1 School of Traditional Chinese Medicine, Henan University of Chinese Medicine, 450046 Zhengzhou, Henan, China
2 School of Rehabilitation Medicine, Henan University of Chinese Medicine, 450046 Zhengzhou, Henan, China
†These authors contributed equally.
Abstract
Background: As a fibrinolytic enzyme from fermented soybean,
nattokinase has been shown to be potentially beneficial for cardiovascular
health, but current clinical evidences regarding the nattokinase supplementation
on cardiovascular risk factors are various. This study aims to evaluate the
cardiovascular efficacy of nattokinase. Methods: Four electronic
databases were systematically searched to collect eligible randomized
controlled trials. Data were extracted and summarized in a pre-designed form by
two independent reviewers. Review Manager 5.4 software (Cochrane Library Software, Oxford, U.K.) was used for meta-analysis
and bias risk assessment. Results: Six studies were eligible for
quantitative analysis with 546 participants. The overall methodological quality
of included studies was high. Relatively low total dosage of nattokinase had a
negative effect on blood total cholesterol (MD [mean difference] = 5.27, 95% CI
[confidence intervals]: 3.74 to 6.81, p
Keywords
- nattokinase
- cardiovascular disease
- cardiovascular risk factor
- meta-analysis
Cardiovascular diseases (CVDs) remain the most common causes of premature mortality and disability globally [1]. The major risk factors of CVD, such as coagulation abnormality, hypertension, dyslipidemia, and hyperglycemia have been well established [2]. Dietary modification is a fundamental strategy for the prevention of CVD, and adequate dietary choices may promote cardiovascular health [3]. Previous research has established that dietary intake of soybeans is negatively associated with the risks of CVD [4], which shows the promise of soy food as a dietary therapy for CVD.
Over past decades, traditional Japanese diets have attracted growing attention because of substantially low CVD morbidity and the highest life expectancy of Japanese population [5]. Natto is a famous traditional Japanese food made from fermented soybeans, which contains a variety of functional ingredients, including nattokinase. As a serine protease produced by Bacillus subtilis, nattokinase has potential anti-coagulatory, thrombolytic, anti-atherosclerotic, lipid-lowering, and anti-hypertensive effects [6, 7]. In addition to these favourable cardiovascular profiles, nattokinase can be orally administered with inexpensive cost, proven safety and preventative efficacy [8]. Hence, nattokinase consumption is growing in both healthy and CVD individuals around the world, especially in Asian countries.
Nattokinase has a stronger fibrinolytic activity than plasmin in vivo, and can even hydrolyze fibrin directly [9]. Oral administration of nattokinase not only promotes the release of tissue plasminogen activator from vascular endothelial cells, but inhibits the level of plasminogen activator inhibitors [10, 11]. Recently, experimenters also provide a new insight that nattokinase is able to prevent arteriosclerosis and thrombosis by exerting anti-inflammatory, anti-oxidative stress and anti-apoptotic effects [6, 12, 13, 14]. In addition, a number of in vitro and animal experiments have established that nattokinase suppresses hypertension via inhibiting angiotensin-converting enzyme and plasma angiotensin II level [8, 15, 16].
Although recent review studies suggest that nattokinase is a promising alternative in the prevention and treatment of CVD [17, 18, 19], cardiovascular benefits of nattokinase, such as its lipid-lowering effect, remain controversial [20, 21, 22, 23]. This study thus aims to assess the efficacy of nattokinase on cardiovascular risk factors and to provide evidence-based recommendations for clinical decision-making.
This systematic review and meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) recommendations [24], and the protocol had been registered on PROSPERO (CRD42022315020).
Electronic searches for pertinent studies were conducted in PubMed, Web of Science, Embase, and Cochrane Library from their inceptions to February 14, 2023, using the keyword “nattokinase” in the title or abstract. In order to adequately identify eligible studies, no restrictions were placed on terms of cardiovascular risk factors. Two authors separately screened retrieved records, and discrepancies were resolved by consulting with other authors. Moreover, references of included literature were checked to find more eligible studies.
(Ⅰ) Type of study: prospective parallel-group randomized controlled trials (RCTs) with at least a 1-month follow-up.
