- Academic Editors
†These authors contributed equally.
Background: Total arterial revascularization (TAR) has gradually become
accepted and recognized, but its effect and safety in diabetic patients are not
clear. We performed a systematic review and meta-analysis to summarize the safety
and efficacy of TAR and additionally evaluated the clinical outcomes of arterial
revascularization using different arterial deployments in
patients with diabetes. Methods: PubMed, Embase, and the Cochrane
Library databases from inception to July 2022 for studies that studied the effect
of arterial revascularization in diabetic patients undergoing isolated coronary
artery bypass graft (CABG) were searched. The primary outcome was long-term
(
Coronary artery bypass grafting (CABG) has been identified as the preferred revascularization strategy for patients with multivessel disease and diabetes. As an important factor influencing the clinical outcomes of those receiving surgery, graft selection has gradually attracted investigators’ attention in recent years. Compared with conventional surgery involving saphenous venous grafts (SVGs), using the left internal mammary artery (LIMA) to bypass a stenotic left anterior descending artery (LAD) improves outcomes and is thus considered the standard of care. However, with SVGs failure rating up to 10% to 20% after 1 year and an additional 5% failure rate for each subsequent year [1, 2, 3], debates began to surround the application of additional arterial grafts. An increasing number of studies have detailed the association between total arterial revascularization (TAR) and improved long-term survival in the general population [4, 5, 6]. Nevertheless, before TAR can be widely performed in clinical practice, it needs further development because of its association with increased surgical difficulty and risks caused by some specific comorbidities, such as diabetes.
Oftentimes, patients with diabetes mellitus (DM) have complex, three-vessel coronary artery lesions. Consequently, surgeons usually have to carefully select the best graft to serve as the adjunct to the LIMA. Despite a prolonged operation time and increased surgical difficulty, the primary reasons hindering the application of multiarterial/ total-arterial coronary revascularization (MAR/TAR) are the increased risks of sternal wound infection and perioperative mortality. Therefore, whether DM patients can get consistent long-term benefits from arterial grafts, which may overweigh the short-term risk, is a critical issue that requires investigation. Moreover, the clinical outcomes of arterial revascularization via different arteries are also not clear. In this context, we conducted this systematic review and meta-analysis to provide the latest evidence to answer these issues above.
This study was registered on INPLASY (INPLASY2022120003). We performed and reported this work in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis statements [7]. All data used in this study were extracted from individual studies. The authors declare that all supporting data are available within the article and the supplementary documents.
We searched PubMed, Embase, and Cochrane from inception to July 2022 for studies evaluating the outcome of arterial revascularization in diabetic patients undergoing isolated CABG. The search strategies and related terms are provided in the Supplemental file. Two reviewers (GL and TL) screened each study by title and abstract for inclusion eligibility, reviewed the full texts of eligible studies, and then extracted the data independently. All disagreements were resolved by discussion. The references of selected articles and conference proceedings were also screened.
Inclusion criteria: (1) studies evaluating patients with a primary diagnosis of
diabetes according to the International Classification of Diseases, 10th
Revision, and receiving insulin or oral treatment before isolated CABG; (2)
studies reporting on any one of the following comparisons: outcomes of TAR and
conventional revascularization with veins (CVR) in diabetic patients, outcomes in diabetic and nondiabetic patients following
TAR grafting, outcomes of BIMA/RA access and right gastroepiploic artery (RGA)
access in patients with DM; (3) postoperative sternal wound infection rate
(superficial and deep infections, SWIs), and Kaplan‒Meier survival curves of
all-cause death and cardiovascular death or hazard ratio (HR) for the two
outcomes; and (4) randomized and nonrandomized controlled trials published in
English. We defined long-term (
Two researchers (LG and LT) independently extracted the following information
from each work: the first author, publication year, type of study, and
participant characteristics. The reviewers extracted the following outcomes of
interest: early death, any SWI, any Kaplan–Meier curve for long-term overall
survival, or cardiac mortality-free survival. For studies that reported the
results of propensity-score–matched (PSM) analyses, we also abstracted and
pooled the PSM data separately. When studies performed stratified analysis
according to the number of arterial conduits used, we included the patients who
received
Risk ratios (RRs), hazard ratios (HRs), and their corresponding 95% confidence
intervals (CIs) were calculated to describe short-term results and long-term
survival results. The I
Fig. 1 details the PRISMA systematic review flowchart. After review, a total of
34 observational studies and one RCT [4, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44], covering 178,274 diabetic
patients, were included in this meta-analysis. The characteristics of the
included studies are provided in Table 1 (Ref. [4, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44]). The average duration of
follow-up in the studies evaluating the long-term outcomes was 71.1 months. In
all the studies, the mean age was 63.8 years, with an apparent male predominance
(75.1%). Obesity, chronic cardiac insufficiency, chronic renal disease and
pulmonary insufficiency were common in the setting of diabetes. Among the studies
exploring the effect of TAR [4, 14, 16, 17, 22, 38, 41]. RA was the artery most
frequently selected as the adjunct to the LIMA. After reviewing these studies,
the overall patient profile was similar between the groups. The funnel plots of
the comparisons of the BIMA and the SIMA suggested the possible existence of
publication bias. The overall risk of bias was considered moderate in the RCT.
