1 Department of Cardiovascular Medicine, Ruijin Hospital, Shanghai Jiaotong University School of Medicine, 200025 Shanghai, China
Abstract
Depression is common among patients with acute coronary syndrome (ACS). Although multiple studies have confirmed that depression is an independent risk factor for poor outcomes in ACS, general awareness of this issue is still limited. Ongoing research has described detailed aspects of depression in ACS, with various mechanistic hypotheses put forward to explain the complexity of this comorbidity. Several investigations have explored management strategies in this subgroup of patients, including screening for depression, antidepressant treatment, and cardiac rehabilitation. However, evidence of long-term improvement in clinical outcomes is still scarce, and a more comprehensive understanding of the underlying mechanisms that link depression with ACS is required to further improve disease management.
Keywords
- acute coronary syndrome
- depression
- prognosis
- management
Interest in the role of depression in acute coronary syndrome (ACS) has surged in recent years because depression is recognized to be a major cause of mortality, disability and quality of life impairment [1, 2]. Depression is a frequent complication of ACS, with a literature review by Thombs et al. [3] finding that major depression was present in 19.8% of patients hospitalized for recent acute myocardial infarction (AMI), as assessed by a structured clinical interview. Depending on the questionnaire and rating scale used, the prevalence of significant depressive symptoms ranged from 7.3% to 31.1%. A recent meta-analysis found the pooled prevalence of depression among patients with myocardial infarction (MI) was 28.7% [4].
Several studies have consistently shown that patients with ACS and depression have a higher risk of recurrent cardiovascular events and mortality [5], poorer quality of life [6], and higher health care costs [7]. Some studies have tried to evaluate the complexity of comorbidities from the perspective of cardiac disease severity or other ACS risk factors [8, 9]. Other studies have focused on the nature of depression and found that different aspects of depression, such as specific symptoms [10] or the time of onset [11], were associated with adverse outcomes in ACS patients.
Various biological and behavioral mechanisms have been proposed to explain the relationship between depression and ACS, including inflammation [12], autonomic dysfunction [13], platelet reactivity, endothelial function [14], neuroendocrine disturbances [15], lifestyle factors, and other unmodifiable risk factors [16]. A complex interplay between these systems is likely to modulate cardiac and neuropsychiatric functions.
The role of depression in patients with ACS has generally been overlooked over the past few decades. According to a survey conducted in 2006, only half of all cardiovascular physicians considered that depression was a risk factor for coronary artery disease (CAD) [16]. With accumulating evidence of a correlation between depression and ACS, the American Heart Association (AHA) declared in 2014 that depression was an independent risk factor for ACS. This was based on an extensive review of 53 publications and four meta-analyses [17]. However, interpretation of the prognostic association between depression and ACS remains challenging due to the heterogeneity of these studies in terms of the demographic characteristics of the study sample, the definition and measurement instruments for depression, and the follow-up periods. Experts have developed several guidelines for the screening and treatment of depression in patients with coronary heart disease (CHD) [18]. Nevertheless, a cross-sectional study found that patients with these two conditions were inadequately managed for their depression by physicians, with just 6.6% being offered psychotherapy and 4.1% offered medication [19]. One possible explanation for the poor adherence to guidelines was a lack of awareness at the provider level of the connection between depression and ACS [20]. Here, we comprehensively review the correlation between depression and ACS with the aim of raising awareness of the comorbidity of these two diseases. We also summarize the possible mechanisms for this complex biobehavioral pathway, and provide further details on the robust correlation between ACS and depression. In addition, we examine the consistency of results between various studies, and discuss the optimal management strategy for this important subgroup of patients with ACS.
Left ventricular dysfunction is one of the most important predictors of
mortality in patients with ACS. In the Myocardial Infarction and
Depression-Intervention Trial (MIND-IT), a dose-response-like relationship was
observed between the severity of depressive symptoms and left ventricular
dysfunction in patients with AMI. This should be considered when evaluating the
prognostic impact of depression on clinical outcomes from ACS [8]. Depression
appears to reflect the severity of ACS. Meijer et al. [5] conducted the
largest meta-analysis to date that examined depression as a risk factor for
adverse medical outcomes in patients with ACS. These workers identified 29
studies that included 16,889 patients with MI. After a 24-month follow-up period,
the pooled odds ratios (ORs) for all-cause mortality, cardiac mortality, and
fatal or non-fatal cardiac events were 2.25 [95% confidence interval [CI],
1.73–2.93; p
The role of gender has been a major research topic in ACS in recent years. The
incidence of hospitalization of young women due to ACS has increased over the
past two decades [23]. In addition, the mortality rate from ACS is higher in
elderly women than in men, and depression is almost twice as common in women than
in men [24]. In the Variation in Recovery: Role of Gender on Outcomes of Young
AMI Patients (VIRGO) study, young women (
Patients with advanced age have a high risk of mortality from ACS, which may be
explained by a higher prevalence of comorbid diseases and delayed presentation to
hospital [33]. Late-life depression refers to depressive syndrome in adults
Diabetes mellitus is another strong predictor of recurrent ischemic events and mortality in patients with ACS [38]. Depression is present in approximately one in five adults with type 2 diabetes and is associated with increased risks of mortality, work absenteeism, poor disease management, and poor health outcomes [39]. Depression and diabetes share common mechanisms including inflammation, neuroendocrine dysfunction, and insulin resistance [40]. These play vital roles in the progression of macro- and microvascular lesions in patients with ACS.