(Ⅱ) Subjects: adults with or without established cardiovascular risk factors.
(Ⅲ) Intervention and control measures: comparison between nattokinase supplementation and placebo intervention without limitations on oral dosage and frequency.
(Ⅳ) Outcome measurements: blood lipids, blood pressure, blood glucose, metabolic factors, hemorheological parameters, coagulation indexes, and adverse effects.
Studies in which the outcome measurements could not be synthetically evaluated were included for qualitative analysis but excluded from the meta-analysis. This study did not include trials that observed fermented soybeans (natto) on cardiovascular risk factors, because fermented soybean product contained a high amount of nutrients, some of which had cardiovascular benefits (not just nattokinase). Furthermore, we excluded cohort studies, case control studies, case report/series, and review studies.
Collected data were summarized in a pre-designed form by two authors, and any difference was solved by consensus, which included basic information of research, population characteristics, medication administration details, and outcome measurements. Quality appraisal was undertaken by two reviewers based on the Cochrane Collaboration’s tool, and another author made a final decision regarding any disagreement.
This meta-analysis was performed using the RevMan 5.4 version software (Cochrane
Library Software, Oxford, U.K.). Quantitative analyses were carried out if more
than one study reported the same outcome data that were available and consistent
on clinical grounds. Between-study heterogeneity was tested by the Q-test
(Chi-square) and quantified by the I
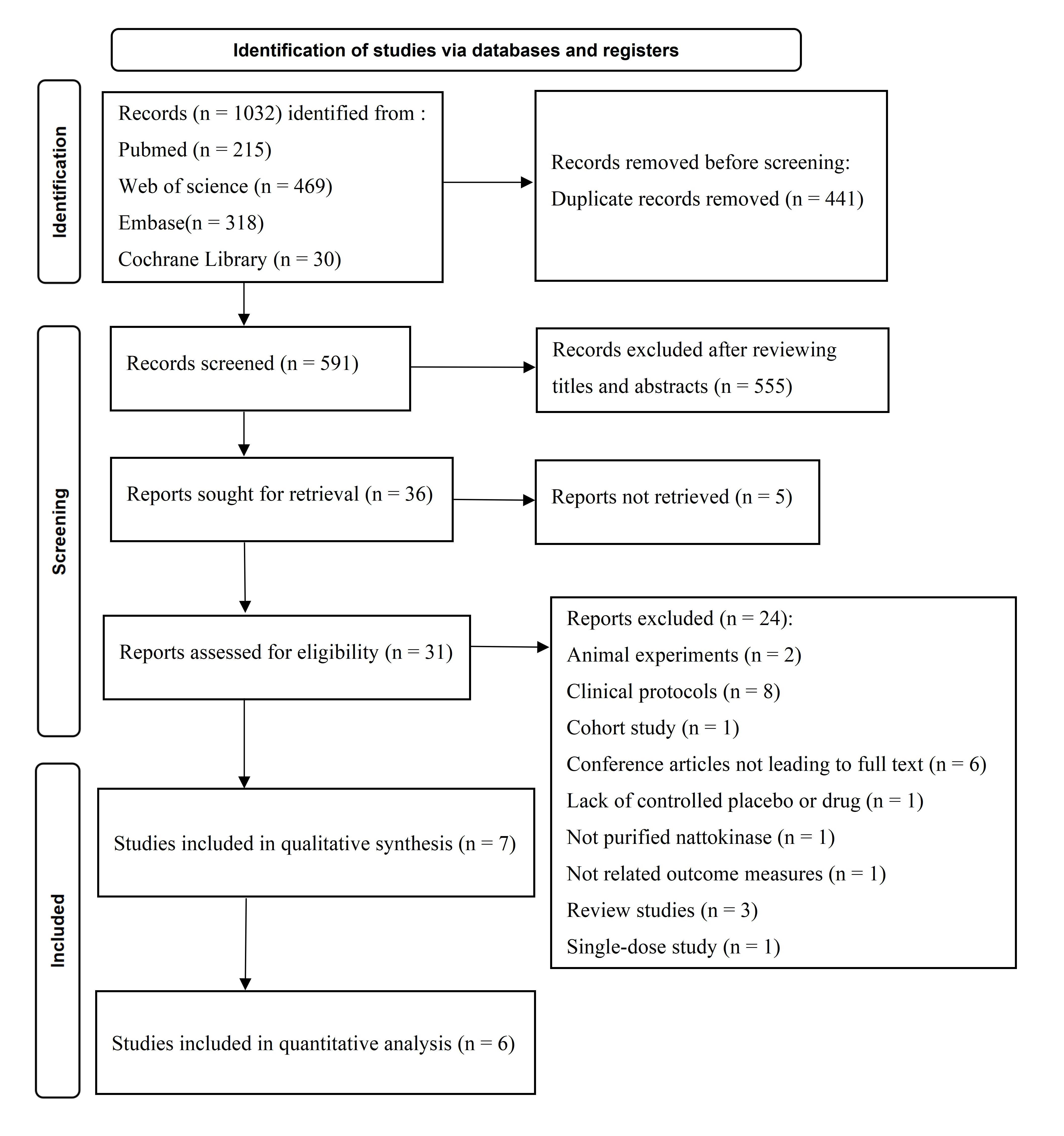
The first search yielded 1032 studies, and thirty-six remained after excluding duplicates and irrelevant studies. Based on the selection criteria, 24 studies were removed, and 7 RCTs were finally included in the qualitative analysis [27, 28, 29, 30, 31, 32, 33]. Data from the 6 RCTs could be synthesized for the meta-analysis [27, 28, 29, 31, 32, 33]. The PRISMA flow chart presents the detailed retrieval process (Fig. 1).
 Fig. 1.
Fig. 1.The PRISMA flow diagram of study selection.
