- Academic Editors
Background: Small vessel disease (SVD) widely exists in patients with
acute coronary syndrome. However, the plaque characteristic of SVD has not been
investigated. Methods: Optical coherence tomography (OCT) of culprit
lesion was examined in 576 patients with ST-segment elevation myocardial
infarction (STEMI) and finally 404 patients with qualified images were analysed of plaque
phenotypes and microstructure. The cohort was divided into three groups according
to vessel diameters of culprit lesion which were measured by OCT. Major adverse
cardiac events (MACEs) were recorded of each patient and compared among patients
with different vessel diameters and plaque phenotypes. Results: Gender,
age and body mass index (BMI) were significantly different among patients with
different diameters of culprit vessels (98.4% vs. 85.7% vs.71.4%, p
Small vessel disease (SVD) has emerged as an intriguing issue of atherosclerotic coronary artery disease nowadays. SVD was reported to account for 30% to 67% of patients undergoing percutaneous coronary intervention (PCI) [1, 2]. Moreover, SVD also existed in patients with acute coronary syndrome (ACS), or even ST-segment elevation myocardial infarction (STEMI) [3, 4, 5]. Compared with large vessel disease (LVD), SVD had poorer prognosis [6] and more easily appeared in female patients with diabetes and chronic kidney disease [6, 7]. Although drug eluting stent (DES), drug coated balloon (DCB) or bioresorbable scaffolds (BRS) were proven to be effective for treating SVD [8, 9], the most challenge issue of SVD is equivocal definition and optimal treatment which depended on more refined classification.
Although previous observation studies illustrated the clinical features of SVD, whether the intravascular structure in lesions of small vessels differs from large vessels remained unknown. Moreover, the relationship between plaque morphology and vessel size was not investigated yet. Optical coherence tomography (OCT) is one of the powerful intracoronary image devices which not only identified plaque characteristics but also determined lumen size of culprit lesion more accurately than intravenous ultrasound (IVUS) or quantitative coronary analysis (QCA), especially in small coronary vessels [10, 11]. Herein, we measured diameters of culprit lesion determined by OCT and investigated its association with plaque characteristics.