Except for the study of Raza et al. [34] published in 2013, the quality
evaluation of non-RCTs based on the Newcastle-Ottawa scale found that all scores
were
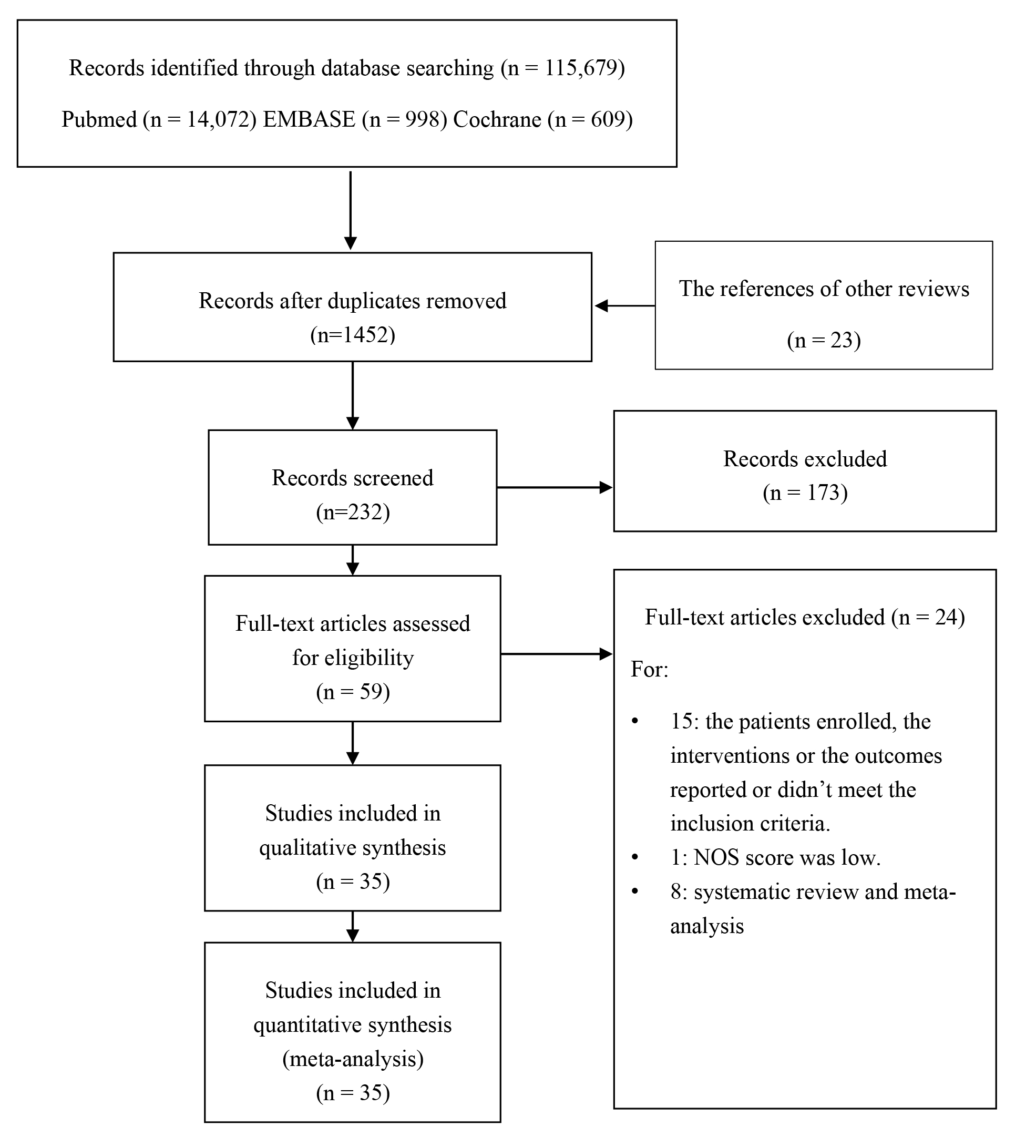 Fig. 1.