The main study characteristics are presented in Table 1 (Ref. [27, 28, 29, 30, 31, 32, 33]). A total of 311 participants received nattokinase and 296 received matching placebo. The mean number of participants per study was 86, ranging from 28 to 265. The average age of participants was significantly varied in each trial, and approximately 62% of the population was female. Five studies recruited subjects with cardiovascular disease risk factors, including hypertension [28, 29] and hyperlipidemia [31, 32, 33]. One trial recruited patients diagnosed with sub-acute ischemic stroke [30], and another trial included healthy subjects without any clinical evidence of cardiovascular risk factors [27]. Nearly 56% of the included participants were Americans and the others were Asians.
| Trial | Location | Study design | Participants | Age (year) | Nattokinase supplementation | Control | Outcomes for quantitative analysis |
| N/C | Mean (SD)/[Range] | ||||||
| Hodis et al. (2021) [27] | USA | RCT; prospective; single-center; double-blinded | 132/133 | 65.3 [60.6–72.3] | 2000 FU/day | Matching placebo | Total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, blood glucose |
| 36 months | 36 months | ||||||
| Jensen et al. (2016) [28] | USA | RCT; prospective; multi-center; double-blinded | 39/35 | 53.4 [20.8–82.8] | 2000 FU/day | Matching placebo | Blood pressure |
| 8 weeks | 8 weeks | ||||||
| Kim et al. (2008) [29] | South Korea | RCT; prospective; single-center; double-blinded | 39/34 | N: 47.6 |
2000 FU/day | Matching placebo | Blood pressure |
| C: 46.5 |
8 weeks | 8 weeks | |||||
| Pham et al. (2020) [30] | Vietnam | RCT; prospective; single-center; single-blinded | 31/30 | 60.1 [30–70] | 1200 FU/day | Matching placebo | Blood pressure |
| 60 days | 60 days | ||||||
| Wu et al. (2009) [31] | Taiwan | RCT; prospective; single-center; double-blinded | 15/15 | N: 54.8 |
8000 FU/day | Matching placebo | Total cholesterol, triglyceride, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol |
| C: 51.6 |
8 weeks | 8 weeks | |||||
| Yang et al. (2009) [32] | Taiwan | RCT; prospective; multi-center; double-blinded | 18/10 | N: 51.6 |
7000 FU/day | Matching placebo | Total cholesterol, triglyceride, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol |
| C: 56.3 |
6 months | 6 months | |||||
| Yoo et al. (2019) [33] | South Korea | RCT; prospective; single-center; double-blinded | 37/39 | N: 54.3 |
6000 FU/day | Matching placebo | Total cholesterol, triglyceride, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, blood glucose |
| C: 53.1 |
8 weeks | 8 weeks |
Abbreviations: N, nattokinase group; C, control group; FU, fibrinolytic unit; RCT, randomized controlled trial; SD, standard deviation.