From March 2017 to January 2020, 576 patients with STEMI who underwent OCT imaging of culprit lesions in Fuwai Hospital were consecutively recruited (Fuwai Hospital Optical Coherence Tomography Examination in Acute Myocardial Infarction (OCTAMI) Registry, clinical trials.gov: NCT03593928). After excluding patients without preintervention OCT images (n = 14), patients with poor OCT image quality (n = 93), patients with in-stent restenosis (n = 48), patients with other etiology of ACS (n = 17), the remaining 404 patients with plaque rupture (n = 197), plaque erosion (n = 188) and calcified nodules (n = 19) were ultimately included for analysis. The study flow chart is displayed in Fig. 1. This study was performed in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of Fuwai Hospital. All patients provided written informed consent.
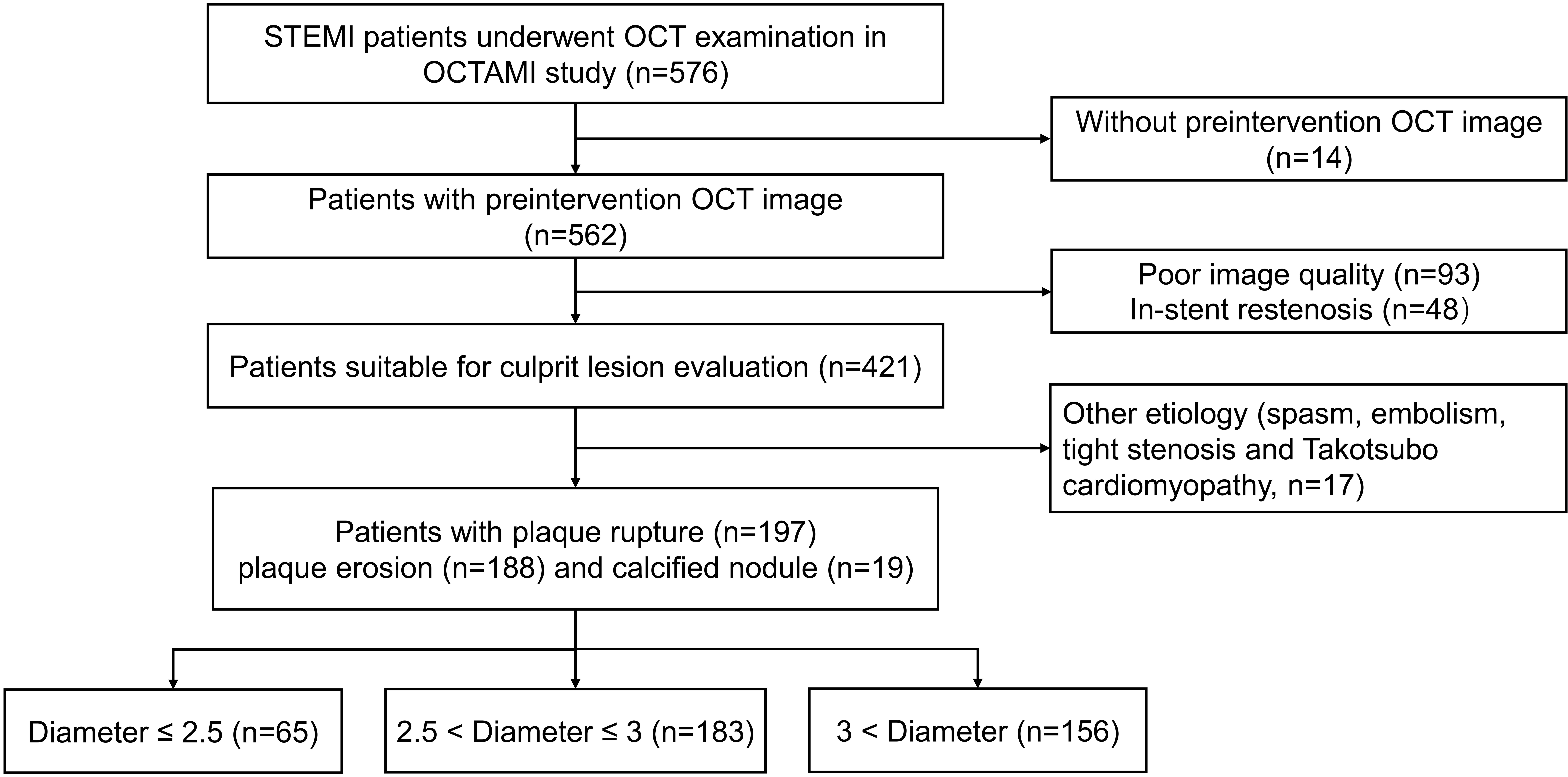 Fig. 1.
Fig. 1.Study flow chart. OCT, optical coherence tomography; STEMI, ST-segment elevation myocardial infarction; OCTAMI, Optical Coherence Tomography Examination in Acute Myocardial Infarction.
Patients were administered 300 mg aspirin, 180 mg ticagrelor, or 600 mg clopidogrel, and 100 IU/kg heparin before the interventional procedure. Percutaneous coronary intervention was performed via radial or femoral access. Thrombus aspiration was used to reduce the thrombus burden and restore the antegrade coronary flow. OCT images of the culprit lesions were acquired with the frequency domain ILUMIEN OPTIS OCT system and a dragon fly catheter (St. Jude Medical, Westford, MA) after the antegrade blood flow was restored, according to the intracoronary imaging technique previously described. Intracoronary injection of nitroglycerin is generally performed before OCT imaging in most of cases except hypotension. Reference vessel diameter (RVD) of culprit lesions was evaluated by OCT before intervention. RVD was determined where at least 180° of external elastic lamina (EEL) could be visualized within 5 mm from target lesion [12]. If EEL is invisible, RVD was estimated on basis of comparatively regular vessel segment besides culprit lesion or according to diameters of stent.
