- Academic Editors
Background: This study aimed to assess the clinical significance of generating a volumetric stent expansion index for tapering lesions through intravascular ultrasound (IVUS). Previous IVUS studies have used minimal stent area (MSA) to predict adverse outcomes. Methods: A total of 251 tapering lesions were treated in this study via IVUS guidance in 232 patients. Eight stent expansion indices were evaluated to determine the association of these indices with device-oriented clinical endpoints (DoCEs) after two-year follow-ups. These were the ILUMIEN III and IV standards, the ULTIMATE (Intravascular Ultrasound Guided Drug Eluting Stents Implantation in “All-Comers” Coronary Lesions) standard, the IVUS-XPL (Impact of Intravascular Ultrasound Guidance on the Outcomes of Xience Prime Stents in Long Lesions) standard, the minimal volumetric expansion index (MVEI) using the Huo-Kassab or linear model, the MSA/vessel area at the MSA cross-section, the traditional stent expansion (MSA/mean proximal and distal reference lumen cross-sectional area), and MSA. Results: The MVEI was the only stent expansion index that correlated significantly with the two-year DoCEs (hazard ratio [HR], 1.91; 95% confidence interval [CI]: 1.16–3.96; p = 0.028). In the ROC analysis, the area under the curve for the MVEI was 0.71 (p = 0.002), with an optimal cut-off value of 62.2 for predicting the DoCEs. Conclusions: This is the first study to use IVUS for tapering lesions and demonstrate that the MVEI is an independent predictor of two-year DoCEs.
Coronarytapering lesions (CTLs) refer to a type of lesion where there is a significant mismatch in the lumen diameter between the distal and proximal reference segments of the target lesion [1, 2]. Although interventional and stent techniques have shown rapid progress, the treatment of CTLs remains challenging and is associated with poorer clinical outcomes [3, 4]. The stenting of CTLs is associated with greater in-stent restenosis and risk of stent thrombosis [5]. In light of the adverse events associated with CTLs and the need for more lesion preparation (e.g., using intravascular imaging to assess the vessel size and lesion characteristics) and post-stenting improvement (e.g., using non-compliant balloons with various sizes or pressure) [3], the interventional standard requires urgent modification to improve the outcomes for CTLs.