In the present meta-analysis, nattokinase supplementation as an intervention to manage cardiovascular risk factors was compared with the matching placebo. The daily dosage of nattokinase was highly variable among the included trials, ranging from 1200 to 8000 FU (a fibrin unit used to quantify the ability of nattokinase to lyse fibrin in vitro) [34]. Both nattokinase and placebo were produced in the same capsule form. Five studies performed a follow-up evaluation at the eighth week [28, 29, 30, 31, 33]. The follow-up time of the other two studies was 6 months [32] and 3 years [27]. All included studies evaluated potential risks of CVDs, including blood coagulation and fibrinolysis factors, blood lipids, blood pressure, and blood glucose. Six of included trials observed adverse events encountered with nattokinase and control interventions [27, 28, 30, 31, 32, 33]. Four studies evaluated compliance by counting returned capsules [27, 28, 32, 33].
All the studies were randomized and provided information about randomization and allocation concealment. Double-blinded method was reported in six trials [27, 28, 29, 31, 32, 33] and one trial had a single-blinded design [30]. Two studies reported no patient drop-outs [29, 30], and five studies provided numbers and reasons for dropping out [27, 28, 31, 32, 33], so they all were considered to have low risk of attrition bias. All included trials reported the main results as planned, and four of them were judged to have a low risk of selective reporting bias [27, 29, 32, 33]. Table 2 (Ref. [27, 28, 29, 30, 31, 32, 33]) presents the risk of bias assessment results.
| Trial | Random sequence generation | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data | Selective reporting | Other bias |
| Hodis et al. (2021) [27] | L | L | L | L | L | L | L |
| Jensen et al. (2016) [28] | L | L | L | U | L | U | U |
| Kim et al. (2008) [29] | L | L | L | U | L | L | L |
| Pham et al. (2020) [30] | L | L | U | U | L | U | U |
| Wu et al. (2009) [31] | L | L | L | U | L | U | U |
| Yang et al. (2009) [32] | L | L | L | L | L | L | L |
| Yoo et al. (2019) [33] | L | L | L | L | L | L | L |
Abbreviations: L, low risk of bias; U, unclear or unrevealed risk of bias.
Blood coagulation and fibrinolytic parameters. In a trial conducted
among healthy subjects, nattokinase showed no detectable effects on any observed
coagulation and fibrinolytic factor, such as prothrombin time, activated partial
thromboplastin time, von Willebrand factor antigen and tissue plasminogen
activator antigen, at time points of 1 week and 1, 3, and 6 months relative to
placebo [27]. Jensen et al. [28] found that average level of von
Willebrand factor was reduced by 15% in patients with hypertension after
nattokinase supplementation, whereas consistent change was not found for subjects
consuming placebo after 8 weeks (p
Degree of atherosclerosis. Results of a 3-year intervention with nattokinase supplementation in healthy individuals showed that annualized rate of carotid artery intima-media thickness progression was 0.013 mm (95% CI, 0.010 to 0.015) per year in the nattokinase group, and 0.011 mm (95% CI, 0.009 to 0.013) per year in the placebo group (p = 0.31). In addition, the mean rate of carotid arterial stiffness was not significantly different between two groups [27].
