) | 6 (5.5) | 4 (8.5) | 0.49 | ||
| Thrombus aspiration | 62 (39.5) | 35 (31.8) | 27 (57.4) | 0.005 | ||
| Drug-coated balloon use | 36 (22.9) | 25 (22.7) | 11 (23.4) | 1.00 | ||
| Drug-eluting stent implantation | 119 (75.8) | 83 (75.5) | 36 (76.6) | 1.00 | ||
| Medication during the procedure | ||||||
| Tirofiban | 21 (13.4) | 9 (8.2) | 12 (25.5) | 0.008 | ||
| Nicorandil | 10 (6.4) | 5 (4.5) | 5 (10.6) | 0.28 | ||
| Final TIMI flow grade 3 | 153 (97.5) | 110 (100) | 43 (91.5) | 0.002 | ||
| caIMR in the IRA | 32.9 |
24.3 |
52.6 |
0.002 | ||
| caIMR in the reference vessel | 27.4 |
25.9 |
30.9 |
0.009 | ||
| Clinical outcomes | n = 155 | n = 108 | n = 47 | |||
| MACEs at 3 months | 21 (13.5) | 9 (8.3) | 12 (25.5) | 0.009 | ||
| Cardiac death | 8 (5.2) | 6 (5.6) | 2 (4.3) | 1.00 | ||
| TVR | 3 (1.9) | 1 (0.9) | 2 (4.3) | 0.22 | ||
| Rehospitalization | 10 (6.5) | 2 (1.9) | 8 (17.0) | 0.001 | ||
| MACEs at 1 year | 29 (18.7) | 15 (13.9) | 14 (29.8) | 0.04 | ||
| Cardiac death | 8 (5.2) | 6 (5.6) | 2 (4.3) | 1.00 | ||
| TVR | 5 (3.2) | 2 (1.9) | 3 (6.4) | 0.16 | ||
| Rehospitalization | 16 (10.3) | 7 (6.5) | 9 (19.1) | 0.02 | ||
Values are mean
caIMR, coronary angiography-derived index of microcirculatory resistance; SBP,
systolic blood pressure; DBP, diastolic blood pressure; LAD, left anterior
descending artery; LCX, left circumflex; RCA, right coronary artery; LM, left
main; TIMI, thrombolysis in myocardial infarction; IRA, infarct-related artery;
MACEs, major adverse cardiac events; TVR, target vessel revascularization;
Rehospitalization, rehospitalization for coronary heart failure, myocardial
infarct, and angina.
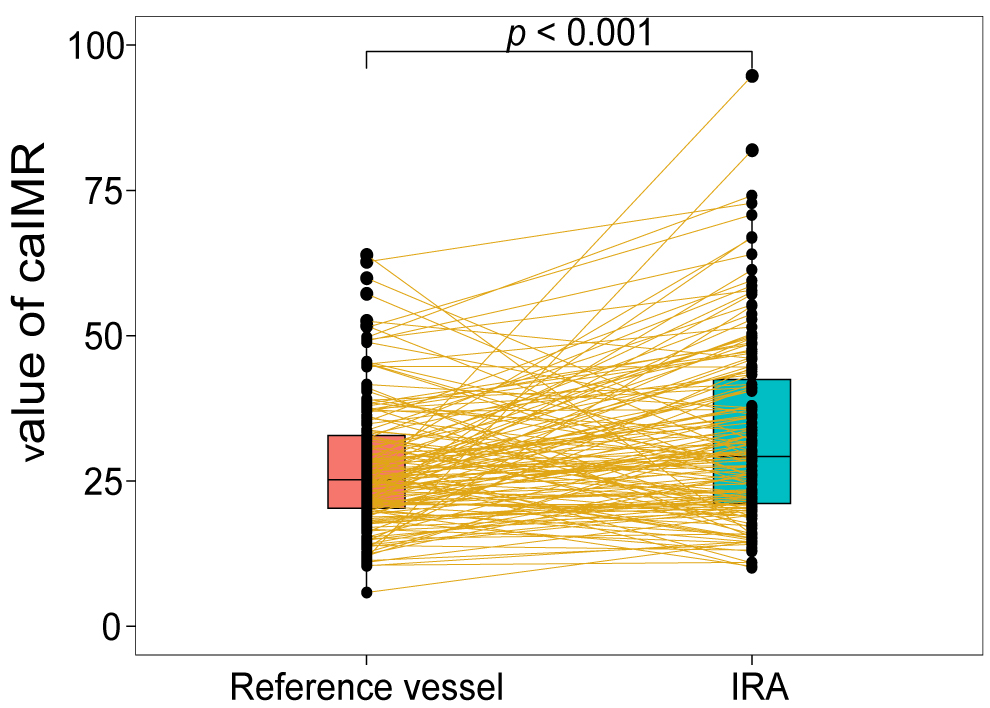 Fig. 3.
