- Academic Editor
†These authors contributed equally.
Background: Acute kidney injury (AKI) is common after cardiac
interventional procedures. The prevalence and clinical outcome of AKI in patients
with acute myocardial infarction (AMI) after undergoing intra-aortic balloon pump
(IABP) implantation remains unknown. The aim of this study was to investigate the
incidence, risk factors, and prognosis of AKI in specific patient populations.
Methods: We retrospectively reviewed 319 patients with AMI between
January 2017 and December 2021 and who had successfully received IABP
implantation. The diagnostic and staging criteria used for AKI were based on
guidelines from “Kidney Disease Improving Global Outcomes”. The composite
endpoint included all-cause mortality, recurrent myocardial infarction,
rehospitalization for heart failure, and target vessel revascularization.
Results: A total of 139 patients (43.6%) developed AKI after receiving
IABP implantation. These patients showed a higher incidence of major adverse
cardiovascular events (hazard ratio [HR]: 1.55, 95% confidence interval [CI]:
1.06–2.26, p = 0.022) and an increased risk of all-cause mortality (HR:
1.62, 95% CI: 1.07–2.44, p = 0.019). Multivariable regression models
found that antibiotic use (odds ratio [OR]: 2.07, 95% CI: 1.14–3.74, p
= 0.016), duration of IABP use (OR: 1.24, 95% CI: 1.11–1.39, p
Acute kidney injury (AKI) is a common occurrence after cardiac interventions, particularly in patients with baseline renal dysfunction, and results from improper or excessive use of contrast during the interventional procedure [1, 2]. A previous real-world study reported an AKI incidence of 11.6% and in-hospital mortality of 8.8% [3]. Several large cohort studies have also reported a higher incidence of AKI in patients with acute myocardial infarction (AMI) [4, 5, 6], suggesting that impaired renal function may be associated with worse clinical outcomes [7, 8, 9]. Moreover, the incidence of AKI increased to approximately 33% when complicated by cardiogenic shock (CS) [10], primarily due to the significant reduction in cardiac output [11, 12]. Current guidelines discourage intra-aortic balloon pump (IABP) implantation due to limited improvement in prognosis [13, 14]. IABP implantation is nevertheless thought to be helpful for stabilizing hemodynamics in some patients, and is commonly used in developing countries [15, 16]. Since peripheral blood flow in CS patients is further reduced by IABP implantation, this leads to further compromise of kidney function [17]. Therefore, additional studies are needed to determine the risk factors and clinical outcomes for AKI in AMI patients. To address this, we evaluated the incidence and risk factors for AKI in AMI patients who underwent IABP implantation.
This was a single-center observational study of patients hospitalized at the
coronary care unit, Nan Jing First Hospital, from January 2017 to December 2021.
Patients were retrospectively assessed for eligibility using the following
inclusion criteria: aged 18–85 years; diagnosis of AMI with CS; received
successful IABP implantation. The diagnostic criteria for AMI and CS were based
on prior descriptions [13, 18]. Patients with CS who were characterized by
sustained hypotension (systolic blood pressure [SBP]
Clinical follow-up was conducted through visits to the clinic or by telephone
calls, ranging from 1 to 18 months after discharge. The composite endpoint was a
major adverse cardiovascular event (MACE), which included all-cause mortality,