All OCT images were anonymously analysed on a St Jude OCT Offline Review
Workstation by 3 independent investigators blinded to the other data. According
to the previously established criteria [13], plaque rupture (PR) was identified by a disrupted
fibrous cap with clear cavity formation. Thin-cap fibroatheroma (TCFA) was
defined as LRP with the thinnest part of the fibrous cap being
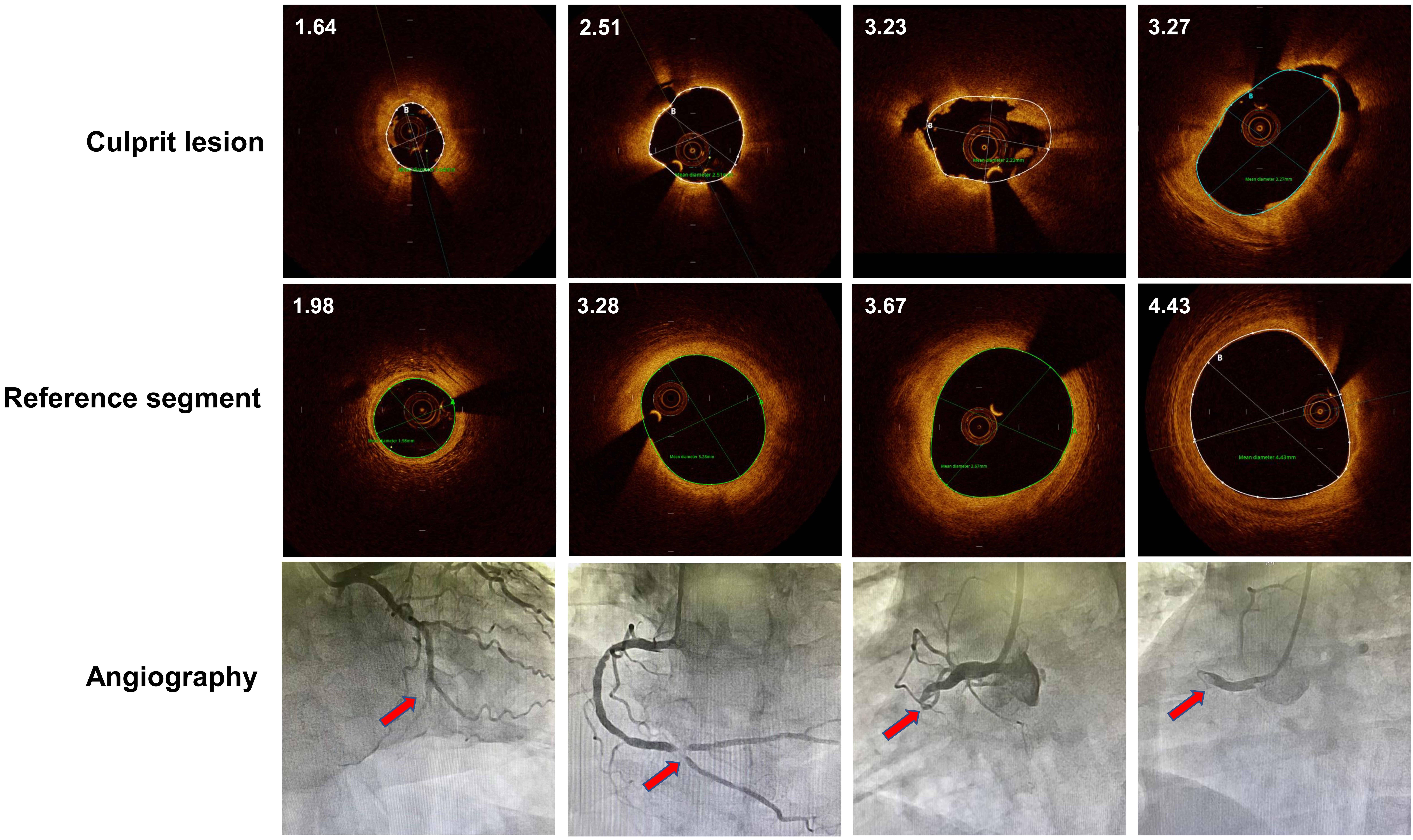 Fig. 2.
Fig. 2.OCT image of culprit lesion and reference segment with small to large diameters. Every column indicated representative OCT and angiography images indicating luminal size from left to right. Mean diameters of coronary lumen were labelled on the left upper corner of OCT images. Red arrow indicated culprit lesion site. OCT, optical coherence tomography
Major adverse cardiac events (MACEs) were defined as composite of all-cause death, recurrence of myocardial infarction, heart failure, stroke and unplanned revascularization. Follow-up was performed by well-trained physicians who were blinded to the routine clinical data at 1, 6, and 12 months after discharge via outpatient visits or phone interviews and then annually after 1-year follow-up.
