- Academic Editors
Background: Physical activity (PA) is an important component of
secondary prevention after myocardial infarction (MI). The mortality risk of MI
survivors varies at different post-MI periods, yet the time-varying effect of
total PA is unclear. We aimed to investigate the association between different
volumes and patterns of total PA and mortality at different post-MI periods.
Methods: Using data from the China Patient-centered Evaluative
Assessment of Cardiac Events Million Persons Project, we divided the screened MI
survivors into within-1-year and beyond-1-year groups based on the duration
between their baseline interview and MI onset. Total PA was divided into
insufficient (
In China, the number of patients with myocardial infarction (MI) has been dramatically increasing, and is estimated to reach 23 million by 2030 [1]. MI compromises cardiac function and remodels the cardiac structure, and thus cardiac structure and function may vary in different post-MI periods [2, 3, 4]. The mortality risk of MI survivors is still high [5], and varies at different post-MI periods (within and beyond 1 year) [6]. Physical activity (PA) has been shown to reduce all-cause mortality and cardiovascular mortality of MI patients by 8%–37% and 7%–38%, respectively [7]. Aerobic PA and other lifestyle interventions have been reported to reduce coronary atherosclerosis to different degrees within 1 and 5 years [8]. And PA and exercise-based cardiac rehabilitation have been recommended as important components of secondary prevention of MI and cardiovascular disease (CVD) [9, 10, 11, 12, 13]. The guideline for cardiac rehabilitation in China recommends regular aerobic PA within 1 year after MI and individualized training beyond 1 year after discharge [14]. The possible mechanisms of mortality reduction associated with PA may include improved cardiorespiratory fitness and cardiac remodeling [15]. Thus, we hypothesized that PA may have a different impact on mortality reduction among MI survivors at different post-MI periods.
However, evidence is lacking on the association between different volumes and patterns of total PA and the mortality risks at different post-MI periods. First, there is limited evidence on the association between PA collected at different post-MI periods and mortality among MI survivors. Prior studies primarily used longitudinal cohort follow-up through repeated surveys [16, 17, 18, 19, 20], and focused on PA at 6–10 weeks post-MI [19], or the change of levels of PA after MI [16, 17, 18, 20]. Second, there is a lack of evidence on non-leisure time PA (household, occupational, and transport PA) or total PA (both leisure and non-leisure time PA) among MI patients. Specifically, PA was mainly performed during non-leisure time rather than leisure time in China and other low- and middle-income countries [21]. This differed greatly from those in high-income countries, and the previous evidence mainly focused on the high-income ones [22]. Besides, previous analyses mainly focused on certain populations with small sizes [17, 18, 20]. A comprehensive understanding of the time-varying effect of physical activity on mortality is needed in clinical practice for developing a more tailored strategy for secondary prevention after MI.
We analyzed data from the China Patient-centered Evaluative Assessment of Cardiac Events (PEACE) Million Persons Project (MPP), a population-based cohort study covering 31 provinces in mainland China, to explore the time-varying effect of different volumes and patterns of total PA (including leisure and non-leisure time PA) on mortality risk among MI survivors.
The China PEACE MPP is a government-funded public health project for the screening and management of high CVD risk subjects. The detailed study design has been previously published [23]. From August 2014 to December 2021, permanent residents aged 35–75 years with a history of MI were identified from 349 rural counties or urban districts (210 rural counties, 139 urban districts) in 31 provinces in mainland China. These participants received a detailed questionnaire survey on lifestyle and medical history at baseline, and then were followed up annually (Supplementary Section 1). We excluded the participants who reported difficulty in mobility, self-care, or usual activities in the European Quality of Life–5 Dimensions (EQ-5D) questionnaire, as well as those who had incomplete data for PA and covariates (Supplementary Fig. 1). Eligible participants were divided into two groups based on the duration between baseline and their MI onset (within and beyond 1 year).
The project protocol was approved by the central ethics committee at Fuwai Hospital, Beijing, China (Approval No. 2014-574). Written informed consent was obtained from all enrolled participants.
Data on PA was collected through standardized in-person questionnaire interviews. The questions on PA were adapted from validated questionnaires used in other cohort studies [24, 25, 26]. Participants were asked about detailed information on PA across all domains of life (leisure time, household, occupational, and transport) during the past 12 months. Leisure time PA included swimming, running, or aerobic exercise as vigorous-intensity activities, and ball games, walking, gymnastics, folk dancing, Tai-Chi, and qigong as moderate-intensity activities. Non-leisure time PA included PA in the household, occupational, and transport domains. To quantify the volume of PA, each activity was assigned an intensity level based on the updated 2011 Compendium of Physical Activities [27]. One metabolic equivalent of task (MET) is equivalent to 1 kcal/kg/hour and equal to the energy cost of sitting quietly. The level of each activity was calculated by multiplying its MET value and the minutes spent per week. Domain-specific PA levels were calculated by summing all the MET minutes per week spent in non-sedentary leisure time, household, occupational, and transport domains. Total PA was calculated as a sum of all domain-specific PA (Supplementary Section 2).