recurrent myocardial infarction, rehospitalization for heart failure, and target
vessel revascularization. Based on guidelines from the Kidney Disease Improving
Global Outcomes (KDIGO) [19], the diagnostic criteria for AKI used in the present
study was an increase in the serum creatinine (SCr) concentration by
Experienced interventionists oversaw all procedures following accepted
standards. The use of glycoprotein IIb/IIIa inhibitors, pre-dilation or
post-dilation, and the type of implanted drug eluting stent (DES) were at the
discretion of the interventionist. A loading dose of clopidogrel (300 mg) or
ticagrelor (180 mg) was routinely administered prior to percutaneous coronary
intervention (PCI) procedures. A standard dual antiplatelet therapy consisting of
aspirin (100 mg/d) and a P2Y
Continuous variables were expressed as the mean
A total of 319 consecutive AMI patients who underwent successful IABP
implantation were included in the study cohort. Of these, 139 (43.6%) were
diagnosed with AKI after receiving IABP implantation, while remaining patients
were classified as the non-AKI group (n = 180). The flow chart used for the
selection of study participants is shown in Fig. 1, while the baseline
characteristics of eligible patients are summarized in Table 1. Significant
differences in heart rate (HR) and Killip classifications were observed between
the AKI and non-AKI groups. Additionally, patients with AKI had a significantly
increased incidence of ventricular fibrillation and were more likely to be
treated with vasopressors (58.3% vs. 40.6%, p = 0.002) and antibiotics
(69.1% vs. 39.4%, p
| Characteristic | Non-AKI (N = 180) | AKI (N = 139) | p value | |
|---|---|---|---|---|
| Age, yrs | 70.0 (61.0 to 77.0) | 69.0 (60.5 to 77.0) | 0.735 | |
| Male, n% | 135 (75%) | 108 (78.3%) | 0.585 | |
| Hypertension, n% | 132 (73.3%) | 91 (65.5%) | 0.163 | |
| Diabetes | 71 (39.4%) | 63 (45.3%) | 0.347 | |
| Stroke, n% | 30 (16.8%) | 31 (22.3%) | 0.271 | |
| Dyslipidemia, n% | 110 (61.1%) | 84 (60.4%) | 0.994 | |
| BMI, kg/m |
23.7 (21.6 to 25.7) | 23.9 (21.4 to 26.0) | 0.610 | |
| HR, bpm | 88.3 |
93.3 |
0.041 | |
| SBP, mmHg | 122.3 |
120.5 |
0.512 | |
| DBP, mmHg | 78.0 (68.0 to 89.0) | 77.0 (68.0 to 90.0) | 0.898 | |
| Killip classification | 0.015 | |||
| Killip I | 54 (30.3%) | 28 (20.1%) | ||
| Killip II | 50 (28.1%) | 31 (22.3%) | ||
| Killip III | 17 (9.6%) | 27 (19.4%) | ||
| Killip IV | 57 (32%) | 53 (38.1%) | ||
| LVEF, % | 45.0 (37.0 to 52.0) | 44.0 (34.0 to 50.0) | 0.106 | |
| STEMI, n% | 120 (66.7%) | 94 (67.6%) | 0.952 | |
| CRRT, n% | 4 (2.2%) | 2 (1.4%) | 0.700 | |
| Ventricular fibrillation, n% | 23 (12.8%) | 34 (24.5%) | 0.011 | |
| Antibiotic administration, n% | 71 (39.4%) | 96 (69.1%) | ||
| Vasopressor administration, n% | 73 (40.6%) | 81 (58.3%) | 0.002 | |
| BUN, mmol/L | 8.3 (5.7 to 12.4) | 9.5 (7.1 to 14.6) | 0.006 | |
| SCr, umol/L | 81.2 (67.2 to 106.0) | 117.0 (80.3 to 175.6) | ||
| eGFR, mL/min | 134.3 (98.4 to 155.4) | 103.7 (61.3 to 135.6) | ||
| Serum albumin, g/L | 36.1 (33.8 to 38.7) | 34.6 (32.1 to 37.3) | ||
| Serum potassium, mmol/L | 3.9 (3.7 to 4.2) | 4.1 (3.8 to 4.4) | 0.011 | |
| Serum phosphorus, mmol/L | 1.1 (0.9 to 1.4) | 1.2 (1.0 to 1.5) | ||
AKI, acute kidney injury; BMI, body mass index; HR, heart rate; SBP, systolic blood pressure; DBP, diastolic blood pressure; LVEF, left ventricular eject fraction; STEMI, ST segment elevated myocardial infarction; CRRT, continuous renal replacement therapy; BUN, blood urea nitrogen; SCr, serum creatinine; eGFR, estimated glomerular filtration rate.