Fig. 3.Paired boxplot between the caIMR in the IRA and reference vessel. caIMR, coronary angiography-derived index of microcirculatory resistance; IRA, infarct-related artery.
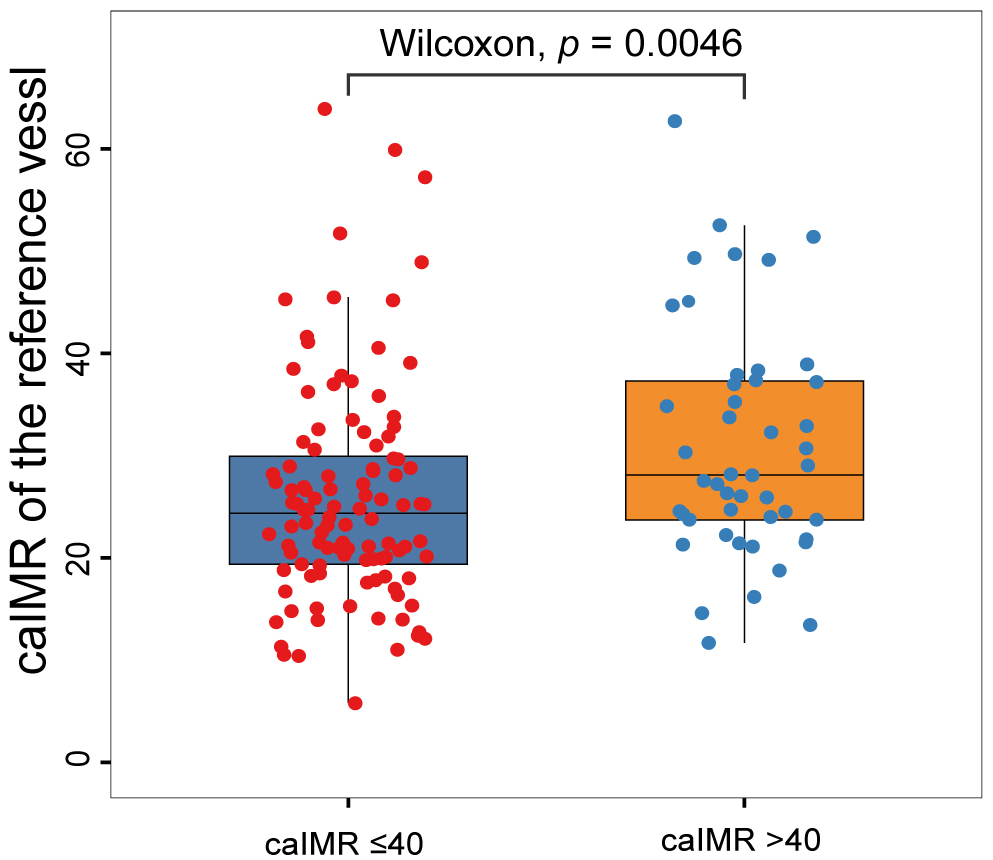 Fig. 4.
Fig. 4.Distribution of caIMR in the reference vessel according to the caIMR in the IRA. caIMR, coronary angiography-derived index of microcirculatory resistance; IRA, infarct-related artery.