Extensive research has confirmed the positive effect of stent implantation with
guidance from intravascular ultrasound (IVUS) [6, 7]. Adequate stent expansion,
measured by IVUS, is recognized as a critical aspect of stent improvement for
reducing the failure rate [8]. The minimal stent area (MSA) provides a measure of
stent expansion through the use of either optical coherence tomography (OCT) or
IVUS. The MSA has been extensively confirmed as a strong predictor of adverse
clinical events, with cut-off values for the prediction of stent failure reported
as 4.5 to 5.5 mm
This retrospective observational study was conducted at the Xiangtan Central
Hospital from March 2015 to November 2019. A total of 1058 lesions were selected
from 961 consecutive patients subjected to IVUS-guided percutaneous coronary
intervention (PCI) for de novo lesions. Amongst them, 232 cases
possessed 251 CTLs. The exclusion criteria were: (1) non-tapering lesions (n =
541), (2) left main coronary artery lesions (n = 35), (3) ostial lesions (n =
86), (4) chronic total occlusion (CTO) lesions (n = 59), (5)
administration with drug-coated balloons (n = 47), and (6)
non-satisfactory angiographic or IVUS image quality (n = 39) (Fig. 1). CTLs were
defined by IVUS and were based on differences in the proximal and distal
references for each lesion of
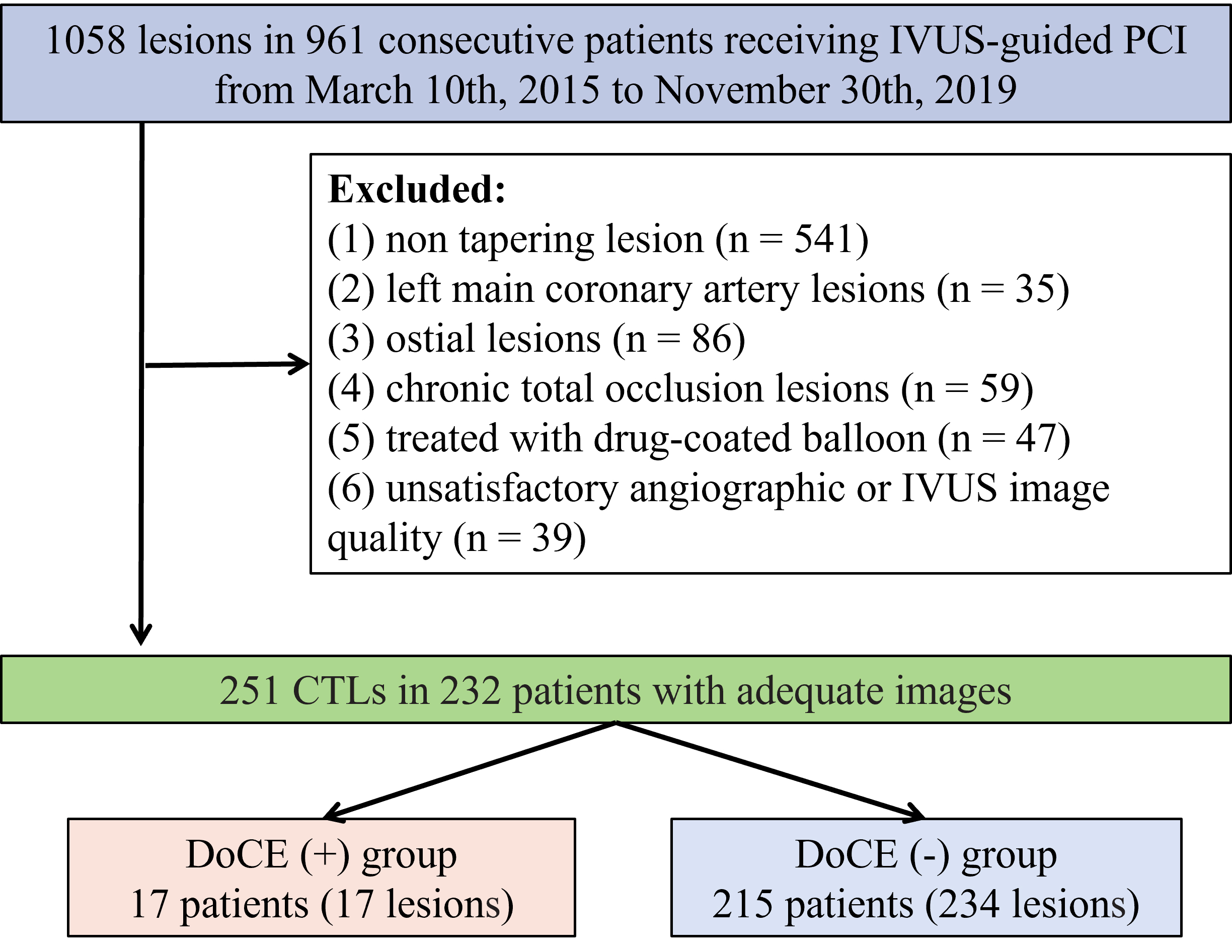 Fig. 1.
Fig. 1.Schematic of the study flow. PCI, percutaneous coronary intervention; IVUS, intravascular ultrasound; CTLs, coronary tapering lesions; DoCEs, device-oriented clinical endpoints.
Procedure-associated strategies were decided upon by the operator.
Second-generation drug-eluting stents were used in all cases. Preintervention
IVUS was employed to analyze CTLs prior to balloon dilatation. Once stenting was
complete, another IVUS was carried out to verify the results for stent
deployment. For ineffective cases with MSA
An offline, commercially available software (QAngio® XA, Medis, Leiden, the Netherlands) was employed for quantitative coronary angiography (QCA) of CTLs. QCA analysis included the minimal lumen diameter, percent diameter stenosis, lesion length, reference vessel diameter, calcification, etc. [14]. The three epicardial arteries were divided into left main (LM) (5), distal (3, 4, 8–10, 12, 14, 15), mid (2, 7, 13), and proximal (1, 6, 11) segments, in accordance with the American Heart Association classification [15].