There is a phenomenon known as the obesity paradox in ACS patients, whereby low-weight and overweight patients with ACS have a higher risk of mortality than normal-weight patients [41]. This paradox might be explained by follow-up time and obesity indices. In the long-term, the protective effect of obesity is no longer present or may even be reversed. When using waist circumference to evaluate central obesity, a greater waist circumference was associated with adverse outcomes [42]. Depression and obesity frequently co-occur and have a reciprocal relationship [43]. A recent study reported that obesity was predictive of depression after ACS [44], with the association being stronger in patients with central obesity [45]. Several hypotheses have been proposed to explain this relationship. Depression, obesity and ACS are all associated with lifestyle risk factors, such as poor diet and physical inactivity [46]. Biological pathways such as inflammation and hyperactivation of the hypothalamic-pituitary-adrenal (HPA) axis might be involved in the co-occurrence of these conditions. However, in the Enhancing Recovery in Coronary Heart Disease patients (ENRICHD) study [47], no interaction was found between weight change and depression in patients with ACS. More studies are therefore required to fully elucidate this relationship.
Unlike other diseases that can be diagnosed using imaging techniques or
serum biomarkers, the diagnosis of depression relies mainly on evaluation of
clinical symptoms. This means that various measurement instruments used in
different studies could influence the apparent prognostic effects of depression.
The most widely used self-reported depression scale for studying the association
between ACS and depression is the Beck Depression Inventory (BDI). This is
available in different versions including BDI-1A, BDI-II, and BDI-Fast Screen
(BDI-FS). Other commonly used self-report questionnaires include the Hospital
Anxiety and Depression Scale (HADS) [48], the Patient Health Questionnaire (PHQ)
[49], and the Zung Self-Rating Depression Scale [50]. A cut-off score is used to
distinguish patients who present with “clinically significant” depression. In
some studies, patients with abnormal screening results underwent additional
psychiatric evaluation to diagnose major depressive disorder using a standardized
psychiatric interview by trained interviewers. Interviews were conducted based on
the standard criteria outlined in the Diagnostic and Statistical Manual of Mental
Disorders, or the International Classification of Diseases. Not all patients
classified as having clinically significant depression met the criteria for major
depression. In a systematic review of the accuracy of depression-screening
instruments in ACS [51], BDI-II displayed an overall sensitivity of 90% [95%
CI, 86%–92%] and specificity of 80% [95% CI, 68%–88%] compared with a
validated criterion standard. Both these sensitivity and specificity results were
deemed acceptable. Brief approaches, such as the selection of just one or two
items from traditional screening tools, may have consistent diagnostic accuracy
metrics with longer screens and are easier to apply in clinical practice. In
secondary analyses of the meta-analysis mentioned above [6], all-cause mortality
was different between studies that used interview-based instruments or
self-report instruments [OR 3.69 for interview-based instruments, 95% CI,
2.05–6.63; p
Much interest has been focused on specific depressive symptoms and cardiac prognoses. Studies on the association between depressive symptoms and ACS have often divided depressive symptoms into three dimensions: somatic, cognitive, and affective. Somatic symptoms of depression can be defined as bodily sensations of concern, such as sleep and appetite changes, fatigue, shortness of breath, and pain. Cognitive symptoms include deficits in executive function, attention, short-term memory, and psychomotor skills. Affective symptoms are related to emotional symptoms such as depressed mood and anhedonia. In the MIND-IT study [53], researchers created a new dimensional structure of depressive symptoms in patients with MI by combining explorative and confirmatory factor analyses of the BDI items. They distinguished three factors (somatic/affective, cognitive/affective, and appetitive) and categorized the symptoms in BDI items according to these factors. For example, fatigue is associated with both somatic and affective factors, and was therefore defined as a somatic/affective symptom in the study. The authors found that somatic/affective symptoms were correlated with cardiovascular mortality and cardiac events after an average of 2.5-years of follow-up. This association was partially confounded by cardiac health status, but remained significant after adjustment. Conversely, cognitive/affective symptoms were negatively associated with cardiovascular death in multivariate analyses. Researchers in the ENRICHD clinical trial used the same analytical method, with the symptoms divided into two dimensions of somatic and cognitive [10]. In this study, somatic symptoms at 12 months post-MI predicted outcomes, but not at baseline. However, other studies reported that cognitive symptoms were correlated with a high risk of cardiac mortality [54, 55]. A meta-analysis that investigated the association between specific depressive symptoms and cardiovascular prognosis found that both somatic/affective and cognitive/affective symptoms were associated with cardiac prognosis [56]. The correlation between somatic/affective factors and cardiac prognosis was stronger, as the association remained significant in fully adjusted analyses. Somatic symptoms are related to autonomic dysfunction and neuroendocrine disturbances, which are the underlying mechanisms in the vicious cycle of depression and ACS [57]. These studies suggest that treatment of depression in patients with ACS should target somatic symptoms to improve cardiac prognosis. There are several methodological inconsistencies between studies, such as in the classification of symptoms. For example, sadness was defined as a somatic/affective symptom in MIND-IT, but as a cognitive symptom in the ENRICHD study. Thus, the superiority of specific symptoms for cardiac prognosis remains inconclusive [58]. Sleep disturbance is a common somatic symptom [59]. In the Escitalopram for DEPression in ACS (EsDEPACS) study [60], sleep disturbance during the acute phase of ACS increased long-term all-cause mortality in patients with ACS [HR 1.08–1.59]. Further trials are warranted on the interventions needed to improve sleep disturbances and their effect on cardiac outcomes.