Blood pressure and associated regulators. Pham et al. [30]
found that nattokinase supplementation (60 days) led to statistically significant
reductions (p
Overall, four studies with a total of 399 participants included measures of
total cholesterol, high-density lipoprotein cholesterol (HDL-C), and low-density
lipoprotein cholesterol (LDL-C) [27, 31, 32, 33]. There was high heterogeneity
for the above three meta-analyses (I
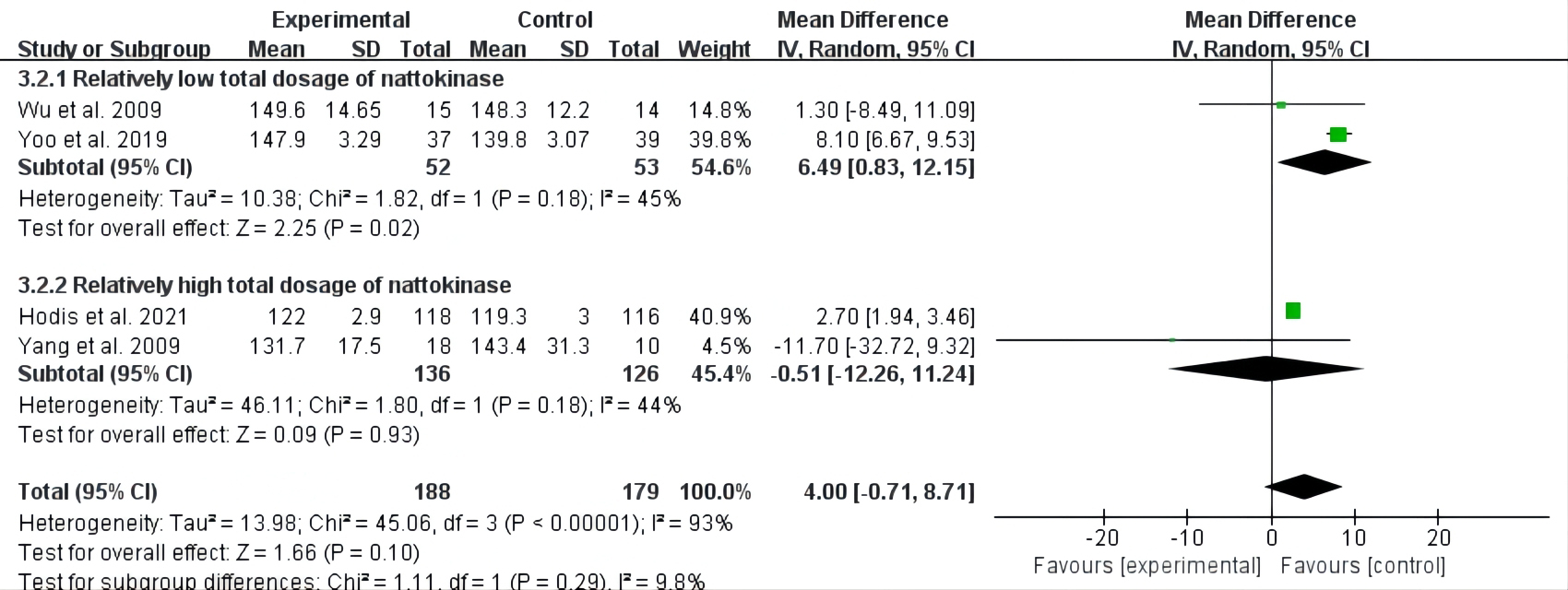
The subgroup of relatively low total nattokinase dosage showed that there was a
positive association between nattokinase supplementation and total cholesterol
(MD = 5.27, 95% CI: 3.74 to 6.81, p
 Fig. 2.
Fig. 2.Forest plot of RCTs investigating the effect of nattokinase on total cholesterol. RCTs, randomized controlled trials; SD, standard deviation; CI, confidence intervals.
The aggregated results of these studies showed that nattokinase group with
relatively low total dosage had more HDL-C levels reduced (MD = –2.76, 95% CI:
–3.88 to –1.64, p
 Fig. 3.
Fig. 3.Forest plot of RCTs investigating the effect of nattokinase high-density lipoprotein cholesterol. RCTs, randomized controlled trials; SD, standard deviation; CI, confidence intervals.
 Fig. 4.
Fig. 4.Forest plot of RCTs investigating the effect of nattokinase on low-density lipoprotein cholesterol. RCTs, randomized controlled trials; SD, standard deviation; CI, confidence intervals.
As shown in Fig. 5, data from three studies with 134 participants were pooled to assess the effect of nattokinase on triglyceride [31, 32, 33]. No significant effect of intake of nattokinase was found in improving triglyceride levels (MD = –0.7, 95% CI: –4.45 to 3.025, p = 0.71).