Fig. 1.The flow diagram of literature searching and selection.
| Study | Pts | Study design | Trial group | The major arterial conduct | Control group | Study period | Follow-up, m | Age, y | Male (%) | Prior MI (%) | Prior Re. (%) | Obesity, %/BMI, kg/m |
HBA1c (%) (mean) | Diabetes on insulin (%) | CHI/LVEF (%) | CKD (%) | Pulmonary insufficiency/COPD (%) | Smoker (%) | NOS or Rob2 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buxton 2012 [14] | 206 | Retrospective; PSM | TAR in DM | RA | CVR in DM | 1996–2008 | 93.6 | Age |
84.0 | 50.5 | 10.2 | 18.9 /NA | NA | 18.9 | 1.9 | Creatinine |
3.9 | 23.3 | 8 |
| DiBacco 2019 [17] | 269 | Retrospective; PSM | TAR in DM | BIMA and RA | CVR in DM | 2005–2015 | 101 | 70.1 | 77.3 | 40.4 | 16.7 | NA | NA | 13.6 | NA/50.2 | 12.1 | 13.1 | NA | 9 |
| Tatoulis 2015 [4] | 11,642 | Retrospective; PSM | TAR in DM | RA | CVR in DM | 2001–2012 | 58.8 | 66.0 | 75.7 | 56.2 | 14.6 | NA/30.0 | NA | NA | 19.1/NA | Preoperative dialysis: 2.7 | 12.7 | 66.1 | 8 |
| Hwang 2010 [22] | 558 | Retrospective | TAR in DM | BIMA and RGA | TAR in NDM | 1998–2004 | 81 | 61.7 | 75.1 | NA | NA | 42.7/NA | NA | 13.6 | 6.1 (LVEF |
5.9 | NA | 45.3 | 8 |
| Suzuki 2015 [38] | 602 | Retrospective | TAR in DM | BIMA and RGA | TAR in NDM | 2002–2013 | 52.8 | 67.0 | 84.1 | 33.1 | 29.4 | NA/23.8 | HbA1c |
29.6 | 27.4 | 10.1 | 17.9 | 63.3 | 9 |
| Choi 2005 [16] | 517 | Prospective | TAR in DM | BIMA and RGA | TAR in NDM | 1998–2003 | 34.0 | 61.4 | 76.0 | 19.9 | NA | 1.5/NA | NA | 12.6 | 6.0 | 5.6 | NA | 45.1 | 7 |
| Schwann 2018 [41] | 3992 | Retrospective; PSM | TAR in DM | RA | TAR in NDM; CVR in DM | 1994–2011 | 104.4 | 64.0 | 66.8 | 54.4 | 19.0 | NA/ |
NA | NA | 15.2/48.0 | 0 | 22.2 | NA | 9 |
| Muneretto 2006 [31] | 200 | Retrospective; PSM | BIMA | BIMA | SIMA | 1999–2003 | 34 | 68.5 | 58.0 | 56.0 | NA | 11.0/NA | NA | NA | NA/LVEF |
NA | 26.0 | 13.0 | 7 |
| Lev-Ran 2004 [28] | 285 | Retrospective | BIMA | BIMA | SIMA | 1996–1998 | 63.0 | 65.8 | 66.9 | Acute MI |
14.0 | NA/25.7 | NA | 0 | 21.0/NA | NA | NA | NA | 8 |
| Lev-Ran 2003 [29] | 124 | Retrospective | BIMA | BIMA | SIMA | 1996–2001 | 55.0 | 65.9 | 58.9 | NA | 28.2 | NA/25.0 | NA | 100.0 | 37.9/NA | 13.7 | 11.3 | 37.1 | 8 |
| Abelaira 2021 [12] | 152 | Retrospective | BIMA in diabetics | BIMA | BIMA in non-diabetics | 2004–2017 | 3.0 | 62.2 | 85.0 | 34.2 | NA | 23.0/NA | 5.9 | 78.7 | 15.8 (LVEF% |
Hemodialysis: 1.3 | 14.5 | 42.1 | 7 |
| Agrifoglio 2008 [13] | 81 | Prospective; PSMPU | BIMA | BIMA | SIMA | 2006 | 12.0 | 66.5 | 64.4 | 34.1 | NA | NA/27.4 | 8.4 | 34.6 | NA/55.9 | 9.9 | 12.3 | 64.2 | 7 |