Emerging studies suggest that the association between depression and cardiac outcomes in patients with ACS may depend on the time of onset of depression. Patients with ACS and depression were categorized into two groups according to the sequence of events. One group without a history of depression and constituting nearly half of all cases experienced their first depressive episode at the time of ACS [61, 62]. The second group had a history of depression before ACS and experienced ongoing or recurrent depression after ACS. In the Depression After Myocardial Infarction (DepreMI) study, only incident, post-MI depression was associated with cardiovascular prognosis [11]. Another study also compared these two groups. First-ever post-MI depression was related to cardiac function, revascularization during hospitalization, and arrhythmic events, which may be triggered by severe MI [63]. Ongoing or recurent depression was associated with neuroticism and exacerbated depression before MI. Identification of the different subtypes could help to formulate the appropriate treatment strategies. First-ever post-MI depression may require a treatment strategy that targets the consequences of MI, rather than a purely depression-oriented treatment. However, other studies have suggested that patients with ACS who experienced recurrent depression were at a particularly high risk of cardiac death [64]. In a nationwide, population-based cohort study [65], patients with MI and a previous diagnosis of depression had a higher mortality risk than those without previous depression. A meta-analysis aimed at evaluating the timing of depression and cardiac prognosis did not reach any firm conclusion due to inconsistent findings [66]. This inconsistency may have derived from selection bias, study quality, definition of incidence periods in various studies, and recall bias [66]. More solid evidence is needed to establish the prognostic significance of depression onset before or after ACS.
One study evaluated the onset of depression at different times following ACS [67]. Depression within 2 weeks or 1-year after ACS was associated with adverse outcomes. The patient subgroup with depression at both the baseline and at 1-year follow-up had the highest risk of mortality from cardiac events. This finding indicates that screening for depression should be recommended during both the early and late phases of ACS, and that more attention should be given to patients with persistent depression.
Several studies have demonstrated the effects of inflammation on the prognosis
of ACS. Inflammation participates in cardiac repair after ACS, the severity of
which can affect cardiac function and events [68]. It contributes to plaque
instability and predisposes patients to recurrent atherothrombotic events [69, 70]. Various inflammatory biomarkers are elevated in ACS and have been linked to
poor cardiac outcomes, including C-reactive protein (CRP) [71], interleukin-6
[72], and tumor necrosis factor-
Ischemia directly damages the cardiac autonomic nerves in ACS. Consequently, an
imbalance of sympathetic and parasympathetic activity occurs to preserve
hemodynamic changes [80]. As the stimuli persist, the functional and structural
maladaptation in the cardiac autonomic nervous system (ANS) worsens and confers
additional risks of cardiac events and mortality. Alterations in heart rate are
easily measured clinically from electrocardiogram recordings and are used to
quantify cardiac autonomic modulations, such as heart rate variability (HRV)
[81]. HRV is defined as beat-to-beat variations in the heart rate [82]. In
response to environmental changes, the central nervous system receives signals
through neuroception and activates specific components of the vagal system via
neural circuits, resulting in different functional changes in the somatomotor and
visceromotor cortices [83]. HRV is the result of adaptation of the ANS to
environmental changes, which may be impaired in psychiatric disorders. Thus, HRV
may be used as a warning sign of psychiatric disorders, while also serving as “a
bridge” between the heart and brain. Frequency-domain methods are used to study
HRV by dividing the heart rate signal into its constituents (frequencies) and
quantifying their relative intensities (power). High-frequency HRV primarily
reflects parasympathetic vagal activity, while low-frequency (LF) HRV is complex
and may have both sympathetic and parasympathetic influences. The long periods of
rhythm and circadian, neuroendocrine rhythm are reflected by very low frequency
(VLF) and ultra-low frequency HRV, respectively. Other HRV metrics include the
time-domain index (a method to quantify variations within a specific time) and
non-linear index (a method to quantify the irregularity and unpredictability of
the heart rate based on chaos theory) [84]. The HRV is low during sympathetic
activation and high during parasympathetic activation. According to the
Framingham Heart Study, reduced HRV is an independent prognostic factor for
adverse cardiac events [85]. A recent meta-analysis found that HRV was lower in
patients with depression compared with healthy controls [86]. In the ENRICHD
clinical trial [13], the four indices of HRV measured using the frequency domain
method were significantly lower in patients who developed depression following
ACS. The HR for all-cause mortality [HR 2.8, 95% CI, 1.4–5.4; p
Platelets play a critical role in ACS and are associated with recurrent thrombotic events after ACS. Increasing evidence shows that many functional aspects of platelets, including platelet activation and aggregation, affect the comorbidity of ACS and depression. Platelet function was reported to be hyperactive in patients with ACS and depression [93]. Some important signaling pathways for platelet aggregation, such as serotonin and adenosine diphosphate signaling, were altered in these patients and linked to the occurrence of cardiac events [93, 94]. However, inconsistencies exist in the alteration of indices among these studies. Currently, there are no well-established methods for assessing platelet function. Furthermore, studies that investigate specific platelet indices as risk factors for the association between ACS and depression are lacking. As more methods are developed for assessing platelet function, the role of platelet dysfunction in the association between depression and ACS can be further elucidated.