Continuous data were presented as the means
According to the diameter of vessel in culprit lesion, patients were divided
into three groups of diameter (D)
| Variables | Group 1 | Group 2 | Group 3 | p value | ||||||
| D |
2.5 |
3.0 |
overall | 1 vs. 2 | 1 vs. 3 | 2 vs.3 | ||||
| Patient characteristics | ||||||||||
| Age (mean |
40.0 |
54.9 |
68.9 |
|||||||
| Male, n (%) | 62 (98.4) | 150 (85.7) | 105 (71.4) | 0.006 | 0.002 | |||||
| BMI (mean |
28.4 |
25.8 |
25.2 |
0.126 | ||||||
| Past history | ||||||||||
| Smoking, n (%) | 52 (80.0) | 137 (75.3) | 103 (66.0) | 0.055 | 0.440 | 0.039 | 0.062 | |||
| Hypertension, n (%) | 37 (56.9) | 99 (54.1) | 98 (62.8) | 0.264 | 0.694 | 0.413 | 0.105 | |||
| Dyslipidemia, n (%) | 58 (89.2) | 164 (89.6) | 138 (88.5) | 0.943 | 0.930 | 0.869 | 0.734 | |||
| Diabetes mellitus, n (%) | 17 (26.2) | 56 (30.6) | 49 (31.4) | 0.731 | 0.499 | 0.437 | 0.872 | |||
| Stroke, n (%) | 0 (0) | 17 (9.3) | 20 (12.8) | 0.011 | 0.011 | 0.002 | 0.307 | |||
| CKD, n (%) | 2 (3.1) | 0 (0) | 7 (4.5) | 0.018 | 0.017 | 0.629 | 0.004 | |||
| Myocardial infarction, n (%) | 2 (3.1) | 11 (6.0) | 18 (11.5) | 0.051 | 0.362 | 0.046 | 0.070 | |||
| PCI, n (%) | 3 (4.6) | 11 (6.0) | 22 (14.1) | 0.014 | 0.675 | 0.042 | 0.012 | |||
| Laboratory data | ||||||||||
| Total cholesterol (mmol/L) | 4.8 (4.2–5.4) | 4.4 (3.8–5.1) | 4.2 (3.6–4.9) | 0.003 | 0.116 | |||||
| LDL cholesterol (mmol/L) | 3.0 (2.3–3.6) | 2.8 (2.4–3.3) | 2.6 (2.0–3.1) | 0.006 | 0.208 | 0.005 | 0.017 | |||
| HDL cholesterol (mmol/L) | 1.0 (0.9–1.2) | 1.1 (0.9–1.2) | 1.1 (1.0–1.3) | 0.010 | 0.086 | 0.003 | 0.071 | |||
| TG (mmol/L) | 2.0 (1.3–3.0) | 1.5 (1.0–2.0) | 1.2 (0.8–1.7) | 0.002 | 0.001 | |||||
| Serum creatinine (mmol/L) | 83.8 (76.3–93.1) | 78.6 (68.3–91.7) | 83.3 (70.4–94.2) | 0.031 | 0.020 | 0.668 | 0.040 | |||
| WBC (10 |
10.7 |
10.0 |
9.4 |
0.007 | 0.126 | 0.003 | 0.039 | |||
| hs-CRP (mg/dL) | 8.5 (4.0–10.9) | 5.7 (2.3–10.9) | 5.8 (2.5–10.7) | 0.375 | 0.185 | 0.222 | 0.784 | |||
| HbA1c (%) | 6.6 |
6.6 |
6.6 |
0.906 | 0.846 | 0.890 | 0.658 | |||
| cTNI (ng/mL) | 1.1 (0.2–5.3) | 1.3 (0.1–4.9) | 0.8 (0.1–5.9) | 0.436 | 0.761 | 0.275 | 0.287 | |||
| NT-proBNP (pg/mL) | 109.1 (32.3–353.5) | 179.9 (45.4–507.6) | 240.0 (83.2–850.0) | 0.002 | 0.064 | 0.001 | 0.021 | |||
| LVEF (%) | 55.5 |
54.8 |
54.5 |
0.515 | 0.421 | 0.250 | 0.622 | |||
| Angiography data | ||||||||||