The caIMR in the IRA or the reference vessel was comparable between patients
with and without MACEs at 3 months and 1 year (Fig. 5). Table 2 displays the
clinical outcomes observed at the 3-month and 1-year follow-ups. The caIMR
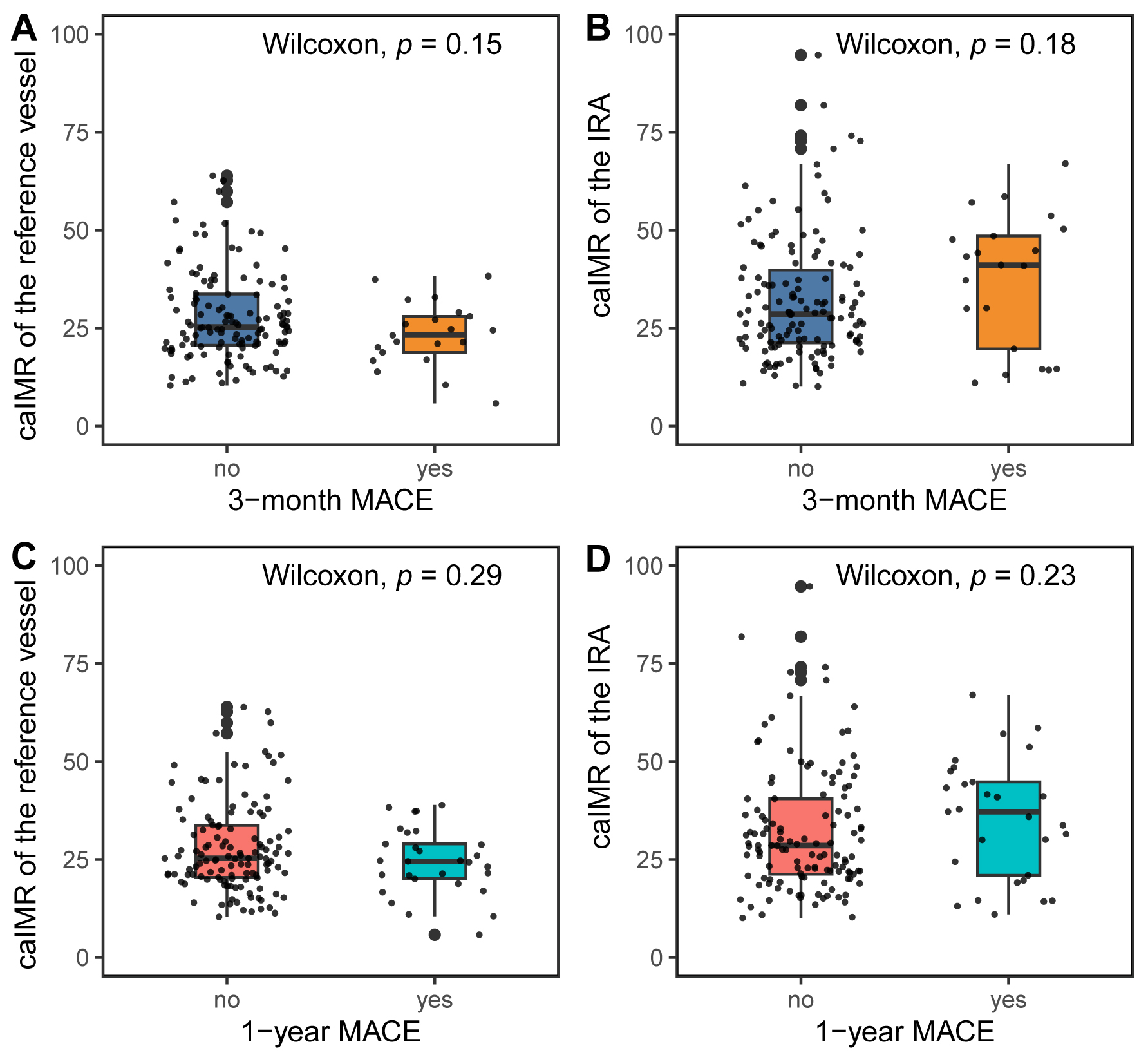 Fig. 5.
Fig. 5.CaIMR in the IRA and reference vessel according to MACEs. (A) CaIMR in the reference vessel according to MACEs at 3 months. (B) CaIMR in the IRA according to MACEs at 3 months. (C) CaIMR in the reference vessel according to MACEs at 1 year. (D) CaIMR in the IRA according to MACEs at 1 year. MACEs, major adverse cardiac events; caIMR, coronary angiography-derived index of microcirculatory resistance; IRA, infarct-related artery.
 Fig. 6.
Fig. 6.Kaplan–Meier curves for MACE-free survival according to the caIMR in the IRA. (A) Curve for MACE-free survival at 3 months. (B) Curve for MACE-free survival at 1 year. caIMR, coronary angiography-derived index of microcirculatory resistance; IRA, infarct-related artery; MACE, major adverse cardiac event; HR, hazard ratio; CI, confidence interval.