The theoretical minimum-risk exposure level for total PA was 3000–4500 MET
minutes/week recommended by the Global Burden of Disease study [28]. Thus, total
PA of less than 3000 MET minutes/week was considered insufficient PA [29].
Sufficient PA was divided into moderate (3000–4500 MET minutes/week) and high
(
We also collected information on participants’ sociodemographic factors (i.e., age, sex, household income, occupation type, and education level), other lifestyle factors (i.e., diet, smoking, and alcohol consumption), medical history, and medication use during the in-person interviews. Physical measurements (i.e., height, weight, and blood pressure) were collected, and blood lipid and blood glucose levels were determined following standardized protocols by trained medical staff (Supplementary Section 3).
Outcomes of interest in this study were all-cause mortality and cardiovascular mortality, which were identified through the National Mortality Surveillance System and Vital Registration of the Chinese Center for Disease Control and Prevention. All events were coded using the International Classification of Diseases, 10th edition (ICD-10). Cardiovascular mortality was defined as death due to CVD (ICD-10: I01-I99). Mortality data were available up to December 31, 2021. Follow-up was censored at this time or date of death, whichever came first.
Baseline characteristics were described for the entire cohort, stratified by two post-MI periods and different volumes and patterns of PA. Baseline PA was described for the entire cohort, and the within-1-year and beyond-1-year period. Continuous variables were summarized as means (standard deviations) or medians (interquartile range [IQR]) as appropriate, and categorical variables as frequencies and percentages. Differences in characteristics were examined by one-way analysis of variance for continuous variables and the Chi-square test for categorical variables.
Cox proportional hazard models were fitted to calculate independent hazard ratios (HRs) and 95% confidence intervals (CIs) for the association between different volumes and patterns of PA with all-cause mortality and cardiovascular mortality. We used age instead of time-on-study as the time scale [30, 31, 32]. Multivariable-adjusted models included age, sex, household income, occupation type, education level, high blood pressure, high blood glucose, high total cholesterol, high body mass index, high alcohol consumption, current smoking, unhealthy diet, history of heart failure, history of chronic kidney disease, and medication use including angiotensin-converting enzyme inhibitors or angiotensin receptor blockers, beta-blockers, statins, and aspirin. The interaction between different volumes and patterns of PA and post-MI periods was considered in the statistical models. Using age as the time scale, restricted cubic splines were fitted with 4 knots by treating the volume of total PA as a continuous variable using the “rms” R package. The reference value for PA was set at 3000 MET minutes/week. To reduce potential bias from reverse causation, we also did sensitivity analyses to assess the effect of different volumes and patterns of total PA using inverse probability weighting. Specifically, we included the participants who reported difficulty in mobility, self-care, or usual activities in the EQ-5D questionnaire, and calculated the propensity scores based on the EQ-5D score and covariates of the multivariable-adjusted models. Then the Cox proportional hazards models were weighted to inverse probability weighting.
All analyses were performed in SAS 9.4 (SAS Institute Inc., Cary, NC, USA) and R software, version 4.1.2 (The R Foundation for Statistical Computing, Vienna, Austria).
We enrolled 29,913 participants with prior MI in the China PEACE MPP. After
excluding participants with difficulty in mobility, self-care, and usual
activities (1293, 4.3%), and those with incomplete information on PA (5463,
19.1%) and covariates (2504, 10.8%), 20,653 participants were finally included
(Supplementary Fig. 1). Among the included patients, the median age was
62 years and 47.7% were female (Table 1). Patient characteristics differed in
most features across the two post-MI periods, except for sex (p =
0.620), history of chronic kidney disease (p = 0.928), statins use
(p = 0.499), and aspirin use (p = 0.109). Baseline
characteristics were also stratified by different volumes and patterns of PA
(Supplementary Table 1). Baseline PA at each post-MI period is presented
in Table 2. MI patients in the within-1-year group had a higher volume of total PA
(median 4200 versus 3780 MET minutes/week, p