| Dorman 2012 [18] | 828 | Retrospective: PSM | BIMA | BIMA | SIMA | 1972–1994 | 106.8 | 65.7 | 79.3 | 57.7 | NA | NA | NA | NA | 15.0/LVEF |
1.0 | NA | 58.2 | 8 |
| Calafiore 2004 [15] | 1140 | Prospective: PSM | BIMA | BIMA | SIMA | 1986–1999 | 87.6 | 60.8 | 81.6 | 49.0 | 0.0 | NA /NA | NA | NA | 2.8/59.4 | 2.3 | 2.9 | NA | 9 |
| Endo 2003 [19] | 467 | Retrospective; PSM | BIMA | BIMA | SIMA | 1985–1998 | 97.2 | 61.6 | 80.1 | 67.2 | NA | 60.0/NA | NA | 10.7 | NA/52.2 | NA | NA | 71.3 | 8 |
| Gansera 2017 [20] | 250 | Retrospective: PSM | BIMA | BIMA | SIMA | 2000–2011 | 111.6 | 59.7 | 83.2 | 35.1 | 20.0 | NA | NA | 38.0 | 34.8 (LVEF% |
NA | NA | NA | 7 |
| Hirotani 2003 [21] | 303 | Retrospective | BIMA | BIMA | SIMA | 1991–2003 | NA | 64.4 | 75.9 | 80.2 | 3.0 | NA | NA | 49.2 | 48.4/NA | NA | NA | NA | 7 |
| Iribarne 2017 [23] | 430 | Retrospective: PSM | BIMA | BIMA | SIMA | 1992–2014 | 111.6 | NA | 73.9 | MI within 7 days 14.9 | 17.4 | NA/NA | 11.7 | NA | 15.1/NA | 5.9 | 11.7 | NA | 8 |
| Kainuma 2021 [24] | 124 | Retrospective | BIMA | BIMA | SIMA | 1995–2015 | 68.0 | 68.0 | 87.1 | NA | NA | NA/23.0 | 7.1 | 40.3 | 100.0/32.8 | eGFR |
NA | NA | 8 |
| Kazui 2021 [25] | 16,741 | Retrospective: PSM | BIMA | BIMA | SIMA | 2008–2016 | 1.0 | 60.0 | 84.5 | NA | NA | NA/29.7 | NA | NA | 13.1/53.6 | Dialysis 1.6 | 17.8 | 32.4 | 8 |
| Konstanty-Kalandyk 2012 [27] | 147 | Retrospective | BIMA | BIMA | SIMA | 2006–2008 | 3.0 | 65.0 | 61.2 | 67.3 | NA | BMI |
NA | 52.4 | NA/51.2 | 8.8 | 6.8 | NA | 6 |
| Kinoshita 2010 [26] | 340 | Retrospective: PSM | BIMA | BIMA | SIMA | 2002–2009 | 38.4 | 69.5 | 75.3 | 45.0 | 28.5 | NA/23.1 | 6.2 | 48.8 | 23.5 (LVEF |
27.6 | 19.4 | 50.3 | 8 |
| Momin 2005 [30] | 920 | Retrospective | BIMA | BIMA | SIMA | 1992–2002 | 120.0 | 63.4 | 71.0 | 59.3 | NA | NA/28.3 | NA | 28.4 | NA/LVEF |
17.4 | 6.2 | 11.5 (current); 57.2 (history of smoking) | 7 |
| Pevni 2017 [32] | 980 | Retrospective: PSM | BIMA | BIMA | SIMA | 1996–2010 | 146.4 | Age |
70.7 | Recent MI |
23.4 | NA/ BMI |
NA | 14.8 | 28.8/NA | 15.8 | 7.3 | NA | 8 |
| Puskas 2012 [33] | 1445 | Retrospective | BIMA | BIMA | SIMA | 2002–2010 | 108 | 62.6 | 70.9 | 53.0 | NA | NA/29.4 | NA | NA | 21.2/50.2 | 6.8 | 14.7 | 60.3 | 8 |
| Raza 2017 [11] | 564 | Retrospective; PSM | BIMA | BIMA | SIMA+RA | 1994–2011 | 88.8 | 58.0 | 88.1 | 52.1 | NA | NA/29.0 | NA | NA | Left ventricular dysfunction: 44.3/NA | NA | 5.5 | NA | 8 |
| Raza 2014 [34] | 9404 | Retrospective | BIMA | BIMA | SIMA | 1972–2011 | 93.6 | 62.0 | 72.1 | 56.5 | NA | NA/30.0 | NA | 23.0 | 15.7/NA | 2.4 (dialysis) | NA | NA | 8 |
| Sajja 2012 [35] | 1211 | Retrospective | BIMA | BIMA | SIMA | 2004–2010 | During the hospitalization. | 58.2 | 86.8 | 28.7 | NA | NA/25.9 | NA | NA | LVEF |