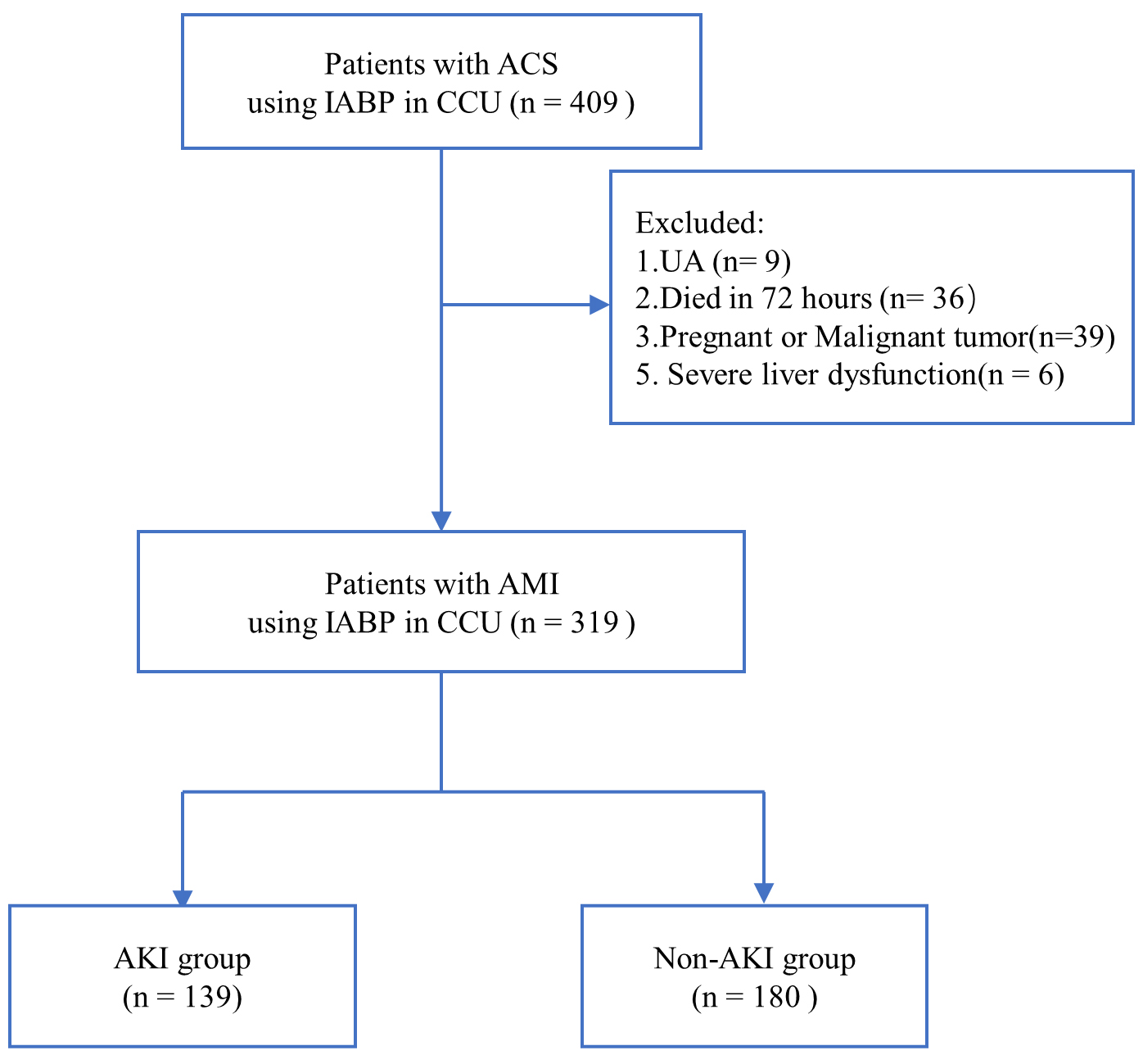 Fig. 1.
Fig. 1.Flow chart showing patient selection. ACS, acute coronary syndrome; CCU, coronary care unit; UA, unstable angina; AMI, acute myocardial infarction; IABP, intra-aortic balloon pump; AKI, acute kidney injury.