Because the endothelium is essential for vasodilatation, anticoagulation and
fibrinolysis, endothelial dysfunction is closely related to the pathophysiology
of ACS [95]. The most commonly used method for evaluating endothelial function is
flow-mediated dilatation (FMD). An increase in flow is induced by the inflation
and subsequent release of a sphygmomanometer cuff on the distal forearm, and the
impact of this ‘physiological’ stimulus on artery diameter above the elbow was
assessed by high resolution ultrasound. FMD reflects endothelial-dependent
vasodilatation, which depends on the bioavailability of local nitric oxide [96].
In a recent meta-analysis, patients with depression were found to have lower FMD
[97]. In a study involving patients with CHD [98], FMD was significantly
impaired in depressed patients compared to non-depressed patients [adjusted mean
Dysfunction of the HPA axis has been implicated in the pathophysiology of depression. An increase in plasma cortisol level is the most consistent finding in depression. It results from excessive cortisol release induced by stress and impaired glucocorticoid receptor-mediated feedback inhibition [24]. Hyperactivity of the HPA is associated with hypertension and hyperglycemia, which in turn increase the risk of CVD. A recent study found that high cortisol levels were associated with ACS severity and mortality during hospitalization [104]. Activation of the HPA axis is prolonged in patients with ACS [105], while a blunted cortisol awakening response was also observed in these patients [15]. Rather than the hyperactive HPA axis often observed in depression, a flatter cortisol rhythm was seen in patients with CAD and depression [106], indicating an increased vulnerability to inflammation. The central corticosteroid signaling pathway, which was found to be impaired in rodent models, may contribute to the poor outcome of patients with depression after MI [107]. These findings provide evidence of HPA dysfunction in patients with ACS and depression, although its effect on cardiac outcomes remains unknown.
Depression is associated with lifestyle factors that increase the risk of ACS
[108]. Smoking is more frequent in adults with depression [109], while the
cessation of smoking after ACS may improve depressive symptoms [110]. Depression
is also correlated with a Western-style dietary pattern characterized by elevated
consumption of high-fat products and low consumption of fruit and vegetables,
which further contributes to ACS [111]. Physical activity is another important
aspect of lifestyle. Based on self-report questionnaires, depression was
associated with low physical activity in patients with ACS [112, 113]. A study
that applied objective measures to evaluate physical activity found that
Poor medication adherence is a common problem among patients with ACS and
depression [118]. Depression has been linked to inadequate treatment with
antiplatelet therapy,
Several studies have shown that the gut microbiome plays a role in ACS. Alterations in gut microbiota composition can result in changes in appetite, production of inflammatory factors, and accumulation of the pro-atherogenic metabolite trimethylamine N-oxide (TMAO) [121]. These risk factors further contribute to the development of ACS. The gut microbiome is also closely associated with depression. It can modulate brain development and function through microbial metabolites and immune mediators that subsequently trigger changes in neurotransmission, neuroinflammation, and behavior [122]. Several studies have identified similar alterations in the gut microbiota in both ACS and depression. For example, the relative abundance of the phyla Firmicutes decreased in patients with ACS and depression, whereas Proteobacteria increased [123, 124]. The shift in gut microbiota composition could lead to a “leaky gut” that allows lipopolysaccharides to enter the circulation and to trigger systemic inflammatory processes [125]. Increased systemic inflammation affects the progression of both depression and ACS. Alterations in the composition of gut microbiota causes changes in gut-derived metabolites, most of which are absorbed into the blood and cause perturbations in serum metabolomic patterns. Some metabolites including uremic toxins, tryptophan and its derivatives, short-chain fatty acids and TMAO were shown to be involved in the pathophysiology of ACS and depression [126]. These findings reveal new paradigms and therapeutic directions for the comorbidity of ACS and depression.
Despite the high prevalence and robust association between depression and increased morbidity and mortality after ACS, the utility of routine screening for depression in patients with ACS remains debatable. The AHA and the American Academy of Family Physicians both recommend regular screening for depression using validated questionnaires in patients with CHD or MI [18, 127]. The European Society of Cardiology guidelines recommend screening for depression as a risk modifier in patients at high risk of CVD [128]. The European Society of Preventive Cardiology also recommends that screening for depression be included in cardiac rehabilitation programs [129]. However, some studies have pointed out that screening for depression might not affect clinical outcomes. In a randomized clinical trial that enrolled 1500 patients with ACS [130], screening for depression did not alter quality-adjusted life-years, depression-free days, or self-harm. One explanation for this disappointing result was that screening for depression on its own without further intervention has no impact on cardiac outcomes. In the DEPACS study [131], patients with ACS who screened as depression-positive showed a higher incidence of MACE [adjusted HR, 2.15; 95% CI, 1.63–2.83] over a median 8.4-year follow-up period. Those diagnosed with depressive disorder and treated with escitalopram had the lowest incidence of composite MACE (40.9%) compared to those treated with placebo (53.6%) or those receiving medical treatment for ACS only (59.6%) [131]. Although a direct comparison was not made between the screened and non-screened groups, this study suggests that screening for depression can help to identify patients who are at high risk, as well as improving long-term cardiac outcomes. A recent study reported that approximately 60% of patients undergoing cardiac rehabilitation underwent screening for depression [132]. It is clear from this result that screening practices in routine cardiac rehabilitation are still far from optimal.