When nitroglycerin (0.1–0.2 mg) was used for intracoronary administration,
automated pullback (0.5 mm/s) was employed to obtain the CTL IVUS images (40 MHz
OptiCross™, Boston Scientific, Marlborough, MA, USA) for both
before and after PCI. Two independent readers who were blinded to patient
information evaluated all IVUS images using a frequency domain available offline
software (QIvus®, Medis, Leiden, the Netherlands). The CTL IVUS
measurements were performed every 1 mm for the administered segment (pre-PCI) and
stent, and every 5 mm for the proximal and distal reference segments. We examined
the distal and proximal references in the site, reaching the maximal lumen 5 mm
distal and proximal to the stented segment. Reference luminal areas were also
examined in frames with minimal plaque burden. The calculation for each percent
area of stenosis was: ((reference lumen area - minimal lumen area)/reference
lumen area)
The indices for stent expansion were specified in advance and are described below (Fig. 2):
(1) MSA was derived from the automatic minimal cross-sectional lumen area within the post-stented lesion [18].
(2) MSA/vessel area at the MSA cross-sectional [19].
(3) Traditional SEI: MSA/mean proximal and distal reference lumen cross-sectional area.
(4) Minimal volumetric expansion index (MVEI) [13]: (actual
lumen area/ideal lumen area
(5) IVUS-XPL standards, calculated by an MSA
(6) ULTIMATE standards, calculated by an MSA
(7) ILUMIEN IV standards, calculated by an MSA of the proximal
site
(8) ILUMIEN III standards, calculated by mean stent expansion: mean stent area (total of stent area/total of stent length)/mean reference lumen cross-sectional area [23].
 Fig. 2.
Fig. 2.The calculation formula for stent expansion indices. MSA, minimal stent area; IVUS-XPL, Impact of Intravascular Ultrasound Guidance on the Outcomes of Xience Prime Stents in Long Lesions; ULTIMATE, Intravascular Ultrasound Guided Drug Eluting Stents Implantation in “All-Comers” Coronary Lesions; ILUMIEN IV, Observational Study of Optical Coherence Tomography in Patients Undergoing Fractional Flow Reserve and Percutaneous Coronary Intervention IV.
Device-oriented clinical endpoints (DoCEs) included target lesion revascularization (TLR), myocardial infarction (MI) or stent thrombosis associated with the target vessel, and cardiac death [24]. Cardiac death was defined as any death due to cardiac-related causes, procedure-related deaths, and death of unknown cause. MI was reported in accordance with European Society of Cardiology guidelines [25]. TLR refers to an ischemia-driven repeat PCI, or to coronary artery bypass surgery of the target lesion for angiographic target lesion restenosis or ischemia-driven clinical complications. Stent thrombosis refers to either probable or definite stent thrombosis [24]. Periodic clinical follow-up occurred at 6-month intervals through either a telephone interview or a clinical visit. In general, recruited patients were subjected to almost 3-years of clinical follow-ups, and at least one year of follow-ups.
Continuous variables are expressed as mean
A total of 232 consecutive patients with 251 CTLs were assessed. Of these, 17 patients (7.3% of all patients) with 17 lesions (6.8% of all lesions) had 2-year follow-ups for the DoCEs. The average follow-up was 729 days (interquartile range: 705–733 days). As shown in Table 1, no significant differences were observed in any of the clinical characteristics between DoCE(–) and DoCE(+) patient groups. The procedural and angiographic findings were also compared between the two groups. Again, no significant differences were observed between patients who did or did not suffer DoCEs (Table 2).