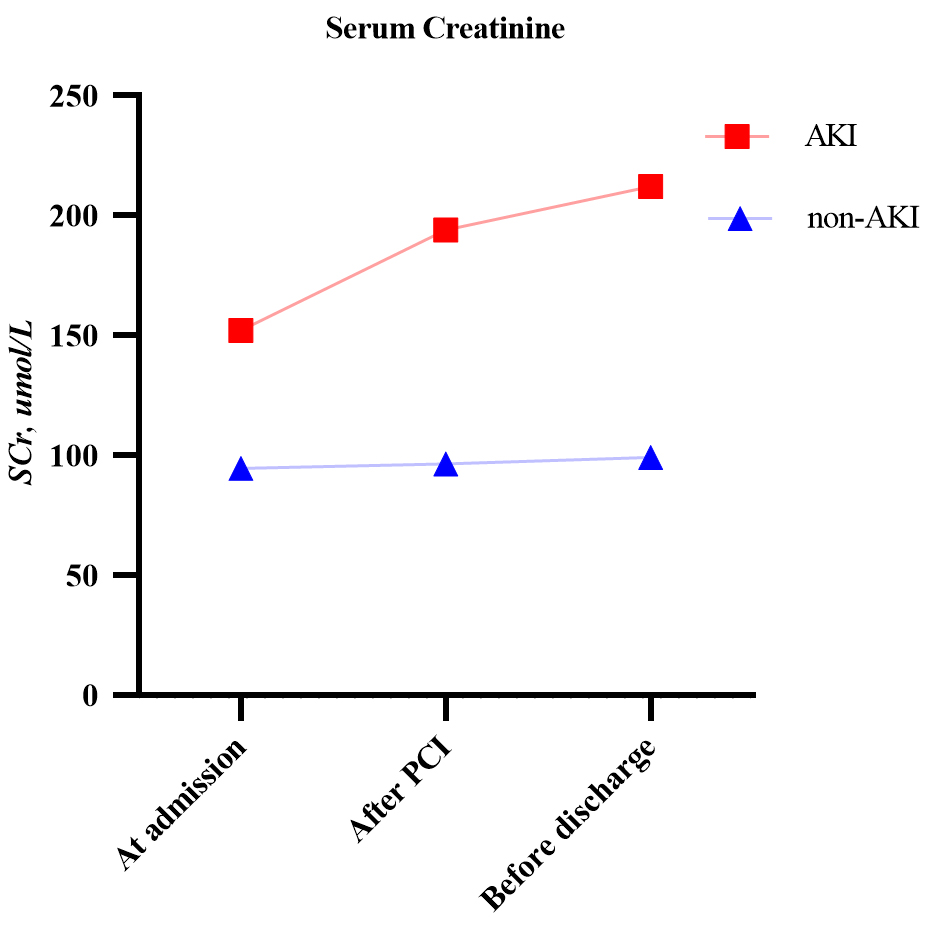 Fig. 2.
Fig. 2.Changes in serum creatinine in the AKI and non-AKI groups. AKI, acute kidney injury; PCI, percutaneous coronary intervention.
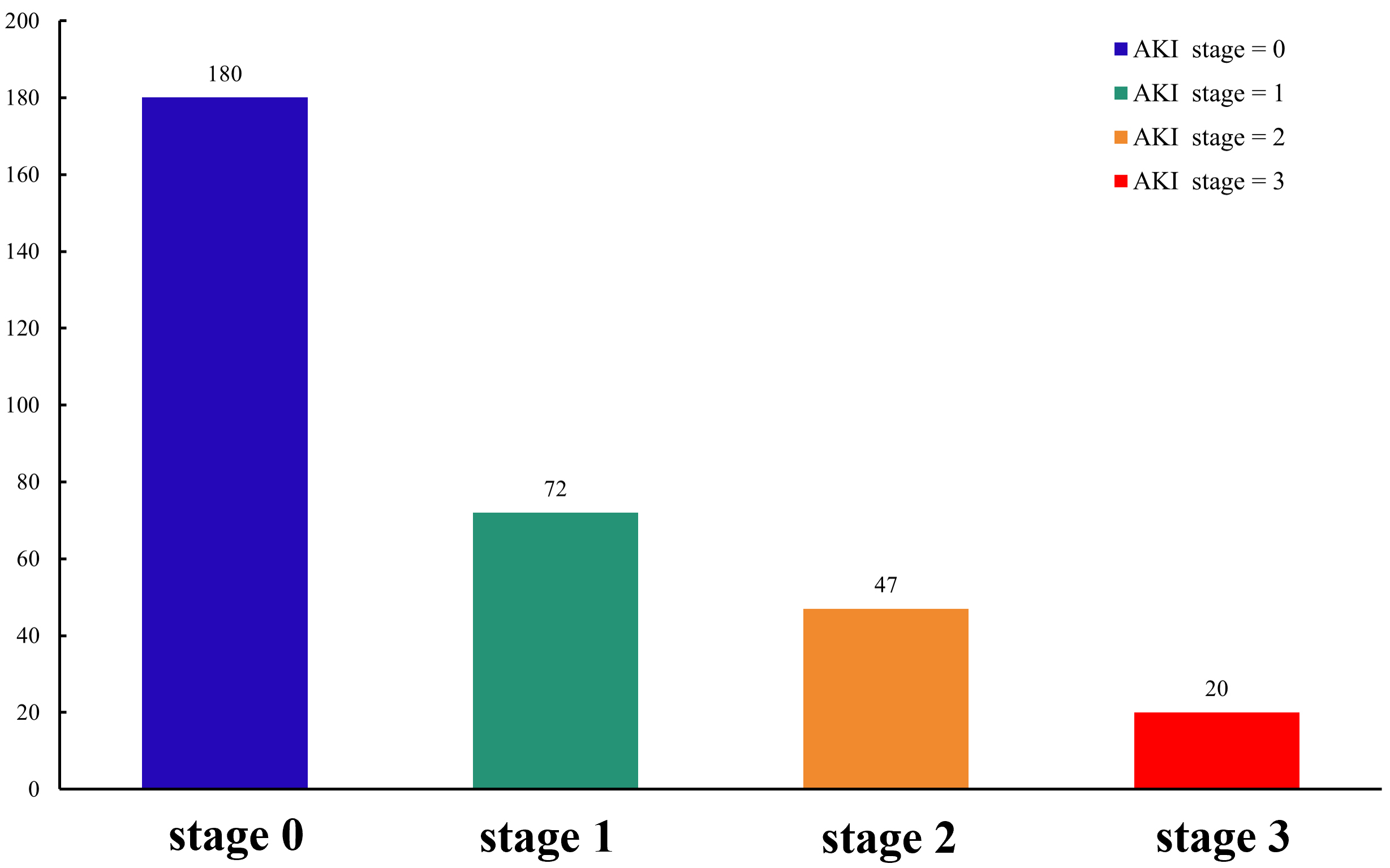 Fig. 3.