 Fig. 5.
Fig. 5.Forest plot of RCTs investigating the effect of nattokinase on triglyceride. RCTs, randomized controlled trials; SD, standard deviation; CI, confidence intervals.
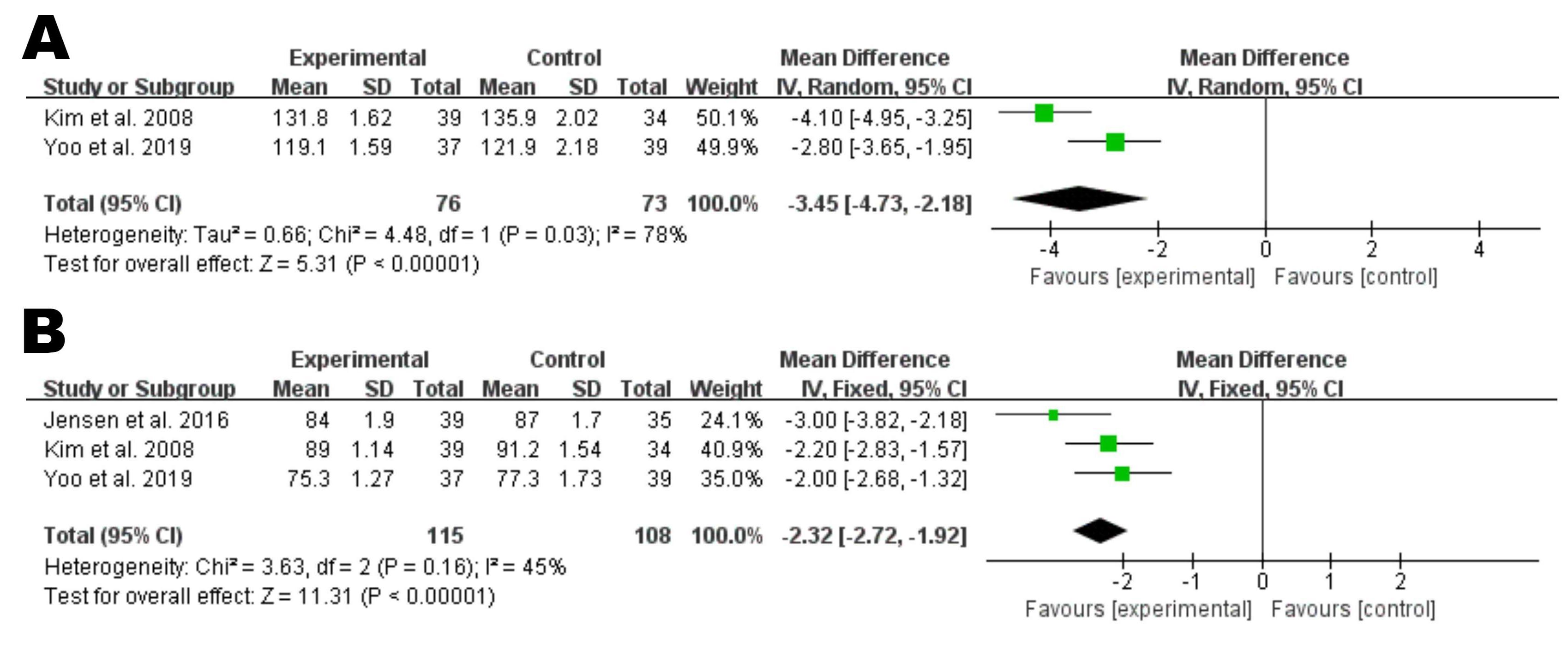
Based on the results of 3 RCTs that included 115 individuals in the nattokinase
group and 108 individuals in the control group [28, 29, 33], nattokinase
supplementation was found to be associated with a significant decrease in SBP (MD
= –3.45, 95% CI: –4.37 to –2.18, p
 Fig. 6.
Fig. 6.Forest plot of RCTs investigating the effect of nattokinase on systolic blood pressure (A) and diastolic blood pressure (B). RCTs, randomized controlled trials; SD, standard deviation; CI, confidence intervals.