| Variables | DoCE(+) (n = 17) | DoCE(–) (n = 215) | p value | |
| Age, mean |
62.1 |
63.4 |
0.223 | |
| Male, n (%) | 5 (29.4) | 68 (31.6) | 0.461 | |
| Body mass index, kg/m |
24.5 |
24.8 |
0.323 | |
| Diabetes mellitus, n (%) | 6 (35.3) | 63 (29.3) | 0.234 | |
| Hypertension, n (%) | 13 (76.5) | 171 (79.5) | 0.695 | |
| Hyperlipidemia, n (%) | 12 (70.6) | 145 (67.4) | 0.737 | |
| Current smoker, n (%) | 4 (23.5) | 54 (25.1) | 0.851 | |
| Chronic kidney disease, n (%) | 2 (11.8) | 23 (10.7) | 0.921 | |
| Prior PCI, n (%) | 1 (5.9) | 8 (3.7) | 0.693 | |
| Prior MI, n (%) | 5 (29.4) | 70 (32.6) | 0.612 | |
| Peripheral arterial disease, n (%) | 2 (11.8) | 21 (9.8) | 0.712 | |
| Clinical presentation, n (%) | 0.804 | |||
| STEMI | 1 (5.9) | 13 (6.0) | ||
| Non-STEMI | 2 (11.8) | 27 (12.6) | ||
| Stable angina | 10 (58.8) | 124 (57.7) | ||
| Others | 4 (23.5) | 51 (23.7) | ||
| Three-vessel coronary disease, n (%) | 7 (41.2) | 100 (46.5) | 0.308 | |
| Left ventricular ejection fraction |
2 (11.8) | 31 (14.4) | 0.482 | |
| Laboratory data | ||||
| Hemoglobin, g/dL, mean |
10.2 |
10.1 |
0.651 | |
| HbA1c, %, mean |
6.5 |
6.4 |
0.830 | |
| LDL-C, mg/dL, mean |
108.0 |
116.3 |
0.439 | |
| HDL-C, mg/dL, mean |
44.1 |
45.6 |
0.406 | |
| Triglyceride, mg/dL, median (interquartile range) | 126.0 (87.1–157.1) | 135.0 (88.4–194.1) | 0.412 | |
| Creatinine, mg/dL, mean |
0.9 |
1.2 |
0.225 | |
| eGFR, mL/min/1.73 m |
57.7 |
55.2 |
0.717 | |
| Medication at discharge | ||||
| DAPT, n (%) | 17 (100) | 215 (100) | 1.000 | |
| Beta-blocker, n (%) | 11 (64.7) | 130 (60.5) | 0.721 | |
| ACE inhibitor/ARB, n (%) | 10 (58.8) | 120 (55.8) | 0.801 | |
| Statin, n (%) | 16 (94.1) | 208 (96.7) | 0.887 | |
ACE, angiotensin-converting enzyme; ARB, angiotensin II receptor blocker; DAPT, dual antiplatelet therapy; DoCE, device-oriented clinical endpoint; HDL-C, high density lipoprotein cholesterol; MI, myocardial infarction; LDL-C, low density lipoprotein cholesteroll; PCI, percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction; eGFR, estimated glomerular filtration rate; SD, standard deviation; HbA1c, hemoglobin A1c.
| Variables | DoCE(+) (n = 17) | DoCE(–) (n = 234) | p value | |
| Lesion location, n (%) | 0.209 | |||
| RCA | 2 (11.8) | 18 (7.7) | ||
| LAD | 11 (64.7) | 160 (68.4) | ||
| LCx | 4 (23.5) | 56 (23.9) | ||
| % diameter stenosis, mean |
72.4 |
69.7 |
0.672 | |
| Proximal reference diameter, mm, median (interquartile range) | 3.64 (3.36–3.97) | 3.78 (3.40–3.99) | 0.174 | |
| Distal reference diameter, mm, median (interquartile range) | 2.38 (2.06–2.69) | 2.45 (2.15–2.81) | 0.136 | |
| Stent diameter, mm, mean |
3.5 |
3.3 |
0.114 | |
| Stent length, mm, mean |
29.4 |
29.7 |
0.854 | |
| Multiple stents, n (%) | 13 (76.5) | 175 (74.8) | 0.628 | |
| Predilatation, n (%) | 11 (64.7) | 156 (66.7) | 0.271 | |
| Postdilatation, n (%) | 17 (100) | 234 (100) | 1.000 | |
| Maximal inflation pressure, atm, mean |
18.5 |
18.1 |
0.708 | |
LAD, left anterior descending artery; LCx, left circumflex artery; RCA, right coronary artery; DoCE, device-oriented clinical endpoint; SD, standard deviation.