Fig. 3.Stage of AKI in the study population. AKI, acute kidney injury
The procedural details for the two patient groups are shown in Table 2. Patients
in the non-AKI group underwent PCI more often than the AKI group (86.7% vs. 77%
respectively, p = 0.035), of which 39.1% received an emergency
procedure. However, there was no difference in the number of implanted DES (2.0
vs. 2.0, p = 0.872) between the two groups. There were also no
significant differences in the use of glycoprotein IIb/IIIa inhibitors or in the
dose of infused contrast. The AKI group had a significantly longer duration of
IABP (7 vs. 5 days, p
| Characteristic | Non-AKI (N = 180) | AKI (N = 139) | p value | |
|---|---|---|---|---|
| CAG, n% | 161 (89.4%) | 110 (79.1%) | 0.017 | |
| PCI, n% | 156 (86.7%) | 107 (77%) | 0.035 | |
| Emergency PCI, n% | 63 (39.1%) | 21 (19.3%) | ||
| Number of stents | 2.0 (1.0 to 2.0) | 2.0 (1.0 to 2.0) | 0.872 | |
| Culprit artery, n% | 0.358 | |||
| LM | 26 (15.7%) | 20 (16.4%) | ||
| LAD | 83 (50.9%) | 68 (55.8%) | ||
| LCX | 13 (8.0%) | 12 (9.8%) | ||
| RCA | 41 (25.2%) | 22 (18.0%) | ||
| Number of lesion vessels | 0.334 | |||
| 1 | 28 (17.8%) | 13 (12.1%) | ||
| 2 | 42 (26.8%) | 26 (24.3%) | ||
| 3 | 87 (55.4%) | 68 (63.6%) | ||
| CTO, n% | 41 (26.1%) | 31 (28.4%) | 0.780 | |
| Contrast volume, mL | 160.0 (140.0 to 200.0) | 150.0 (130.0 to 220.0) | 0.824 | |
| Heparin, IU | 7000 (5775, 8500) | 7230 (6000, 9050) | 0.248 | |
| Bivalirudin, n% | 75 (49%) | 44 (40.7%) | 0.232 | |
| Tirofiban, n% | 46 (29.3%) | 22 (20.2%) | 0.125 | |
| Duration of PCI, min | 40.0 (30.0 to 60.0) | 40.0 (25.0 to 65.0) | 0.984 | |
| Duration of IABP use, days | 5.0 (4.0 to 6.0) | 7.0 (5.0 to 11.0) | ||
AKI, acute kidney injury; CAG, coronary angiography; PCI, percutaneous coronary intervention; LM, left main coronary artery; LAD, left anterior descending coronary artery; LCX, left circumflex artery; RCA, right coronary artery; CTO, chronic total occlusion; IABP, intra-aortic balloon pump.
After an 18-month follow-up period, no significant differences were observed between the two groups for the incidence of recurrent myocardial infarction (AKI vs. non-AKI: 1.4% vs. 1.7%, p = 0.871), rehospitalization for heart failure (5.3% vs. 3.9%, p = 0.620) and target vessel revascularization (3.8% vs. 3.4%, p = 0.957) (Table 3). However, Kaplan–Meier analyses found that patients with AKI had a higher risk of MACEs than those without AKI (41.7% vs. 28.3%, p = 0.022, Fig. 4A), which likely also contributed to the higher incidence of all-cause mortality (36.7% vs. 23.3%, p = 0.019, Fig. 4B). Kaplan–Meier analyses were also performed according to the stage of AKI. The severity of AKI was found to be associated with an increased risk of MACEs (stage 2 vs. stage 0: p = 0.024; stage 3 vs. stage 0: p = 0.038, Fig. 5A), which contributed to an increased incidence of mortality (stage 2 vs. stage 0: p = 0.036; stage 3 vs. stage 0: p = 0.026, Fig. 5B).