Candidate predictors found in the univariate analysis included caIMR in the IRA,
caIMR in the reference vessel, caIMR in the IRA of
| Variables | Univariate Analysis | Multivariate Analysis | ||||
| HR | 95% CI | p value | HR | 95% CI | p value | |
| caIMR in the IRA | 1.017 | 0.994–1.041 | 0.16 | |||
| caIMR in the reference vessel | 0.959 | 0.914–1.006 | 0.09 | |||
| caIMR in the IRA |
3.398 | 1.431–8.068 | 0.006 | 3.459 | 1.363–8.779 | 0.009 |
| Age | 1.042 | 1.008–1.077 | 0.02 | 1.006 | 0.967–1.045 | 0.77 |
| Female sex | 2.627 | 1.107–6.237 | 0.03 | 2.079 | 0.741–5.826 | 0.16 |
| Hypertension | 4.181 | 1.232–14.197 | 0.02 | 2.161 | 0.570–8.191 | 0.26 |
| Diabetes mellitus | 0.910 | 0.367–2.255 | 0.84 | |||
| Dyslipidemia | 0.573 | 0.222–1.478 | 0.25 | |||
| Current smoker | 2.285 | 0.886–5.889 | 0.09 | |||
| LVEF before discharge |
3.566 | 1.306–9.736 | 0.01 | 2.075 | 0.696–6.186 | 0.19 |
| eGFR | 0.974 | 0.957–0.992 | 0.006 | 0.981 | 0.959–1.003 | 0.09 |
| Multivessel disease | 1.807 | 0.729–4.478 | 0.20 | |||
| Door-to-balloon time | 1.002 | 0.998–1.007 | 0.25 | |||
| Thrombus aspiration | 0.733 | 0.296–1.815 | 0.50 | |||
| Final TIMI flow grade 3 | 0.510 | 0.069–3.804 | 0.51 | |||
MACEs, major adverse cardiac events; HR, hazard ratio; CI, confidence interval; caIMR, coronary angiography-derived index of microcirculatory resistance; IRA, infarct-related artery; LVEF, left ventricular ejection fraction; eGFR, estimated glomerular filtration rate; TIMI, thrombolysis in myocardial infarction.
| Variables | Univariate Analysis | Multivariate Analysis | ||||
| HR | 95% CI | p value | HR | 95% CI | p value | |
| caIMR in the IRA | 1.011 | 0.990–1.033 | 0.29 | |||
| caIMR in the reference vessel | 0.970 | 0.934–1.008 | 0.12 | |||
| caIMR in the IRA |
2.446 | 1.180–5.070 | 0.02 | 2.384 | 1.100–5.166 | 0.03 |
| Age | 1.034 | 1.006–1.063 | 0.02 | 0.999 | 0.967–1.032 | 0.96 |
| Female sex | 2.584 | 1.234–5.415 | 0.01 | 2.089 | 0.864–5.047 | 0.10 |
| Hypertension | 6.286 | 1.902–20.773 | 0.003 | 4.026 | 1.144–14.162 | 0.03 |
| Diabetes mellitus | 1.123 | 0.530–2.378 | 0.76 | |||
| Dyslipidemia | 0.632 | 0.288–1.389 | 0.25 | |||
| Current smoker | 1.780 | 0.828–3.830 | 0.14 | |||
| LVEF before discharge |
2.544 | 1.158–5.589 | 0.02 | 1.738 | 0.742–4.073 | 0.20 |
| eGFR | 0.978 | 0.963–0.993 | 0.006 | 0.987 | 0.969–1.005 | 0.15 |
| Multivessel disease | 0.891 | 0.421–1.887 | 0.76 | |||
| Door-to-balloon time | 1.001 | 0.997–1.005 | 0.55 | |||
| Thrombus aspiration | 1.288 | 0.615–2.697 | 0.50 | |||
| Final TIMI flow grade 3 | 0.335 | 0.080–1.410 | 0.14 | |||
MACEs, major adverse cardiac events; HR, hazard ratio; CI, confidence interval; caIMR, coronary angiography-derived index of microcirculatory resistance; IRA, infarct-related artery; LVEF, left ventricular ejection fraction; eGFR, estimated glomerular filtration rate; TIMI, thrombolysis in myocardial infarction.