The two-step PHQ-2 is a self-report questionnaire recommended by the AHA as the
first step in depression screening for patients with ACS. It includes two items
(sad mood and anhedonia) with yes/no options that can be asked verbally by
physicians. Patients who answer “Yes” to either question in the two-step PHQ-2
should be assessed with PHQ-9, which is a more comprehensive questionnaire that
assesses each of 9 domains that define depression. Those with PHQ-9 scores
Intervention in the culprit vessels is the standard treatment strategy for ACS, with PCI considered to be one of the primary approaches. Emerging evidence suggests that depression is associated with poorer clinical outcomes in patients undergoing PCI. In a study of 1112 patients with stable angina pectoris or ACS who underwent PCI [134], depression after PCI was associated with a 77% increased risk of all-cause mortality after 10 years of follow-up [HR, 1.77; 95% CI, 1.36–2.29]. A similar effect on patients who underwent PCI was observed in a recent meta-analysis [relative risk, 1.57; 95% CI, 1.28–1.92] [135], with neither the assessment time or the follow-up time affecting the relationship. However, most studies did not report separate results according to the indications for PCI, and hence caution should be exercised when generalizing the results to patients with ACS.
Coronary artery bypass grafting (CABG) is another approach for revascularization
in patients with ACS when PCI fails or the coronary occlusion is not amenable to
PCI. Unlike PCI, CABG is rarely performed in emergency settings. Thus, in one
study, patients who underwent CABG and had depression were divided into
preoperative and postoperative depression groups [136]. In a study on
postoperative depression in patients undergoing elective CABG surgery [137],
depressive symptoms one year after surgery were associated with a slightly higher
mortality rate over an 11-year follow-up period [adjusted HR, 1.05; 95% CI,
1.01–1.10; p = 0.03 for males; adjusted HR, 1.07; 95% CI, 1.02–1.13;
p = 0.013 for females]. Other studies have focused on the association
between preoperative depression and clinical outcomes in CABG patients. In a
meta-analysis that included seven studies with a combined study population of
89,490 [138], patients with preoperative depression exhibited a pooled HR of 1.46
[95% CI, 1.23–1.73; p
Aspirin is an antiplatelet drug recommended for ACS. In a recent meta-analysis [139], aspirin use was associated with a lower risk of depression [OR, 0.85; 95% CI, 0.75–0.97; p = 0.02]. Consistent with this result, poor aspirin adherence has been shown to account for a substantial proportion of the excess prognostic risk associated with depressive symptoms after ACS [140]. While the protective effect of aspirin can be attributed to its anti-inflammatory properties, its antidepressant effect is still open to debate. Evidence suggests that aspirin use in elderly individuals may increase the risk of depression [35], and aspirin use for the long-term management of late-life depression may worsen depressive symptoms [141].
Statins are used to reduce low-density lipoprotein cholesterol levels in ACS patients. In addition to lowering cholesterol, they also have the ability to reduce oxidative stress and modulate inflammation. Statin use has been associated with a reduced risk of depression [HR, 0.91; 95% CI, 0.87–0.94] [142]. In the EsDEPACS study, the use of statins and especially lipophilic statins was associated with high response rates to escitalopram therapy in patients with ACS and depression [143]. Combinations of statins and antidepressants such as selective serotonin reuptake inhibitors (SSRIs) are thought to be safe [144]. Statins appear to be a good add-on option along with standard therapy for post-ACS depression [145].
Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers are believed to exert antidepressant effects by acting on the renin-angiotensin system [149]. However, more clinical trials are needed to confirm this viewpoint.
Given the high prevalence and clinical implications of depression in patients with CAD, cardiologists are advised to initially manage mild-to-moderate depression. Determining whether patients with ACS and depression might obtain long-term clinical benefit from antidepressant treatment is of vital importance. Treatments for depression include antidepressant medications, psychotherapy, and exercise.
SSRIs are recommended for the treatment of depression in patients with ACS. The cardiovascular safety of sertraline was demonstrated in the SADHART study, which randomized 369 patients with depression and ACS to either the sertraline or placebo group in a double-blind manner [150]. Safety outcomes including LVEF (primary outcomes), treatment-emergent increase in VPCs, and QT interval prolongation were similar in both the intervention and control groups at the 24-week follow-up, while the depressive symptoms improved in the treatment group [150]. It is worth noting that sertraline was associated with a lower incidence of severe cardiovascular events than placebo (14.5% vs 22.4%, respectively), although this did not reach statistical significance. Although this trend may indicate a cardioprotective effect of sertraline in the treatment of patients with depression [150], the study was not designed to evaluate the efficacy of sertraline on long-term cardiac outcomes. The ENRICHD randomized clinical trial investigated treatments for depression (mainly psychotherapy, as discussed in the next section) after MI. In post hoc analysis, the use of SSRIs was associated with lower all-cause mortality or recurrent MI over a 4-year follow-up period. However, since SSRIs were not designed as a primary intervention, a cause-and-effect relationship between the use of SSRIs and cardiac outcomes could not be established. The MIND-IT study investigated active treatment with antidepressant medications on long-term improvements in depression and cardiac outcomes in 2177 patients with MI [151]. The treatment modalities included antidepressant medications with noradrenergic actions and specifically, the serotonergic antidepressant mirtazapine as the first choice in a double-blind placebo-controlled fashion. Open treatment with the SSRI citalopram was used in case of refusal or insufficient treatment response. These were combined with other therapies such as psychotherapy and cardiac rehabilitation. At the 18-month follow-up, no significant differences were observed between the intervention and control groups with respect to long-term depression status (13% in the usual care arm vs 14% in the intervention arm, p = 0.76). Moreover, no differences in cardiac outcomes were observed between patients who did or did not receive antidepressant medication [151]. Although negative results were observed in the MIND-IT study, an association between the relief of depressive symptom burden and cardiac outcomes could not be ruled out. In a subgroup analysis [152], the incidence of cardiac events was higher (25.6%) in patients who did not respond to antidepressant medications (at least 50% reduction in the Hamilton Depression Rating scale) compared to 7.4% in responders. This observation indicates that cardiac outcomes may depend in part on improvements in depressive symptoms. The EsDEPACS study investigated the use of escitalopram for the treatment of depression following ACS [153]. A total of 300 patients were randomized to either escitalopram or a placebo group for 24 weeks. Treatment with escitalopram for 24 weeks was associated with a lower risk of composite MACE [HR, 0.69; 95% CI, 0.49–0.96; p = 0.03] and individual MACE after a median of 8.1 years. This is the largest study cohort to report beneficial effects of antidepressant medications on long-term cardiac outcomes. In post hoc analysis, patients with remission of their depression had lower risks of composite MACE, all-cause mortality, and PCI than those without remission. These findings concur with results from the MIND-IT study, and emphasize the need to discover new modalities to deal with treatment-resistant depression.