| Outcome | Total (N = 319) | non-AKI (N = 180) | AKI (N = 139) | HR (95% CI) | p value |
|---|---|---|---|---|---|
| MACE, n% | 109 (34.2%) | 51 (28.3%) | 58 (41.7%) | 1.55 (1.06–2.26) | 0.022 |
| All-cause Mortality, n% | 93 (29.1%) | 42 (23.3%) | 51 (36.7%) | 1.62 (1.07–2.44) | 0.019 |
| recurrent myocardial infarction, n% | 5 (1.6%) | 3 (1.7%) | 2 (1.4%) | 0.86 (0.14–5.23) | 0.871 |
| rehospitalization for heart failure, n% | 14 (4.4%) | 7 (3.9%) | 7 (5.3%) | 1.31 (0.45–3.83) | 0.620 |
| target vessel revascularization, n% | 9 (2.8%) | 5 (3.4%) | 4 (3.8%) | 1.04 (0.27–3.94) | 0.957 |
| CABG, n% | 5 (1.6%) | 4 (2.2%) | 1 (0.7%) | 0.32 (0.03–2.89) | 0.319 |
AKI, acute kidney injury; HR, hazard ratio; CI, confidence interval; MACE, major adverse cardiovascular events; CABG, coronary artery bypass grafting.
 Fig. 4.
Fig. 4.Kaplan-Meier analysis of MACE (A) and mortality (B) in non-AKI and AKI groups. MACE, major adverse cardiovascular event; AKI, acute kidney injury.
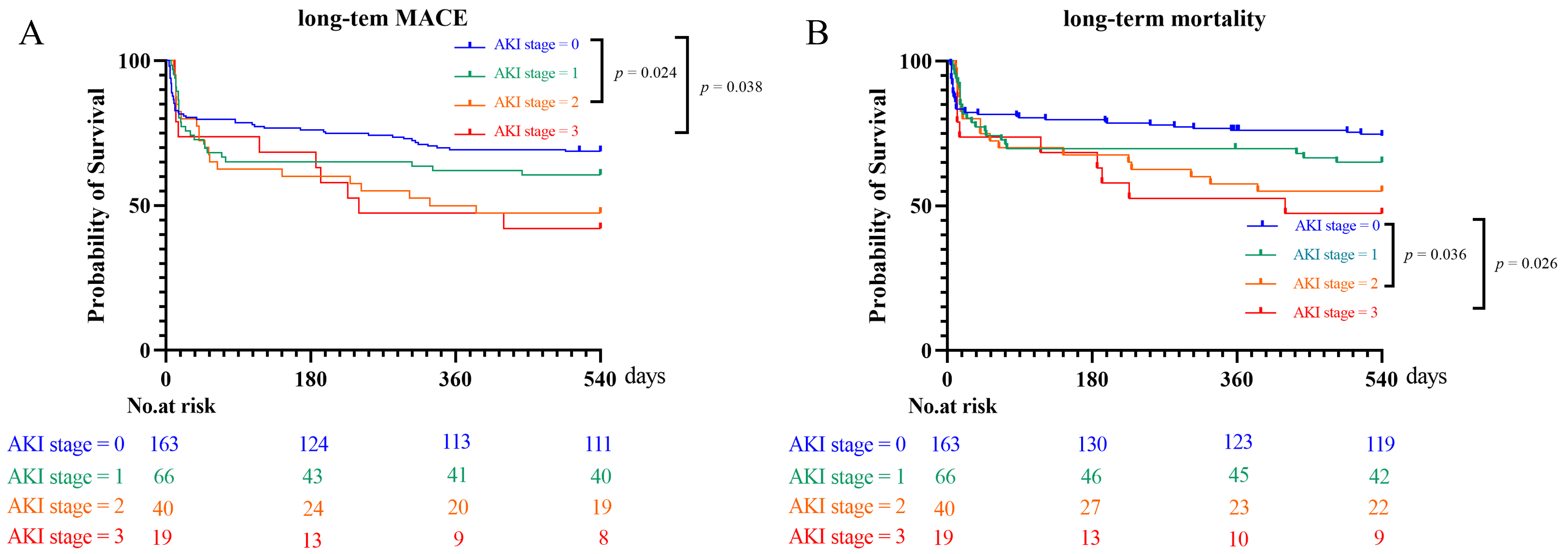 Fig. 5.