| Culprit vessel | ||||||||||
| LAD, n (%) | 28 (43.1) | 107 (58.5) | 56 (35.9) | |||||||
| LCX, n (%) | 26 (40.0) | 15 (8.2) | 4 (2.6) | |||||||
| RCA, n (%) | 11 (16.9) | 60 (32.8) | 96 (61.5) | |||||||
| TIMI flow | 0.406 | 0.692 | 0.899 | 0.122 | ||||||
| 0 | 42 (64.6) | 105 (57.4) | 106 (67.9) | |||||||
| 1 | 2 (3.1) | 10 (5.5) | 5 (3.2) | |||||||
| 2 | 7 (10.8) | 26 (14.2) | 12 (7.7) | |||||||
| 3 | 14 (21.5) | 42 (23.0) | 33 (21.2) | |||||||
| Stent, n (%) | 55 (84.6) | 177 (96.7) | 152 (97.4) | 0.001 | 0.698 | |||||
| IABP, n (%) | 0 (0) | 4 (2.2) | 3 (1.9) | 0.497 | 0.229 | 0.260 | 0.865 | |||
| Medical therapy | ||||||||||
| Aspirin | 62 (95.4) | 177 (96.7) | 154 (98.7) | 0.314 | 0.621 | 0.129 | 0.227 | |||
| Clopidogrel | 29 (44.6) | 87 (47.5) | 80 (51.3) | 0.624 | 0.685 | 0.366 | 0.492 | |||
| Ticagrelor | 36 (55.4) | 95 (51.9) | 77 (49.4) | 0.708 | 0.630 | 0.414 | 0.639 | |||
| ACEI/ARB | 51 (78.5) | 137 (74.9) | 112 (71.8) | 0.568 | 0.561 | 0.305 | 0.524 | |||
| 61 (93.8) | 163 (89.1) | 132 (84.6) | 0.134 | 0.263 | 0.060 | 0.224 | ||||
| Statin | 64 (98.5) | 176 (96.2) | 153 (98.1) | 0.458 | 0.370 | 0.845 | 0.302 | |||
| Anticoagulant | 4 (6.2) | 2 (1.1) | 2 (1.3) | 0.031 | 0.023 | 0.042 | 0.872 | |||
| PPI | 29 (44.6) | 85 (46.4) | 79 (50.6) | 0.637 | 0.799 | 0.414 | 0.441 | |||
Continuous data are presented as median (interquartile range). Categorical data are presented as number (%). D, diameter; BMI, body mass index; PCI, percutaneous coronary intervention; CKD, chronic kidney disease; WBC, white blood cell; HDL, high density lipoprotein; LDL, low density lipoprotein; TG, triglyceride; hs-CRP, high sensitive C-reactive protein; HbA1c, Hemoglobin A1c; cTNI, cardiac troponin I; LVEF, left ventricle ejection fraction; LAD, left anterior descending artery; LCX, left circumfex artery; RCA, right coronary artery; TIMI, thrombolysis in myocardial infarction; IABP, Intra-aortic balloon pump; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; PPI, proton pump inhibitors; NT-proBNP, N-terminal pro-B-type natriuretic peptide.
The proportion of plaque rupture is significantly higher in group of diameters larger than 3 mm than that between 2.5 mm and 3 mm (57.1% vs. 42.1%, p = 0.004) whereas the presence of plaque erosion is lower in group 3 than group 2 (53.6% vs. 36.5%, p = 0.002). The prevalence of calcified nodule is similar among three groups.