Serum creatinine |
15.9 | 23.0 | 6 |
| Savage 2006 [36] | 120,793 | Retrospective | BIMA | BIMA | SIMA | 2002–2004 | 64.6 | 67.7 | 46.2 | NA | NA/30.8 | NA | 29.6 | 19.6/NA | 8.8 | 19.6 | 19.1 | 6 | |
| Stevens 2005 [37] | 633 | Retrospective | BIMA | BIMA | SIMA | 1985–1995 | 132 | 62.0 | 72.0 | 30.3 | 0.6 | 24.1/NA | NA | NA | 1.6 | NA | 5.9 | NA | 9 |
| Taggart 2019 [39] | 734 | RCT | BIMA | BIMA | SIMA | 2004–2007 | Last 120 months | 63.6 | 85.6 | 41.9 | 15.8 | NA/28.2 | NA | 23.7 | NA/NA | NA | NA | 70.4 | Some concerns |
| Tavolacci 2003 [40] | 256 | Retrospective | BIMA | BIMA | SIMA | 1998–2000 | NA | 66.2 | 78.3 | NA | NA | NA | NA | NA | NA/NA | NA | NA | NA | 7 |
| Toumpoulis 2006 [42] | 980 | Retrospective; PSM | BIMA | BIMA | SIMA | 1992–2002 | 56.4 | 64.1 | 55.6 | 56.9 | 11.4 | NA/BMI |
NA | NA | 21.5/LVEF |
3.6 | 16.4 | 15.6 | 8 |
| Hoffman 2014 [44] | 404 | Retrospective: PSM | RA | RA | BIMA | 1995–2012 | 126.8 | 61.9 | 66.3 | NA | 17.8 | NA | NA | NA | 45.2 | 15.6 | 11.4 | NA | 8 |
| Puehler 2020 [43] | Retrospective: PSM | BIMA | BIMA | SIMA | 2009–2016 | 36.3 | 59.8 | 88.4 | 27.2 | Previous surgery 1.2 | NA/28.3 | NA | NA | NA/58.0 | NA | 4.6 | 51.9 | 8 |
Pts, patients; m, months; y, years; MI, myocardial infarction; Re, revascularization; HBA1c, glycosylated hemoglobin, type A1C; CHI, chronic cardiac insufficiency; EF, ejection fraction; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; PSM, propensity score matching; TAR, total arterial revascularization; SIMA, single internal mammary arteries; BIMA, bilateral internal mammary arteries; RA, radial artery; RGA, right gastroepiploic artery; DM, diabetes mellitus; NDM, non-diabetes Mellitus; NA, not available; BMI, body mass index; eGFR, estimated glomerular filtration rate; CVR, conventional revascularization with veins.
Five observational studies enrolling 15,634 eligible patients, with NOS scores
ranging from 7 to 9, were included in the analysis [4, 14, 17, 28, 31, 41]. The
PSM method was not utilized in one study [28]. In the study by Lev-Ran,
et al. [28], as TAR was performed in 91% of the patients in
the BIMA group, we included this article in this analysis. The incidence of early
death and any SWI in patients with DM are shown in Fig. 2A,B. Compared to
conventional surgery with SVG, TAR was not associated with an increased risk of
early mortality (RR 0.77, 95% CI [0.48–1.23]) or risk of SWI (RR 0.77, 95% CI
[0.46–1.28]). The results of the sensitivity analyses suggest that no study
contributes to residual heterogeneity. Removing them from the
meta-analysis one by one would not influence the results. None of the I
 Fig. 2.