The final overall IVUS MSA after PCI was examined as 5.9
| Variables | DoCE(+) (n = 17) | DoCE(–) (n = 234) | p value | ||
| Pre-PCI IVUS | |||||
| Minimal luminal area site analysis | |||||
| Luminal area, mm |
2.8 (2.6–3.0) | 2.7 (2.5–2.9) | 0.295 | ||
| Vessel area, mm |
13.9 (11.8–15.7) | 13.6 (13.2–14.3) | 0.783 | ||
| Plaque burden, %, median (interquartile range) | 77.1 (72.4–82.1) | 76.6 (73.2–79.7) | 0.743 | ||
| Volumetric analysis | |||||
| Mean luminal area, mm |
5.7 (5.4–5.9) | 5.6 (5.2–6.0) | 0.383 | ||
| Mean vessel area, mm |
13.9 (12.4–15.5) | 14.1 (13.4–14.9) | 0.642 | ||
| Plaque volume, %, median (interquartile range) | 62.1 (59.2–64.9) | 61.2 (59.1–63.5) | 0.211 | ||
| Mean reference area, mm |
6.13 |
6.37 |
0.374 | ||
| Mean distal reference area, mm |
4.08 |
5.22 |
0.131 | ||
| Mean proximal reference area, mm |
6.93 |
7.63 |
0.318 | ||
| Superficial calcium, n (%) | 2 (11.8) | 24 (10.3) | 0.712 | ||
| Calcified nodule, n (%) | 1 (5.9) | 14 (6.0) | 0.832 | ||
| Attenuated plaque, n (%) | 4 (23.5) | 47 (20.1) | 0.214 | ||
| Post-PCI IVUS | |||||
| Minimal stent area, mm |
5.8 |
6.0 |
0.327 | ||
| MSA/vessel area at the MSA, %, median (interquartile range) | 47.9 (39.3–54.5) | 50.1 (44.1–55.9) | 0.072 | ||
| Conventional stent expansion, %, median (interquartile range) | 75.7 (72.4–78.9) | 74.6 (72.8–76.1) | 0.793 | ||
| Minimal volumetric expansion index, %, median (interquartile range) | 65.3 (59.7–70.9) | 72.1 (67.2–76.3) | 0.001 | ||
| IVUS-XPL criteria, n (%) | 4 (23.5) | 47 (20.0) | 0.643 | ||
| ULTIMATE criteria, n (%) | 5 (29.4) | 68 (29.0) | 0.982 | ||
| ILUMIEN IV criteria, n (%) | 2 (11.8) | 23 (9.8) | 0.492 | ||
| ILUMIEN III criteria, %, mean |
103.5 |
97.2 |
0.314 | ||
| Tissue protrusion, n (%) | 6 (35.3) | 79 (33.7) | 0.519 | ||
| Stent edge dissection, n (%) | 3 (17.6) | 36 (15.4) | 0.322 | ||
| Acute stent malapposition, n (%) | 0 (0) | 2 (0.09) | 0.737 | ||
IVUS, intravascular ultrasound; IVUS-XPL, Impact of Intravascular Ultrasound Guidance on the Outcomes of Xience Prime Stents in Long Lesions; MSA, minimal stent area; ULTIMATE, Intravascular Ultrasound Guided Drug Eluting Stents Implantation in “All-Comers” Coronary Lesions; DoCE, device-oriented clinical endpoint; PCI, percutaneous coronary intervention; SD, standard deviation; ILUMIEN, Observational Study of Optical Coherence Tomography in Patients Undergoing Fractional Flow Reserve and Percutaneous Coronary Intervention.