In microstructure of plaque, MLA and presence of macrophage was significant
different among three groups (1.5 [1.2–2.0] vs. 1.7 [1.3–2.1] vs. 2.0
[1.5–2.4], p
| OCT features | Group 1 | Group 2 | Group 3 | p value | ||||
| D |
2.5 |
3.0 |
Overall | 1 vs. 2 | 1 vs. 3 | 2 vs.3 | ||
| Plaque phenotypes | ||||||||
| Plaque rupture | 30 (46.2) | 77 (42.1) | 90 (57.7) | 0.015 | 0.569 | 0.117 | 0.004 | |
| Plaque erosion | 33 (50.8) | 98 (53.6) | 57 (36.5) | 0.006 | 0.699 | 0.050 | 0.002 | |
| Calcified nodule | 2 (3.1) | 8 (4.4) | 9 (5.8) | 0.662 | 0.649 | 0.402 | 0.557 | |
| Microstructure | ||||||||
| Lipid plaque, n (%) | 30 (46.2) | 90 (49.2) | 85 (54.5) | 0.449 | 0.675 | 0.259 | 0.330 | |
| Fibrous plaque, n (%) | 30 (46.2) | 70 (38.3) | 53 (34.0) | 0.233 | 0.265 | 0.088 | 0.414 | |
| Calcification, n (%) | 28 (43.1) | 91 (49.7) | 83 (53.2) | 0.388 | 0.357 | 0.170 | 0.523 | |
| Microchannel, n (%) | 13 (20.0) | 36 (19.7) | 34 (21.8) | 0.884 | 0.955 | 0.766 | 0.630 | |
| Cholesterol crystal, n (%) | 6 (9.2) | 23 (12.6) | 24 (15.4) | 0.446 | 0.472 | 0.224 | 0.455 | |
| Macrophage, n (%) | 24 (36.9) | 75 (41.0) | 86 (55.1) | 0.010 | 0.566 | 0.014 | 0.009 | |
| Thrombus, n (%) | 62 (95.4) | 179 (97.8) | 154 (98.7) | 0.310 | 0.310 | 0.129 | 0.529 | |
| TCFA, n (%) | 15 (23.1) | 44 (24.0) | 54 (34.6) | 0.061 | 0.875 | 0.092 | 0.032 | |
| FCT, µm | 100 (70–130) | 100 (60–120) | 90 (60–120) | 0.177 | 0.421 | 0.090 | 0.182 | |
| MLA, mm |
1.5 (1.2–2.0) | 1.7 (1.3–2.1) | 2.0 (1.5–2.4) | 0.077 | ||||
Continuous data are presented as median (interquartile range). Categorical data are presented as number (%). D, diameter; TCFA, thin-cap fibroatheroma; FCT, fibrous cap thickness; MLA, minimal lumen area; OCT, optical coherence tomography.
The median time to follow-up was 3 years (interquartile range: 2 to 4 years). A
KM curve was drawn according to the luminal diameters of the culprit lesion. No
significant difference in MACEs was observed among three groups. In addition, the
cohort was divided into 4 groups according to vessel size and plaque phenotype:
SVD (diameter
 Fig. 3.
Fig. 3.Kaplan-Meier curve for patients with different diameters of culprit lesion (A) and groups which divided by vessel size and plaque phenotypes (B). SVD, small vessel disease; LVD, large vessel disease; PR, plaque rupture; NPR, nonplaque rupture.
Due to the similar clinical features and OCT findings of group 1 and 2, the
cohort was divided into two groups with diameters
 Fig. 4.
Fig. 4.The impact of diameters of culprit lesion on plaque features,
adjusted for patient characteristics. After adjusting patient characteristics
(sex, age, body mass index, current smoking, hypertension, diabetes, low-density
lipoprotein-cholesterol, triglycerides, and high sensitivity C-reactive protein),
patients with diameters
This single centre study summarized clinical and intracoronary features of
different culprit lesion size in patients with STEMI. The main results of the
current study showed that patients with diameters
In the past few decades, SVD has emerged as an intriguing issue of
atherosclerotic coronary artery disease. Although abundant therapeutic methods
were discovered, the optimal treatment option remained conflicting in various
clinical trials. In patients with de novo lesion
Plaque rupture, plaque erosion and calcified nodule are three main pathological phenotypes of ACS which presented different clinical outcome [21]. Although previous study reported that direct stenting had superior clinical outcome than conventional stenting in patients of STEMI with SVD [4], a recent OCT study demonstrated that ACS patients with plaque erosion benefited from medical therapy without stenting, including SVD [22]. Also, some case reports showed that patients with AMI and plaque erosion accepted successful treatment of thrombus aspiration and balloon without stent under OCT guidance [23]. However, there is still no evidence of safety and efficacy of no stent strategy for patients with plaque rupture. The research of drug-coated balloons treating vulnerable plaques is currently ongoing [24]. For calcified lesion, smaller balloons at higher pressures without coronary injuries was needed before stent implantation [25]. In this study, we found significantly different distribution of plaque phenotypes in patients with small to large diameters of culprit lesion. SVD patients with STEMI were easier to present plaque erosion, which may provide important clues for precise management of patients with SVD. For example, SVD with plaque rupture or erosion may reacted differently to DES or DCB in clinical outcome. Although an observation study demonstrated that DCB is safe and effective for ACS complicated with vulnerable plaque [26]. There is still unknown whether DCB is useful in ACS patients with plaque rupture in SVD. The choice of DES or DCB treating SVD should be based on clinical risk factors, functional assessment and plaque characteristics. Namely, for those patients both with plaque rupture and SVD, whether DCB or DES was more effective remained still unknown.