Fig. 2.Plots for the clinical outcomes of TAR in diabetic patients. (A) (a) Forest plot of early death; (b) Forest plot of sensitivity analysis. (B) (a) Forest plot of any SWI; (b) Forest plot of sensitivity analysis. (C) Overall Kaplan–Meier survival curves based on reconstructed patient data. (a) Aggregated survival curve for long-term overall survival with data of 4 propensity score matched analyses; (b) Aggregated survival curve for long-term overall survival with data of all the cohorts from 5 studies. (D) Kaplan-Meier curves in the diabetic population for survival free from cardiovascular death based on reconstructed patient data from 1 propensity score matched analyses and 1 research with unmatched cohorts. Note: M: the studies with data of matched cohorts; UM: the studies with data of unmatched cohorts; TAR, total arterial revascularization; CVR, conventional revascularization with veins; HR, hazard ratio; CI, confidence interval.
Four observational studies with 1677 patients provided information related to the outcome of TAR in DM and non-DM patients [16, 22, 38, 41]. Three of them did not utilize PSM method to conduct analysis. In the comparisons, as Supplementary Fig. 1 (Supplemental file) shows, the risk of early death (RR 1.50, 95% CI 0.64–3.49) and infection (RR 2.52, 95% CI 0.91–7.00) did not differ between the DM and non-DM groups (Supplementary Fig. 1A). The long-term overall survival rate of diabetic patients was lower than that of patients without diabetes (HR 1.66; 95% CI 1.35–2.03) while cardiovascular survival rate was similar (HR 0.98; 95% CI 0.51–1.90) (Supplementary Fig. 1B).
Twenty-three [13, 15, 18, 19, 20, 21, 23, 24, 25, 26, 27, 28, 29, 30, 32, 33, 35, 36, 37, 39, 40, 42, 43] studies with
NOS scores ranging from 6 to 9 reported the early outcomes of the BIMA and the
SIMA as adjuncts in DM. The short-term risk and long-term effects of BIMA as an
access point are shown in Fig. 3. Compared to the SIMA, the BIMA was associated
with a decreased risk of all-cause death (HR 0.67, 95% CI 0.52–0.85) and CV
death (HR 0.55, 95% CI 0.35–0.87) without resulting in a significantly
increased rate of early death (RR 0.95, 95% CI 0.82–1.11) (Fig. 3B,A(a)).
The results of PSM studies (RR 0.84, 95% CI 0.64–1.10) and non-PSM researches
in the analysis of early death were consistent (RR 1.02, 95% CI 0.85–1.23).
However, the pooled analysis of 4 PSM studies and one RCT suggested no
significant difference in survival gains (HR 0.76, 95% CI 0.52–1.11) between
BIMA and SIMA in diabetes (the result of the random effects model was adopted as
I
 Fig. 3.
Fig. 3.Plots for the clinical outcomes of BIMA and SIMA in diabetic patients. (A) (a) Forest plot of early death in matched and unmatched diabetic cohorts; (b) Forest plot of any SWI in matched and unmatched diabetic cohorts. (B) (a) Forest plot of long-term death among matched and unmatched cohort (85.7 months of average follow-up duration); (b) Forest plot of cardiovascular death among matched and unmatched diabetic cohorts (63 months of average follow-up duration). Note: M: the studies with data of matched cohorts; UM: the studies with data of unmatched cohorts; TAR, total arterial revascularization; CVR, conventional revascularization with veins; HR, hazard ratio; CI, confidence interval; DM, diabetes mellitus patients; non-DM, non-diabetes mellitus patients.
The available literature on the value of the RA and the RGA was limited. As Table 1 shows, most diabetic patients receiving TAR were treated via the RA as the arterial conduit second to the LIMA. the method of LIMA plus RA was applied in more than 80% of participants in the studies by Buxton, Tatoulis, and Schwan [4, 14, 41]. Therefore, we conducted an additional subanalysis for the primary outcome. The results suggested a consistent long-term survival benefit (HR 0.71, 95% CI 0.63–0.79) (Fig. 4). The sensitivity analyses (Supplementary Fig. 3) indicated the results robustness. After reviewing these works, we observed no differences in the risks of early death and SWI between the TAR group in which the RA was used and the CVR group. We failed to conduct additional analysis of RGA due to the absence of research.