Fig. 5.Kaplan-Meier analysis of MACE (A) and mortality (B) in patients with different AKI stages. MACE, major adverse cardiovascular event; AKI, acute kidney injury.
As shown in Table 4, emergency PCI was protective against AKI (odds ratio [OR]:
0.35, 95% CI: 0.18–0.69, p = 0.003). In contrast, antibiotic
administration (OR: 2.07, 95% CI: 1.14–3.74, p = 0.016), duration of
IABP use (OR: 1.24, 95% CI: 1.11–1.39, p
| Characteristic | Univariable | Multivariable | ||
|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | |
| HR | 1.01 (1–1.02) | 0.042 | ||
| Killip I | Ref | |||
| Killip II | 1.46 (0.74–2.89) | 0.276 | ||
| Killip III | 3.48 (1.43–8.43) | 0.006 | ||
| Killip IV | 1.90 (0.97–3.69) | 0.060 | ||
| Ventricular Fibrillation | 2.34 (1.20–4.56) | 0.012 | 1.91 (0.88–4.13) | 0.101 |
| Emergency PCI | 0.35 (0.20–0.64) | 0.35 (0.18–0.69) | 0.003 | |
| Vasopressor administration | 2.05 (1.31–3.22) | 0.002 | ||
| Antibiotic administration | 3.62 (2.14–6.10) | 2.07 (1.14–3.74) | 0.016 | |
| Duration of IABP use | 1.28 (1.15–1.41) | 1.24 (1.11–1.39) | ||
| BUN | 1.05 (1.01–1.09) | 0.016 | ||
| SCr | 1.01 (1.01–1.02) | 1.01 (1.00–1.01) | 0.010 | |
| Serum Albumin | 0.93 (0.87–0.99) | 0.027 | ||
| Serum Phosphorus | 2.38 (1.15–4.92) | 0.019 | ||
AKI, acute kidney injury; OR, odds ratio; CI, confidence interval; HR, heart rate; VSR, ventricular septal rupture; PCI, percutaneous coronary intervention; IABP, intra-aortic balloon pump; BUN, blood urea nitrogen; SCr, serum creatinine.
This retrospective study the assessed clinical outcomes of patients with AMI who underwent successful IABP implantation. The major finding was that the presence of AKI in these patients significantly increased the incidence of MACEs and all-cause mortality. Moreover, a higher severity of AKI was associated with worse prognosis, and the duration of IABP use was found to be an independent predictor of AKI.
It is well established that ischemia and the utilization of nephrotoxic drugs are the two main causes of AKI in the clinic [21, 22]. AMI patients may be more vulnerable to AKI because they have significantly decreased cardiac output and more hemodynamic disorders, especially to the extent seen in CS [10]. Currently, IABP is recommended for CS patients in order to stabilize their hemodynamics [15, 16], despite the possibility of peripheral perfusion deficiency. However, the improper or excessive use of contrast during PCI procedures can exacerbate kidney injury in such patients [23, 24]. Therefore, it is important to identify the potential risk factors for AKI in these specific populations so that their clinical outcome can be improved. The present study was conducted to assess the clinical outcome of AMI patients after receiving successful IABP implantation.
We found the incidence of AKI in this specific patient population was approximately 43.6%. This was likely to have contributed to the increased incidence of all-cause death in AKI patients compared to non-AKI patients (36.7% vs. 23.3% respectively, p = 0.019). An earlier study reported that AKI occurred in almost one third of patients during the first day of CS following AMI [10]. However, this study did not exclude patients who died within 72 hours after admission. Moreover, the proportion of patients who underwent coronary angiography (34.7%) or PCI (16.9%) was much lower than in our study (85.0% and 82.4%, respectively). It is important to note that the association between mechanical circulatory assist devices and renal injury remains controversial. IABP is a commonly used cardiac assist equipment for CS and can improve cardiac output to a certain degree, but with the potential risk of renal hypoperfusion [17]. Achieving a balance between the use of IABP and renal hypoperfusion remains challenging. Several earlier studies reported that mechanical circulatory support (MCS) was the preferred option for patients with CS complicated by kidney injury [25, 26]. Moreover, another study suggested that early implantation in conjunction with coronary circulation reconstruction led to improved prognosis in these patient subsets [27]. Nonetheless, longer utilization of MCS has also been associated with a higher incidence of AKI [28]. Our results also showed that long-term IABP use was an independent risk factor for AKI in these patients. In contrast, emergency PCI was associated with a lower incidence of AKI and was a protective factor in these patients. This may have been a result of improved cardiac function following revascularization. The use of different interventional strategies and patient cohorts with different baseline characteristics could be expected to influence the clinical outcomes. A total of 97 consecutive patients diagnosed with ST segment elevated myocardial infarction and complicated by CS were enrolled in an earlier cohort study [8]. The incidence of AKI amongst these patients was 55%, with binary logistic regression analysis also identifying the initial SCr level as an independent risk factor for AKI.