Small coronary lumen size or area presented with regional hemodynamic change which resulted in distinct atherosclerosis progression compared with the large lumen size [27]. Coronary artery flow velocity was reported to inversely relate to the lumen size and small lumen size may suffer from higher blood flow velocity [28]. Moreover, the previous study revealed that the size of the cavity inside the ruptured plaque was positively related to vessel size [29]. The prevalence of stent restenosis was also higher in small lumen artery than large lumen size [6]. Distinct size of vessel lumen exhibited various reaction to different DES. Previous study revealed sirolimus-Eluting stent acted better than paclitaxel-Eluting Stents by reducing MACE and target lesion revascularization in SVD but not in large vessel disease [30]. Furthermore, the previous study demonstrated that small vessel size was significantly associated with poorer prognosis in patients with STEMI [3]. However, as the development of advanced therapeutic strategy and novel technique of drug-eluting stent and DCB, SVD got similar outcome compared with patients of large coronary vessels [31]. Our results also suggested that vessel size of culprit lesion had less impact on patients’ prognosis. However, the number of patients with SVD in our study is quite small. Thus, whether patients with SVD can benefit from OCT guidance for choosing DES or DCB needs future large sample cohort study.
Numerous studies demonstrated that both plaque phenotype and vessel size were
essential factors for treatment strategy in the past few decades [14, 15, 22, 23]. Previous study revealed that patients with plaque rupture showed higher
prevalence of no-reflow and severer systemic inflammation which needed intensive
antithrombotic and anti-inflammatory treatment [32]. However, patients with
plaque erosion might benefit from drug therapy without stent while distal and
microcirculation embolism should be noticed [33]. Although OCT enabled to
identify plaque phenotypes precisely, it was not widely used in clinics because
of its high price and operational complexity. In the current study, the
association of culprit vessel diameters and plaque features was revealed and size
of culprit vessel might assist to evaluate plaque phenotypes which guided us to
product intervention strategy. For example, small vessel with diameters
The present study suggested that vessel size of culprit lesion is significantly associated with plaque phenotype in patients with STEMI. However, patients with different diameters and plaque phenotypes showed no significant difference of clinical outcomes.
First, this study was a single-centre study with small sample size, more than one fourth of the patients were excluded so that selection bias cannot be excluded. Second, due to adhesion of thrombus in culprit lesion, error may exist in diameter measurement in some cases. Third, some interventional procedures, such as guidewire entry and thrombus aspiration before OCT examination, may change the structure of the underlying plaque. Therefore, some cases of plaque phenotype were misjudged.
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Conceptualization—JNL; methodology—XXZ and HBY; software—YW and PZ; validation—HJZ, RZC and CL; formal analysis—JYZ, SDY and LS; investigation—HBY; resources—SDY; data curation—JNL; writing - original draft preparation—JNL, SDY, XXZ, HBY and YW; writing - review and editing—LS, PZ, HJZ and RZC; revision—JNL, CL, JYZ and YC; visualization—YC; supervision—HJZ; project administration—HBY; funding acquisition—HJZ. All authors have read and agreed to the published version of the manuscript.
This study was performed in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of Fuwai Hospital, Beijing (protocol code: 2017-866, date of approval: 22 January 2017). All patients provided written informed consent.
Not applicable.
This study was supported by the Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (2016-I2 M-1–009), National Natural Science Funds (number: 81970308), the Fund of “Sanming” Project of Medicine in Shenzhen (number: SZSM201911017) and Shenzhen Key Medical Discipline Construction Fund (number: SZXK001).
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