Recent studies have identified the SSRI paroxetine, a novel G protein-coupled receptor kinase-2 inhibitor that is capable of reversing cardiac dysfunction and remodeling in experimental models of AMI [154]. However, in randomized clinical trials paroxetine failed to improve LVEF or LVEF recovery in patients with anterior MI and impaired cardiac function [155, 156]. Despite these negative results, the effects of paroxetine-mediated G protein-coupled receptor kinase-2 inhibition on cardiac remodeling merits further research.
Another issue worth noting is the safety profile of antidepressant medications in patients with ACS. Tricyclic antidepressants and monoamine oxidase inhibitors are rarely used in patients with CVD due to cardiotoxic effects such as QT interval prolongation and hypertension [157]. Most SSRIs, especially citalopram, have the potential to cause some QT interval prolongation [157], with the risk increasing at doses higher than the recommended dose. Serotonin and norepinephrine reuptake inhibitors (SNRIs) have more adverse effects than SSRIs, including hypertension [158]. Before prescription, patients should undergo a screening electrocardiogram for baseline QT interval and later they should also be checked periodically for changes. Patients undergoing antihypertensive therapy should be monitored for blood pressure after initiation of SNRIs. SSRIs are the preferred choice for depression in patients with ACS. Drug–drug interactions between antidepressant drugs and cardiovascular drugs are common, and the combined use of SSRIs and antiplatelet therapy increases the risk of bleeding events in patients with ACS [159]. Some SSRIs, including fluoxetine and fluvoxamine, are cytochrome P450 (CYP)2C19 inhibitors. CYP2C19-inhibiting SSRI therapy in clopidogrel users was associated with a moderate risk of ischemic events [HR, 1.11; 95% CI, 1.01–1.22] [160].
Collectively, there is no solid evidence to support the view that antidepressant medications, including SSRIs, improve cardiac outcomes in patients with ACS and depression. The risk of adverse events and of drug–drug interactions must be considered when initiating pharmacotherapy in these patients.
Psychotherapy refers to a broad range of therapeutic modalities including cognitive behavioral therapy (CBT), interpersonal psychotherapy, and positive psychology therapy (PPT). CBT is the most extensively studied intervention for patients with ACS and depression. It is characterized by the identification and modification of negative thoughts that adversely affect emotions and behaviors. The ENRICHD study is the largest investigation on the effect of psychotherapy for the treatment of depression following ACS [161]. In this study, 2481 patients with depression and perceived low social support after MI were randomized to CBT-based psychosocial intervention and the usual medical treatment groups. The composite primary endpoint of the study was mortality or recurrent MI. After an average follow-up of 29 months, no difference was observed in the primary endpoints between the intervention and control groups. Other forms of psychotherapy, such as problem-solving therapy (a form of psychotherapy to augment patients’ skills in evaluating and addressing individual psychosocial problems), PPT (enhancing well-being by developing individual strengths and positive psychological dimensions) [162], and well-being therapy [163] have shown promise for improving depressive symptoms. Some studies have tried to combine psychotherapy and media, such as telephone and the internet, for the treatment of depression following ACS [164]. In the U-CARE Heart study that enrolled 239 patients with depression following ACS, internet-based CBT was comparable with the usual treatment in reducing depressive symptoms, but showed a trend for increased risk of CVD [HR, 1.8; 95% CI, 0.96–3.4; p = 0.07] [165]. The main concern with internet-based psychotherapy is low treatment adherence, which was below 50% in most studies [166]. Psychotherapy was not the only intervention used in many studies so far, and the combination of psychotherapy and pharmacotherapy might result in better clinical outcomes. In the ENRICHD study, a reduction in depression severity was associated with improved survival only in those patients who received CBT and antidepressant medications [167]. In the Coronary Psychosocial Evaluation Studies trials, a combination of problem-solving therapy and antidepressant medication improved cardiac outcomes and depressive symptoms during active treatment [168]. A meta-analysis showed that CBT-based and PPT-based psychological interventions reduced the risk of cardiovascular events, MI, and angina in patients with CAD [169]. Psychotherapy may therefore be helpful for the treatment of depression following ACS.