| Variables | Hazard ratio | 95% Confidence interval | p value |
| Minimal stent area, mm |
0.95 | 0.89–1.12 | 0.655 |
| MSA/vessel area at the MSA, per 10% | 0.78 | 0.62–1.32 | 0.314 |
| Conventional stent expansion, % | 1.04 | 0.88–1.32 | 0.745 |
| Minimal volumetric expansion index, per 10% | 1.91 | 1.16–3.96 | 0.028 |
| IVUS-XPL criteria | 1.61 | 0.74–3.35 | 0.178 |
| ULTIMATE criteria | 0.92 | 0.53–1.78 | 0.793 |
| ILUMIEN IV criteria | 0.74 | 0.24–2.45 | 0.688 |
| ILUMIEN III criteria, per 10% | 1.53 | 0.35–7.34 | 0.653 |
SEI, stent expansion index; DoCE, device-oriented clinical endpoint; IVUS, intravascular ultrasound; IVUS-XPL, Impact of Intravascular Ultrasound Guidance on the Outcomes of Xience Prime Stents in Long Lesions; MSA, minimal stent area; ULTIMATE, Intravascular Ultrasound Guided Drug Eluting Stents Implantation in “All-Comers” Coronary Lesions; ILUMIEN IV, Observational Study of Optical Coherence Tomography in Patients Undergoing Fractional Flow Reserve and Percutaneous Coronary Intervention IV.
| Variables | Hazard ratio | 95% Confidence interval | p value |
| Maximal inflation pressure, atm | 0.28 | 0.10–0.47 | 0.001 |
| Multiple stents | 1.57 | 0.78–3.32 | 0.682 |
| Plaque volume, per 10% | 1.29 | 0.77–2.92 | 0.326 |
| Lesion length, per 10 mm | 1.08 | 0.51–2.59 | 0.474 |
IVUS, intravascular ultrasound.
| Variables | MVEI |
MVEI |
p value |
| Patients level | (n = 119) | (n = 113) | |
| DoCEs, n (%) | 13 (10.9) | 4 (3.5) | 0.011 |
| Cardiac death, n (%) | 0 (0) | 0 (0) | – |
| Target vessel-related myocardial infarction, n (%) | 3 (2.5) | 2 (1.8) | 0.702 |
| Stent thrombosis, n (%) | 2 (1.7) | 1 (0.9) | 0.649 |
| Target lesion revascularization, n (%) | 8 (6.7) | 1 (0.9) | 0.021 |
| Lesions level | (n = 126) | (n = 125) | |
| DoCEs, n (%) | 13 (10.3) | 4 (3.2) | 0.018 |
| Cardiac death, n (%) | 0 (0) | 0 (0) | – |
| Target vessel-related myocardial infarction, n (%) | 3 (2.4) | 2 (1.6) | 0.820 |
| Stent thrombosis, n (%) | 2 (1.6) | 1 (0.8) | 0.862 |
| Target lesion revascularization, n (%) | 8 (6.3) | 1 (0.8) | 0.038 |
DoCEs, device-oriented clinical endpoints; MVEI, minimal volumetric expansion index.
 Fig. 3.
Fig. 3.Receiver operating characteristic curve analysis. AUC, area under curve.
 Fig. 4.
Fig. 4.Two-year Kaplan–Meier curves for DoCEs. DoCEs, device-oriented clinical endpoints; CI, confidence interval; MVEI, minimal volumetric expansion index.