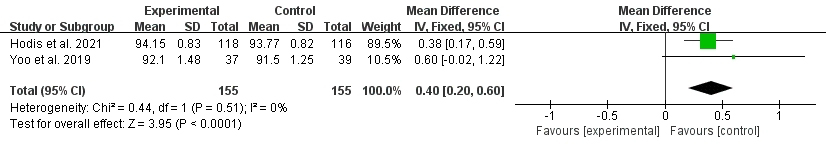
The impact of nattokinase on blood glucose was evaluated in two studies,
including a total of 341 participants (Fig. 7) [27, 33]. The results showed that
nattokinase induced a slight increase of blood sugar with no heterogeneity across
studies (I
 Fig. 7.
Fig. 7.Forest plot of RCTs investigating the effect of nattokinase on blood glucose. RCTs, randomized controlled trials; SD, standard deviation; CI, confidence intervals.
To the best of our knowledge, this is the first systematic review to examine the association between nattokinase supplementation and cardiovascular risk factors. Overall, present study involving 607 participants found that nattokinase might have a beneficial influence on blood pressure, but no significant improvements were observed in blood lipids and blood glucose. Despite the high methodological quality of eligible studies in this meta-analysis, the total number of studies examining any cardiovascular risk factor was small, so these findings should be interpreted cautiously.
Since the fibrinolytic activity of nattokinase was discovered in the 1980s, a variety of animal-based studies have been made to support its strong thrombolytic activity [18, 35, 36, 37, 38]. Even a single dose of oral nattokinase was shown to enhance fibrinolysis and anticoagulation in humans [22, 39]. Moreover, nattokinase positively affected various blood rheological parameters in a dose-dependent manner, including platelet aggregation, red blood cell aggregation, whole blood viscosity and vascular tension, which could be considered as a good candidate in improving blood flow [40, 41, 42, 43]. Clinical data on fibrinolytic and antithrombotic effects of nattokinase were sparse in the present meta-analysis, which limits quantitative analysis of these results; however, it is worth mentioning that in recent years, nattokinase has been used on a much larger scale than previously—researchers have expanded their scopes to other cardiovascular benefits of nattokinase, such as lipid-lowering and hypotensive potentials.
In the meta-analysis of nattokinase on blood lipids, relatively low total dosage of nattokinase supplementation did not exert significant positive effects on levels of total cholesterol, LDL-C, and HDL-C, and even aggravated the dyslipidemia. A previous self-controlled clinical trial also reported no obvious effects of nattokinase (4000 FU/day, 8 weeks) on lipid parameters in both healthy volunteers and patients with cardiovascular risk factors [20]. Our findings were unexpected and inconsistent with results of several animal experiments [13, 44, 45]. This observation may be explained by the fact that all included trials used purified nattokinase products, whereas most previous animal studies used crude natto extracts that contained anti-cholesterol agents, such as soy isoflavones [32, 46].
However, a recent clinical study of 1062 patients with hyperlipidemia showed that nattokinase supplementation at a dosage of 10,800 FU per day for 1 year significantly decreased blood total cholesterol, triglyceride, LDL-C and increased HDL-C [47]. Ren et al. [48] also found that high-dose nattokinase administration over a relatively long period of time (26 weeks, 6500 FU) was effective in inhibiting the progression of atherosclerotic plaques and hyperlipidemia. Noticeably, in the meta-analysis of relatively high total dosage of nattokinase supplementation, the increase in HDL-C and the decrease in LDL-C were detected among included participants. So far, the lipid-lowering mechanism of nattokinase has not been understood, and one possible explanation is that nattokinase has proteolytic activity on some certain proteins involved in lipid metabolism [32]. In general, our observations reflected those of Chen et al. [17] who also proposed that long-term and high-dose nattokinase supplementation seemed to have positive impacts on blood lipids. For future researches, therefore, it is an important issue to determine if the lipid-lowering efficacy of nattokinase is dose-dependent.