Exercise and cardiac rehabilitation are also effective therapies for depression when conducted in cardiac settings. Cardiac rehabilitation involves a combination of exercise, nutritional management, and lifestyle modification to improve the health of patients with CVD. Exercise-based cardiac rehabilitation has been shown to benefit patients with CHD [170]. In the Understanding the Prognostic Benefits of Exercise and Antidepressant Therapy study [171], exercise improved depressive symptoms and HRV in patients with stable CHD, and this presumably also had a favorable effect on cardiac outcomes [171]. In a study of 522 patients with CHD after a recent coronary event [172], cardiac rehabilitation (exercise, dietary intervention, health education) resulted in a 63% reduction in depressive symptoms and a 73% decrease in mortality. In addition to improving physical activity, cardiac rehabilitation helps to improve behavioral aspects related to food consumption, stress management and sleep quality [173]. A recent meta-analysis showed that exercise-based cardiac rehabilitation alleviated depressive symptoms in MI patients [174]. Exercise, or exercise-based cardiac rehabilitation, should therefore be recommended as the basic treatment for patients with CHD and depression.
The gut microbiota is involved in the pathophysiology of both ACS and depression. Targeting of the gut microbiota could therefore be another potential therapy. Preliminary clinical trials have shown that supplementation with probiotics such as Lactobacillus rhamnosus, Bifidobacterium lactis, and/or prebiotics in patients with ACS improved depressive symptoms and alleviated systemic inflammation [175, 176]. These findings suggest new therapeutic directions for the treatment of depression following ACS. However, the evidence is still sparse and there is inconsistency regarding the use of different probiotics. Further research is required to confirm the efficacy and safety of probiotics. Recent studies have suggested that traditional Chinese medicine exerts antidepressant effects in animal models with ACS [177, 178]. These mechanisms may involve anti-inflammatory effects, reduction of cell apoptosis, and alteration of neuroendocrine metabolism. Xinkeshu, a well-known and patented Chinese drug, has been widely studied in patients with CHD and depression after PCI [179]. A meta-analysis showed that Xinkeshu tablets combined with conventional treatments led to improved depressive symptoms and blood lipid profiles compared to conventional treatments alone [180]. Other Chinese herbal medicines have also been reported to exert beneficial effects on post-PCI depression [181]. Since most of the evidence to date originates from small randomized trials conducted in China, more rigorous studies with larger sample sizes are needed to confirm the use of traditional Chinese medicine for the treatment for depression following ACS.
Depression is a common comorbidity that affects approximately 20% of patients with ACS. Such patients are at high risk for long-term adverse clinical outcomes, meaning that more attention should be paid to this subgroup and personalized care plans should be formulated. Depression following ACS is associated with cardiac disease severity and has similar risk factors to ACS. Thus, depression appears to be an independent risk factor for ACS. Evidence of shared biological and behavioral mechanisms for depression and ACS has emerged in recent years. These shared mechanisms may be interconnected and contribute to the reciprocal relationship between the two conditions. Fig. 1 delineates a network of biological and behavioral mechanisms involved in the pathophysiology of ACS and depression. Although the guidelines recommend screening and management of depression in patients with ACS, real world clinical practice is still far from optimal. There is a lack of evidence to support routine screening for depression. Moreover, the inconsistent results between clinical trials on the efficacy of treatment for depression in patients with ACS is a major challenge. Further studies should focus on developing more reliable and easy-to-use screening tools to improve guideline adherence. Although SSRIs appear to be safe for the treatment of depression following ACS, possible adverse outcomes and drug–drug interactions should not be ignored. Other treatments such as psychotherapy and cardiac rehabilitation appear suitable for this group of patients. Shared decision-making should be facilitated so that patients can address problems and actively engage in therapeutic programs. A multidisciplinary approach involving cardiologists, psychologists, and primary healthcare providers is essential for the successful management of complications. For patients who do not respond to treatments, a deeper insight into the underlying mechanisms and new therapeutic directions should help to solve this challenging clinical problem. With a better understanding of the mechanisms involved, the health of this patient subgroup should improve considerably.