 Fig. 4.
Fig. 4.The comparison between the all-cause mortality of TAR with RA versus that of CVR in diabetic patients (85.6 months of average follow-up duration). TAR, total arterial revascularization.
Only two PSM analyses, with a NOS score of 8, effectively compared the RIMA with
the RA in patients with DM [11, 44]. In the propensity-matched analyses by
Raza, et al. [11], in-hospital mortality risk (0.35% versus 0.35%),
the prevalence of deep SWI (1.4% versus 1.4%) and overall survival rate were
similar (p = 0.2) in the LIMA plus RA and BIMA groups. Supporting this
finding, Hoffman’s PSM analysis indicated that long-term mortality was not
significantly different between the use of RA and RIMA (p = 0.01) [44].
However, deep sternal wound infection (p
Our work demonstrated that compared to conventional surgical revascularization with LIMA plus SVG, TAR was associated with a higher rate of long-term overall survival in diabetic patients, without being associated with a significantly increased risk of mortality or SWI. The presence of diabetes did not increase the risk of early death, SWI and long-term cardiovascular death. Regarding the selection of adjunct arteries second to the LIMA, the additional use of RIMA and RA could both exert the consistent survival benefit but did not increase early death. However, the BIMA method was found to result in more occurrences of SWI. Compared to the RIMA, RA might be a similarly effective but safer selection when TAR was applied in DM.
In recent years, multi-arterial grafting has been proven to improve survival rate in general population and is recommended by an increasing number of researchers [45, 46]. Gaudino et al. [45] extensively reviewed the benefits of arterial revascularization in a general population with multivessel disease. In their previous meta-analysis, the use of a third arterial conduit was not associated with a higher operative risk but was associated with superior long-term survival, irrespective of sex and diabetes [45]. However, the outcomes of arterial revascularization in diabetic patients were not well-defined and controversial, hampering its extended application in clinical practice. In this context, our work first systematically summarized the effect and safety of arterial grafts in this specific cohort. According to the results, this surgical approach did not increase perioperative death and SWI risk, but improve the survival rate significantly in diabetic patients. Therefore, the consideration on the perioperative events shouldn’t be the barrier excessively hindering the implementation of TAR into cardiac surgery. To explain the mechanisms underlying the survival gains, we found that the several excellent properties of arterial graft might contribute to this phenomenon. The thin smooth muscle layer and abundant elastic fibers of arterial conduits are relatively protected against the progression of atherosclerosis, resulting in better graft patency compared to SVG. In DM patients, the endothelial function of the coronary artery is depressed, resulting in a decrease in NO and prostacyclin secretion in the coronary artery circulation [47, 48]. In this context, arterial grafts transplanted into the coronary artery system can function not only as a nondiseased living conduit but also as a source of favourable metabolic substances that protect the coronary artery from atherosclerotic progression [49]. Theoretically, better graft patency and salutary metabolic effects on the recipient coronary arteries can lead to survival benefits, especially in DM patients with advanced atherosclerosis and depressed endothelial function.
It is well-known that using the LIMA to bypass a stenotic LAD artery is considered routine in patients eligible for surgery [50]. TAR/MAR has been advocated in recent years, so the selection of arterial conduits second to the LIMA has become a popular topic for discussion. The use of the RIMA was shown to be associated with enhanced survival benefits in people with or without diabetes in previous reviews and meta-analyses [51]. Although a higher occurrence of SWI was observed, they found that the incidence can be reduced by controlling perioperative blood glucose [52] and harvesting in a skeletonized fashion [10, 53]. Our work suggested similar overall survival gains and significantly increased SWI risk. Of note, however, the results of PSM studies and non-PSM analyses differ, and the considerable heterogeneity between researchers made this conclusion less reliable. The Inconsistencies in research indicated that the use of the BIMA in diabetic patients should be re-considered and treated seriously [39, 43]. On the one hand, significantly increased risks of SWI and prolonged preparation and operative times in DM patients require us to balance the pros and cons of accessing the BIMA. On the other hand, advances in medical treatment may narrow the differences in therapeutic effects between arterial revascularization and conventional surgery in the future. Currently, the crossover in the ART trial, one important RCT with a negative result, and multiple confounders in the observation studies made the survival gains of BIMA not very clear. Although most trials supported its application in DM patients, the debate over the selection of arterial grafts is ongoing, and the ROMA (Randomization of Single vs. Multiple Arterial Grafts, NCT03217006) trial, a large RCT in progress comparing the effect of TAR and CVR, is expected to provide more information and answers.