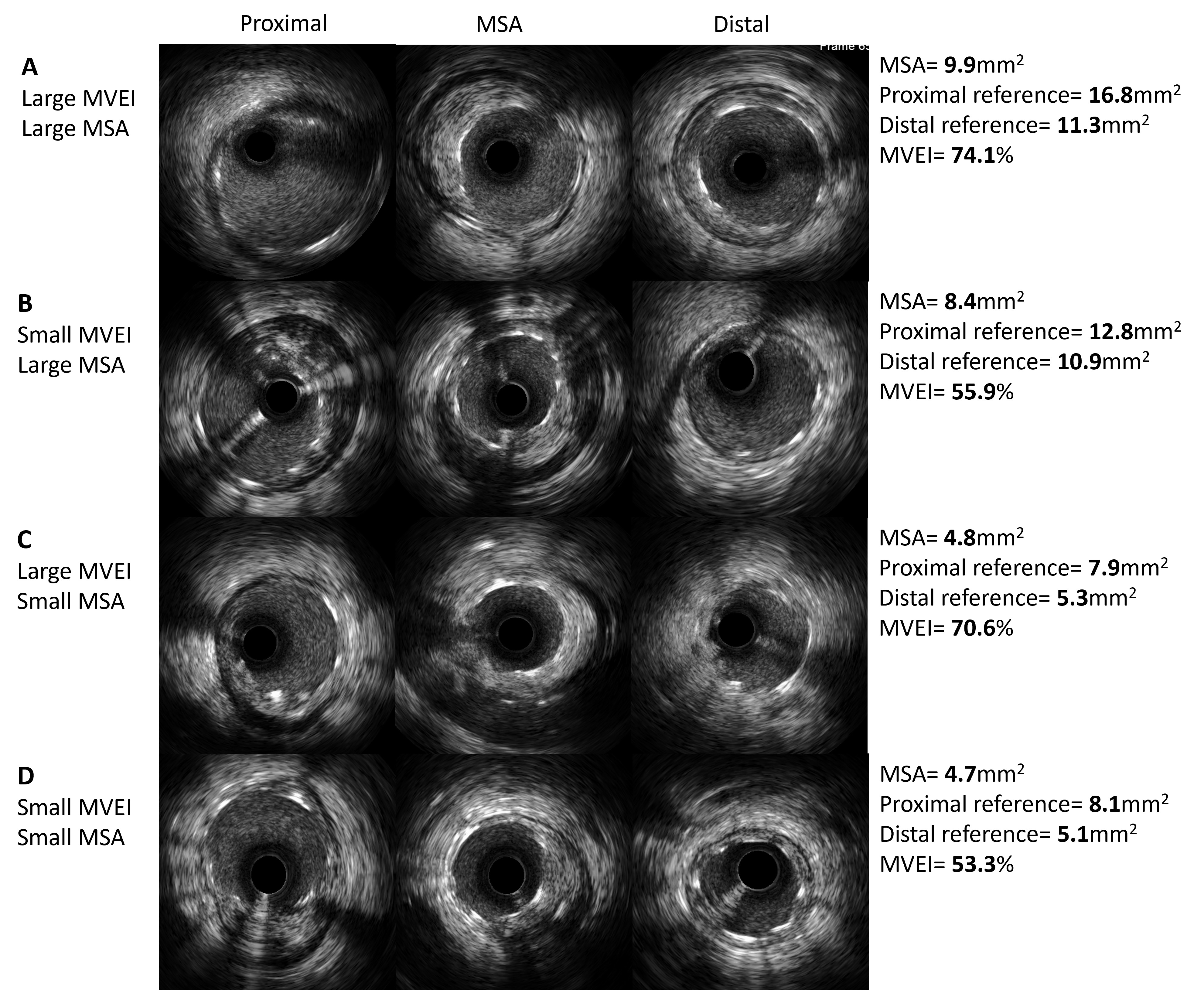 Fig. 5.
Fig. 5.Representative IVUS images with different patterns of MVEI. (A) Both MSA and MVEI are large. (B) MSA is large and MVEI is small. (C) MSA is small and MVEI is large. (D) Both MSA and MVEI are small. MVEI, minimal volumetric expansion index; IVUS, intravascular ultrasound; MSA, minimal stent area.