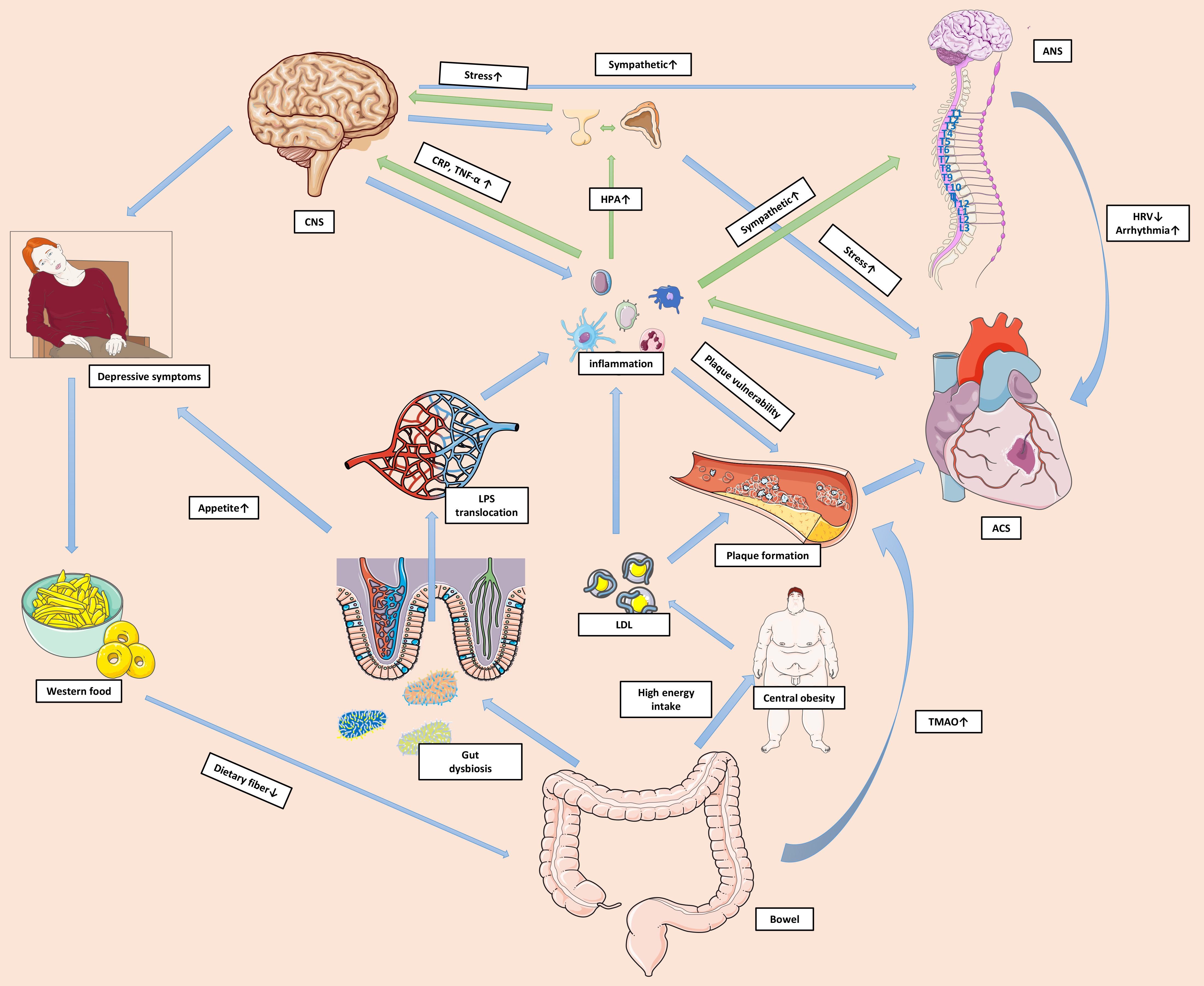 Fig. 1.
Fig. 1.Shared mechanisms of depression and ACS. A shared network of
biological and behavioural mechanisms contribute to the reciprocal relationship
between ACS and depression. ACS prompts substantial activation of local and
systemic inflammation. The unresolved systemic inflammation alters neural
functions and activates the HPA axis and sympathetic system via pro-inflammatory
mediators. Pro-inflammatory mediators impact CNS signaling to regulate mood and
behaviours, resulting in depressive symptoms. Excessive cortisol makes the body
vulnerable to acute stress and may amplify the toxic effects of environmental
threats. Immune cells in the CNS are also activated and perpetuate the systemic
inflammation. Increased catecholamine levels lead to lower HRV and more
arrhythmic events. Inflammation also causes platelet and endothelial dysfunction,
and impacts plaque formation and stability. Furthermore, depressive symptoms can
manifest as excessive intake of food that is high in fat and low in dietary
fiber, resulting in gut dysbiosis. This in turn can increase the permeability of
the gut barrier and facilitate translocation of the pro-inflammatory factor LPS
into the circulation. High energy intake can also cause central obesity and
hyperlipidemia. Some harmful metabolites, such as TMAO, can accumulate and
directly impact cardiac health. The green arrows in the figure represent the
pathophysiological pathways from ACS to depression, while the blue arrows
represent the pathways from depression to ACS. Abbreviations: ACS, acute coronary
syndrome; ANS, autonomic nervous system; CNS, central nervous system; CRP,
C-reactive protein; HPA, hypothalamic-pituitary-adrenal; HRV, heart rate
variability; LPS, lipopolysaccharide; LDL, low-density lipoprotein; TMAO,
trimethylamine N-oxide; TNF-
ACS, acute coronary syndrome; AHA, American Heart Association; AMI, acute myocardial infarction; ANS, autonomic nervous system; BDI, Beck depression inventory; CABG, coronary artery bypass grafting; CAD, coronary artery disease; CBT, cognitive behavioral therapy; CHD, coronary heart disease; CRP, C-reactive protein; CVD, cardiovascular disease; ENRICHD, Enhancing Recovery in Coronary Heart Disease; FMD, flow-mediated dilatation; HPA, hypothalamic-pituitary-adrenal; HR, hazard ratio; HRV, heart rate variability; LVEF, left ventricular ejection fraction; MACE, major adverse cardiac events; MI, myocardial infarction; MIND-IT, Myocardial Infarction and Depression-Intervention Trial; OR, odds ratio; PCI, percutaneous coronary intervention; PHQ, Patient Health Questionnaire; PPT, positive psychology therapy; SSRI, selective serotonin reuptake inhibitors; SNRI, serotonin and norepinephrine reuptake inhibitor; ULF, ultra-low frequency.
RP, QF and RT conceived the presented idea. RP searched the publications and collected the data. RP wrote the original manuscript. QF and RT revised the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
Not applicable.
We would like to express our gratitude to Meng Qiao for polishment of the manuscript, Xiao Zong and Qian Yang for their support in providing writing assistance.
This review was funded by the National Natural Science Foundation of China (grant 82000368 to QF, grant 81970327 to RT).
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.