The RA is also often used as an adjunct to the LIMA and some RCTs have demonstrated its effectiveness in reducing the adverse outcomes in general population, compared with using SVG [54, 55]. However, studies exploring the outcomes of RA in DM are rather limited. The additional analysis of the three studies [4, 14, 41] applying RA as the second arterial grafting demonstrated the consistent long-term survival benefit. And they consistently reported no significant difference in the incidence of adverse early events when compared to the incidence of those associated with traditional surgery. Further systematic analysis of the comparisons between the RIMA and the RA was hampered by the limited number of relevant studies. According to the two PSM analyses, for diabetic patients, SIMA plus RA grafting and BIMA grafting yielded similar long-term survival after CABG. However, accessing the RA instead of RIMA can decrease the risk of SWI and thus might be the preferred conduit for more diabetic patients. While the RA also has its inherent flaws. For instance, it has been proved that RA is more prone to spasms in response to endogenous vasoconstrictors administered to DM patients [56]. Therefore, further data related to the effect comparisons of various arterial deployments from clinical trials are needed to improve the clinical outcomes of TAR in DM.
This study had several limitations. First, the majority of the studies discussed above were based on retrospective rather than prospective longitudinal data, reflecting outcomes after clinical decision-making by treating surgeons. Several studies were not evenly propensity-score matched, so there is a strong possibility of bias due to confounding. Especially in the comparisons between the outcomes of TAR in DM patients and that in non-DM patients, only one study used PSM method. Therefore, the results of analysis should be treated cautiously. Second, we could not study the freedom from clinical events such as recurrent MI, angina, cardiac death, or the need for repeat revascularization as these data were not available to us. Third, therapy using the BIMA grafts was different from applying TAR as other venous conduits can be used. Since studies were limited, we could not assess the outcomes of TAR using different arterial conduits. The outcomes of revascularization using the BIMA grafts was influenced by the application of venous conduits. Last, an evaluation of the RGA effect in DM was not performed due to the absence of relevant research and we failed to systematically summarize the corresponding effect.
Compared with conventional surgery using SVG, TAR was associated with an enhanced survival benefit in DM patients, but not the increased risk of early death and SWI. Given the increased infection risk and uncertain long-term survival gains of using the BIMA in DM patients, its wide use in this cohort should be seriously and cautiously considered. Compared to applying the RIMA, the RA might be a similarly effective but safer option for diabetic patients. However, the reliance of evidence was subjected to the limitation of observational studies and the findings above require the support of RCTs in the future.
CABG, coronary artery bypass graft; TAR, total arterial revascularization; MAR, multiple arterial revascularizations; CVR, conventional revascularization with venous grafts; DM, diabetes mellitus; BIMA, bilateral internal mammary arteries; LIMA, left internal mammary artery; RA, radial artery; RIMA, right internal mammary artery; RGA, right gastroepiploic artery; SVG, saphenous venous grafts; SWI, sternal wound infection; PSM study, propensity-score–matched study; CV death, cardiovascular death.
The datasets generated and analyzed during the current study are available from the corresponding author upon reasonable request.
GL and YP conceived the study and designed the protocol; GL and TL integrated the data and drafted the manuscript; GL, TL, YL, YY, XC and LB were responsible for the study selection, data extraction, assessment of study quality, and analysis and interpretation of data; YP and GL revised the manuscript critically. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
Not applicable.
We gratefully acknowledge the assistance and instruction from Yang Xiong in the surgical department, and Fanfan Shi, et al. in the Department of Clinical Research Management, West China Hospital of Sichuan University on statistic methodology.
This study was supported by Sichuan Science and Technology Program (Grant
numbers: 2021YFS0330, Sichuan, China), Sichuan Provincial Cadre Health Research
Project, China (Sichuan Ganyan ZH2021-101), 1
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