Pooled results of this study suggested that nattokinase produced beneficial influences on CVDs by lowering SBP and DBP levels, which matched those observed in earlier animal studies [8, 15, 49]. It was already known that nattokinase had high gastrointestinal stability, and it might reduce blood pressure by cleaving plasma fibrinogen after absorption in the small intestine [8, 36]. More significantly, degradation products of nattokinase were demonstrated to exert different antihypertensive effects—inhibition of angiotensin I converting enzyme and plasma angiotensin II level [8, 16]. Results of the experiment by Ibe et al. [50] also indicated that nattokinase appeared to inhibit angiotensin converting enzyme related to increases in oral dosage. Renin is a key enzyme in renin-angiotensin system, which has long been considered as an attractive anti-hypertensive target [51]. Although researchers found a decrease in plasma renin activity in nattokinase group compared to controls, the results were not statistically significant [28, 29], and it was still not known whether nattokinase could prevent the elevation of plasma renin activity levels against arterial hypertension.
To date, data about the impact of nattokinase on blood sugar were limited. The
present study found that nattokinase consumption had very little influence on
blood glucose level (MD = 0.4). Combination of nattokinase, aronia, red ginseng,
and shiitake mushroom was found to improve glucose metabolism and diminish
insulin resistance [52]. Several randomized crossover studies also showed that
breakfast accompanied with natto suppressed blood glucose elevation and improved
insulin sensitivity in the early postprandial phase, but this may be attributed
to
In terms of safety, no major adverse events were reported for nattokinase supplementation in all included trials at different doses. A great deal of recent works corroborated that nattokinase was a safe agent for cardiovascular risk factors with low haemorrhagic risk and no toxicologic concerns. For instance, the standard safety margin (haemorrhagic adverse effect) of nattokinase was proven to be three times that of tissue plasminogen activators [56]. Daily nattokinase consumption of 10 mg/kg for 28 days was well tolerated in human volunteers [57], and even no adverse effects were observed when the daily dose of nattokinase was 480,000 FU/kg in mice, which was 1000-fold higher than the recommended daily dose in humans [58]. Nevertheless, several case studies have recently emerged that provide contradictory findings on allergic and bleeding risks of nattokinase [59, 60, 61]. As proposed by Gallelli et al. [23], therefore, although the positive effect of nattokinase on CVD outweighs possible described complications, patients must be always monitored for reference parameters, including clinical condition, coagulation profile, renal function, diet, and weight, and clinicians should make timely and reasonable dose adjustments to ensure the safety of nattokinase administered as monotherapy or in pharmacological combination.
Several limitations of present study should be considered. Firstly, two major limitations were the small total sample size and varied therapeutic dosage of nattokinase for the included studies. Secondly, no restrictions were placed on the cardiovascular health status of included participants. Thirdly, quantitative analysis of the antithrombotic and anticoagulant effects of nattokinase was lacking. Fourthly, the heterogeneity in the corresponding meta-analyses was difficult to estimate because of limited included studies. Fifthly, our search strategies were limited to English papers, which might be linguistically biased. Last but not least, publication bias could not entirely be ignored, since less than 10 studies were pooled in the individual analysis.
Based on the available clinical data, the most obvious finding from this study was that short-term and low-dosage ingestion of nattokinase might have no significant lipid-lowering effects. The second major finding was that nattokinase could be considered as a promising adjunctive tool in the treatment of hypertension. Due to the existing limitations of this work, however, these findings could be considered to be preliminary, and a definitive conclusion on whether nattokinase supplementation was strongly associated with the improvement of any cardiovascular risk factor could not be drawn. Moreover, we inferred that the positive effect of nattokinase on cardiovascular risk factors might be enhanced with increasing oral doses, especially the hypolipidemic effect, and further clinical trials investigating the long-term and high-dose administration of nattokinase on cardiovascular risk factors were strongly recommended.
The data used to support the results of this study are available from the corresponding author upon request.
Conceptualization—XL, JL, and YZ; literature search—XL and JL; data collection—QG and MP; quality assessment—JW and FY; software preparation—XL; quantitative analysis—JL and YZ; qualitative analysis—QG and MP; writing—original draft preparation—XL and JL; writing—review and editing—QG, MP, JW, FY, and YZ; tables and figures—JW and FY; supervision—YZ. All authors have read and agreed to the published version of the manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
Not applicable.
Not applicable.
This research received no external funding.
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.