Several other studies have reported potential risk factors for AKI in AMI patients [29, 30, 31]. The SILVER-AMI study found that HR, left ventricle eject fraction, body mass index, creatinine clearance, presentation of heart failure, prior myocardial infarction and initial hemoglobin were independent risk factors for AKI [30]. An observational study found that hospital-acquired infection, NT-proBNP and prior resuscitation were significantly correlated with acute kidney damage [29]. Several other studies have suggested that age, hypertension, diabetes, chronic kidney disease phase, Killip classification, and extensive anterior myocardial infarction were potential risk factors for kidney impairment [31]. Additionally, the present study found that antibiotic use was an independent risk factor for AKI after adjusting for ventricular fibrillation.
In order to correctly implement the many preventive strategies for AKI, clinicians should take into account the individual characteristics of each patient. Currently, revascularization of the culprit vessel is still strongly recommended for these AMI patients [13, 32]. The risk of contrast-induced AKI in AMI could also be estimated using several clinical prediction models [23, 33]. According to the different risk stratifications, forced diuresis with matched hydration can help prevent AKI following PCI procedures in AMI patients [34, 35, 36]. PCI may therefore be beneficial for patients diagnosed with CS, since fluid management and homeostasis of the inner environment is more challenging in these patients due to the vulnerability of cardiac pump function. CS patients are also more likely to have co-morbidities such as diabetes, stroke and chronic kidney disease. Indeed, several earlier studies have suggested that diabetes can cause an over-inflammatory and thrombotic status [37, 38, 39]. This arises because of severe endothelial injury, which then significantly increases the risk of diabetic vascular complications and leads to poor prognosis, especially in AMI patients. The complete loss of blood flow to the myocardium results in a large amount of cardiomyocyte death, triggering significantly lower cardiac output and the development of hemodynamic disorders. The therapeutic tools available for such patient groups with diabetes are then limited [10, 40]. Treatment with sodium-glucose co-transporter 2 inhibitor (SGLT2i) has been reported to have marked cardioprotective benefits in such patients [38, 41, 42]. This new oral antidiabetic agent has been associated with many potential mechanisms of action. A widely accepted concept is that SGLT2i reduces myocardial apoptosis and the secretion of inflammatory cytokines after AMI. This could help to repair endothelial damage and stabilize hemodynamics, thereby preserving renal function and thus improving prognosis [43]. To prevent AKI, it could therefore be useful to mitigate various risk factors by monitoring serum glucose, lipid levels, signs of infection, and SCr levels.
There are several limitations to our study. First, this was a single-center, retrospective study and hence some selection bias may have occurred. Larger, prospective randomized trials are warranted to overcome this. Second, although we applied the KDIGO criteria to diagnose AKI, several different diagnostic criteria exit and this can influence the selection of patients with AKI. Finally, the lack of information on the use of hypoglycemic medications limited our ability to explore the potential effects of diabetes on AKI.
The results of this study indicate that AKI is associated with a significantly increased risk of all-cause death in AMI patients after IABP implantation. Moreover, a higher severity of AKI is associated with poorer prognosis in these patients. The administration of antibiotics, the duration of IABP use, and the initial SCr level were identified as independent risk factors for AKI, whereas emergency PCI was found to be a protective factor. Finally, renal function should be assessed both before and after IABP implantation in AMI patients so as to facilitate the identification of patients who are at high-risk of AKI, thereby allowing intervention.
All data generated or analyzed during this study are included in this published article.
NLT conceived the project and designed the study. XYZ, ZGF, and KX assessed eligibility, while HMX evaluated and recorded all clinical events. XYZ wrote the manuscript, and NLT critically revised it. XYZ, ZGF, HMX, and KX contributed equally to this study. All authors participated in data analysis, drafting, and critically revising the paper. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
The study was conducted in accordance with the Declaration of Helsinki. It was approved by the Ethical Committee of Nanjing First Hospital affiliated to Nanjing Medical University (No. KY20170904-07). All participants in the study provided written informed consent.
We wish to sincerely thank those colleagues whose names do not appeared in the paper but who contributed diligently to the study. We also appreciate the comments and suggestions of all peer reviewers.
This research received no external funding.
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