This study aimed to evaluate the coronary microvascular function indicated by
caIMR in patients with STEMI undergoing primary PCI. The main findings of our
study are as follows: (1) CaIMR in the IRA of
Achieving a TIMI grade 3 flow in the IRA is the principal objective of primary
PCI, and reperfusion at the myocardial tissue level, manifested by the IMR value,
is increasingly important [14, 15]. Owing to the extra procedure time, discomfort
in patients resulting from adenosine infusion, the risks associated with
manipulating the pressure wire, and additional cost, the use of
pressure‒temperature wire-based IMR has limited applications in STEMI. Some
studies have indicated that caIMR is a promising and reproducible alternative to
wire-based IMR for determining quantitative coronary microvascular function [8, 9, 16]. Since multiple factors are associated with CMVD in primary PCI, the
elevation of caIMR in the IRA could be revealed. In this study, the proportion of
patients with a caIMR in the IRA of
Wire-based IMR is related to the presence and severity of microvascular
obstruction (MVO) and infarct size, as assessed using cardiovascular magnetic
resonance [17, 18]. Similarly, this study found that patients with a caIMR in the
IRA of
In this study, the caIMR in the IRAs was significantly higher than in the
reference vessels. Several potential explanations exist for the difference in the
caIMR between the IRA and reference vessels. First, coronary microembolization
due to the spontaneous or interventional rupture of an epicardial coronary
atherosclerotic plaque may cause physical obstruction in the coronary
microvessels and induce CMVD in the infarct territory subtended by the IRA [23].
Second, reperfusion could paradoxically impact the microvascular function status,
namely, reperfusion injury [24, 25]. Reperfusion injury in STEMI patients is
considered a consequence of a series of pathophysiological mechanisms, including
MVO, intramyocardial hemorrhage, endothelial damage, and extravascular
compression of the microvasculature [26]. Third, the activation of inflammation,
release of oxygen-derived free radicals, and disruption of the coagulation
pathway could worsen CMVD after reperfusion [14, 27]. Otherwise, we observed a
higher proportion of aspiration thrombectomy in the caIMR
It was noteworthy that in this study the caIMR
In our study, the caIMR
There are some limitations in this study. Firstly, this study was a
single-center observational study with a small sample size, and its findings were
not robust due to the absence of a control group. However, we measured the caIMR
in the reference vessels to perform a self-control analysis. The limited number
of events could lead to overfitting in the multivariable Cox survival analysis
model. Therefore, a prospective, randomized trial with larger populations is
necessary. Secondly, more than 50% of patients with multivessel disease were
included in this study, and it was difficult to achieve complete
revascularization in cases of primary PCI. Although we performed multivariate
analysis to control for the potential confounding impact of multivessel disease,
incomplete revascularization has been associated with an unfavorable prognosis
[29]. Thirdly, although it has been reported that the severity of epicardial
stenosis does not influence coronary microcirculatory resistance, the caIMR
measurement was pressure-dependent, which was closely related to Pd
CMVD in patients with STEMI undergoing primary PCI is not a rare situation. A
caIMR in the IRA of
ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor antagonist; BMI, body mass index; CK-MB, creatine kinase-myocardial band; CI, confidence interval; CHF, congestive heart failure; caIMR, coronary angiography-derived index of microcirculatory resistance; CMVD, coronary microvascular dysfunction; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HR, hazard ratio; IMR, index of microcirculatory resistance; IRA, infarct-related artery; LAD, left anterior descending artery; LCX, left circumflex; LDL-C, low-density lipoprotein cholesterol; LM, left main; LVEF, left ventricular ejection fraction; MACEs, major adverse cardiovascular events; MVO, microvascular obstruction; MVD, multivessel disease; MI, myocardial infarction; PCI, percutaneous coronary intervention; RCA, right coronary artery; SBP, systolic blood pressure; STEMI, ST-segment elevation myocardial infarction; TIMI, thrombolysis in MI; TVR, target vessel revascularization; WBC, white blood cell.
The datasets analyzed in the current study are available from the corresponding author upon reasonable request.
HPZ and HA designed the research study. ML, XP, HPZ, and HA performed the research. ML, XP, HPZ, and HA collected, measured, and analyzed the data. NXZ, HL, GJY, GDT, YZ, and FCS interpreted the data and reviewed the results. ML, XP, HA, FCS, and HPZ wrote, reviewed, and/or revised the manuscript. All authors contributed to editorial changes in the manuscript. HPZ supervised the study. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
This study was approved by the Institutional Review Board of Beijing Hospital (Approval No. 2019BJYYEC-021-02), and all patients provided informed consent to participate in this study and underwent the intervention procedure.
Not applicable.
This research was funded by the National High Level Hospital Clinical Research Funding, grant number BJ-2018-201.
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