This is the first evaluation of stent expansions in CTLs. We report an algorithm for volumetric analysis, which is based on IVUS investigations that assess stent expansions. The major findings of this study were firstly that DoCE(+) patients showed lower MVEIs compared to DoCE(–) patients. Secondly, MVEI was found to be the only independent determinant of DoCEs, with none of the other stent expansion indices showing a significant association with clinical outcomes. Thirdly, higher balloon inflation pressures correlated with larger MVEI ratios. Finally, the optimal MVEI cut-off value for predicting DoCEs was 62.2%.
CTLs with reference lumen cross-sectional area mismatching remain difficult to treat by interventional cardiologists, with no optimal interventional strategy confirmed as yet. The remodeling of vessels at the reference site of the target lesion is the primary treatment for CTLs [26]. Although self-expandable stents and tapered stents are employed to revascularize CTLs [27, 28], they have yet to be extensively employed. Moreover, in contrast to existing balloons and symmetrical stents, their efficacy has also yet to be confirmed. Due to the effect of the symmetrical design, and without considering strategies involving stents and balloons, PCI, in terms of tapering lesions through the application of self-expandable stents and tapered stents that are symmetrical, is subject to dissection and overstretching risks within the distal segment, or to incomplete stent apposition and thrombus formation at proximal sites of the tapering lesion. The above sub-optimal conditions for tapering lesions are likely to trigger common PCI complications, such as in-stent restenosis and stent thrombosis [3]. The present study highlights the use of the volumetric expansion index under the guidance of IVUS to assess whether tapering stent expansion is important for reducing adverse clinical outcomes. In addition, the volumetric analysis algorithm could also optimize the success of stent implantations with symmetrical devices.
Current research suggests that PCI following IVUS guidance can optimize stent
expansion by providing accurate lesion assessment, pre-stenting preparation, and
post-dilation improvement. MSA is capable of estimating absolute stent expansion
and is known to be a critical predictor of future stent failures in IVUS and OCT
research. The optimal MSA cut-off values for the prediction of adverse clinical
outcomes are reported to be 4.5 to 5.5 mm
A uniform standard for comparing the minimal luminal area in the intravascular
imaging-guided stent has yet to be established for the proximal or distal
reference luminal area, or the mean luminal area. Meneveau et al. [31],
reported that an optimal cut-off value for stent expansion
The choice of a symmetrical stent for achieving the favorable conformation of a
tapering lesion is very challenging in real-world practice [3]. The use of
post-stenting improvements with a larger-sized balloon or a greater pressure of
inflation helps to address tapering lesions but causes higher rates of stent
failure [33, 34, 35]. The primary clinical endpoint was higher in the present study
(7.3% of patients) than in previous clinical PCI studies (2.9%–3.9% [13, 19, 36]). All cases in our cohort were accepted post-dilatation and with a higher
in-stent balloon inflation pressure (average
Firstly, this was a non-randomized observational investigation that was conducted at a single center. No independent third party was employed to assess the incidence of adverse clinical events. Secondly, some potential selection bias may have occurred in the present study due to the presence of insufficient IVUS images. Thirdly, further additional investigations are required to determine the clinical outcomes after using larger balloons and/or higher pressures in the tapering lesions. Fourth, the measurement of the side branch luminal diameter may be affected by guidewire bias in tortuous vessels and by the oblique orientation of the IVUS catheter. Finally, only 17 patients had a DoCE in the present study. Hence, the effect of the MVIE on clinical events cannot be determined.
For CTLs, MVEI was superior at predicting 2-year DoCEs compared to the traditional methodologies of stent expansion.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
XW and HH had the idea for the paper, reviewed and edited it critically for important intellectual content. HBH and LW performed the literature search and analysis. XW, MXW, HH, JC, ZL and LW substantially contributed to the conception of the paper, drafted and critically revised the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
This study was approved by the Ethics Committee of Xiangtan Central Hospital (approval number: X20221372). Patients were consented by an informed consent process that was reviewed by the Ethics Committee of Xiangtan Central Hospital and certify that the study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki. Before the study was initiated, the respective patient signed written informed consent. No individual patient data will be reported.
We are grateful to Bo Chen for their secretarial assistance.
This research received no external funding.
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