| Characteristics | Total period | Within 1 year of MI onset | Beyond 1 year of MI onset | p-value | ||
|---|---|---|---|---|---|---|
| Number of participants | 20,653 | 5222 (25.3%) | 15,431 (74.7%) | |||
| Socioeconomic factors | ||||||
| Age (years) | 62 [55, 67] | 61 [53, 66] | 63 [56, 68] | |||
| Sex | ||||||
| Female | 9854 (47.7%) | 2507 (48.0%) | 7347 (47.6%) | 0.620 | ||
| Male | 10,799 (52.3%) | 2715 (52.0%) | 8084 (52.4%) | |||
| Household income | ||||||
| 3880 (18.8%) | 1073 (20.5%) | 2807 (18.2%) | ||||
| 15,340 (74.3%) | 3764 (72.1%) | 11,576 (75.0%) | ||||
| Unknown | 1433 (6.9%) | 385 (7.4%) | 1048 (6.8%) | |||
| Occupation type | ||||||
| Light intensity | 2734 (13.2%) | 745 (14.3%) | 1989 (12.9%) | |||
| Medium or heavy intensity | 2973 (14.4%) | 912 (17.5%) | 2061 (13.4%) | |||
| Unemployed or retired | 11,690 (56.6%) | 2676 (51.2%) | 9014 (58.4%) | |||
| Unknown | 3256 (15.8%) | 889 (17.0%) | 2367 (15.3%) | |||
| Education level | ||||||
| Primary school or below | 8557 (41.4%) | 2256 (43.2%) | 6301 (40.8%) | 0.011 | ||
| Middle school or above | 11,918 (57.7%) | 2921 (55.9%) | 8997 (58.3%) | |||
| Unknown | 178 (0.9%) | 45 (0.9%) | 133 (0.9%) | |||
| Metabolic factors | ||||||
| High blood pressure | 10,419 (50.4%) | 2436 (46.6%) | 7983 (51.7%) | |||
| High blood glucose | 5108 (24.7%) | 1181 (22.6%) | 3927 (25.4%) | |||
| High total cholesterol | 5565 (26.9%) | 1247 (23.9%) | 4318 (28.0%) | |||
| High body mass index | 11,400 (55.2%) | 2801 (53.6%) | 8599 (55.7%) | 0.009 | ||
| Lifestyle factors | ||||||
| Current smoking | 4789 (23.2%) | 1150 (22.0%) | 3639 (23.6%) | 0.021 | ||
| High alcohol consumption | 2127 (10.3%) | 467 (8.9%) | 1660 (10.8%) | |||
| Unhealthy diet | 17,991 (87.1%) | 4596 (88.0%) | 13,395 (86.8%) | 0.025 | ||
| Medical History | ||||||
| Heart failure | 948 (4.6%) | 209 (4.0%) | 739 (4.8%) | 0.019 | ||
| Chronic kidney disease | 188 (0.9%) | 47 (0.9%) | 141 (0.9%) | 0.928 | ||
| Medication use | ||||||
| ACEIs or ARBs | 2441 (11.8%) | 540 (10.3%) | 1901 (12.3%) | |||
| Beta-blockers | 2073 (10.0%) | 428 (8.2%) | 1645 (10.7%) | |||
| Statins | 3105 (15.0%) | 770 (14.8%) | 2335 (15.1%) | 0.499 | ||
| Aspirin | 3442 (16.7%) | 833 (16.0%) | 2609 (16.9%) | 0.109 | ||
| Data are presented as mean (SD), median [Q1, Q3 quartiles], or n (%) as
appropriate.
ACEIs, angiotensin-converting enzyme inhibitors; ARBs, angiotensin receptor blockers; MET, metabolic equivalent of task; MI, myocardial infarction; PA, physical activity; SD, standard deviation. | ||||||
| PA (MET minutes/week) | Total period | Within 1 year of MI onset | Beyond 1 year of MI onset | p-value | |
|---|---|---|---|---|---|
| Domains of PA | |||||
| Total PA | 3948 [2352, 6930] | 4200 [2352, 7336] | 3780 [2352, 6741] | ||
| Leisure time PA | 0 [0, 1386] | 0 [0, 1386] | 0 [0, 1386] | ||
| Non-Leisure time PA | 2520 [1176, 5880] | 3360 [1440, 6544] | 2384 [1176, 5760] | ||
| Volumes of total PA | |||||
| Insufficient ( |
7805 (37.8%) | 1802 (34.5%) | 6003 (38.9%) | ||
| Moderate (3000–4500) | 3772 (18.3%) | 952 (18.2%) | 2820 (18.3%) | ||
| High ( |
9076 (43.9%) | 2468 (47.3%) | 6608 (42.8%) | ||
| Patterns of total PA | |||||
| Insufficient ( |
7805 (37.8%) | 1802 (34.5%) | 6003 (38.9%) | ||
| Non-leisure (main domain) | 10,686 (51.7%) | 2971 (56.9%) | 7715 (50.0%) | ||
| Leisure (main domain) | 2162 (10.5%) | 449 (8.6%) | 1713 (11.1%) | ||
| Data are presented as mean (SD), median [Q1, Q3 quartiles], or n (%) as
appropriate.
Total PA (MET minutes/week) was categorized as: insufficient ( | |||||
During the median follow-up of 3.7 years (IQR 2.6–4.8 years), there were 751 all-cause deaths, including 446 cardiovascular deaths (Supplementary Table 2). The associations between different volumes of PA and mortality before and after adjusting for covariates are displayed in Supplementary Table 3 and Fig. 1, respectively.
 Fig. 1.
Fig. 1.HRs (95% CI) for all-cause mortality and cardiovascular
mortality by PA volumes at different post-MI periods. Total PA (MET
minutes/week) was categorized as: insufficient (
Among the survivors within 1 year of MI onset, moderate PA was associated with
significantly lower risk of mortality both in unadjusted analyses (HR 0.59, 95%
CI 0.40 to 0.88, p = 0.009) and fully adjusted models (0.59, 0.40 to
0.88, p = 0.011), while a significant association between high PA and
mortality was only observed in fully adjusted models (0.63, 0.45 to 0.88,
p = 0.008), compared to those with insufficient PA. As shown in Fig. 2,
the risk of mortality showed an inverse non-linear relationship with the volume
of total PA (p for non-linearity
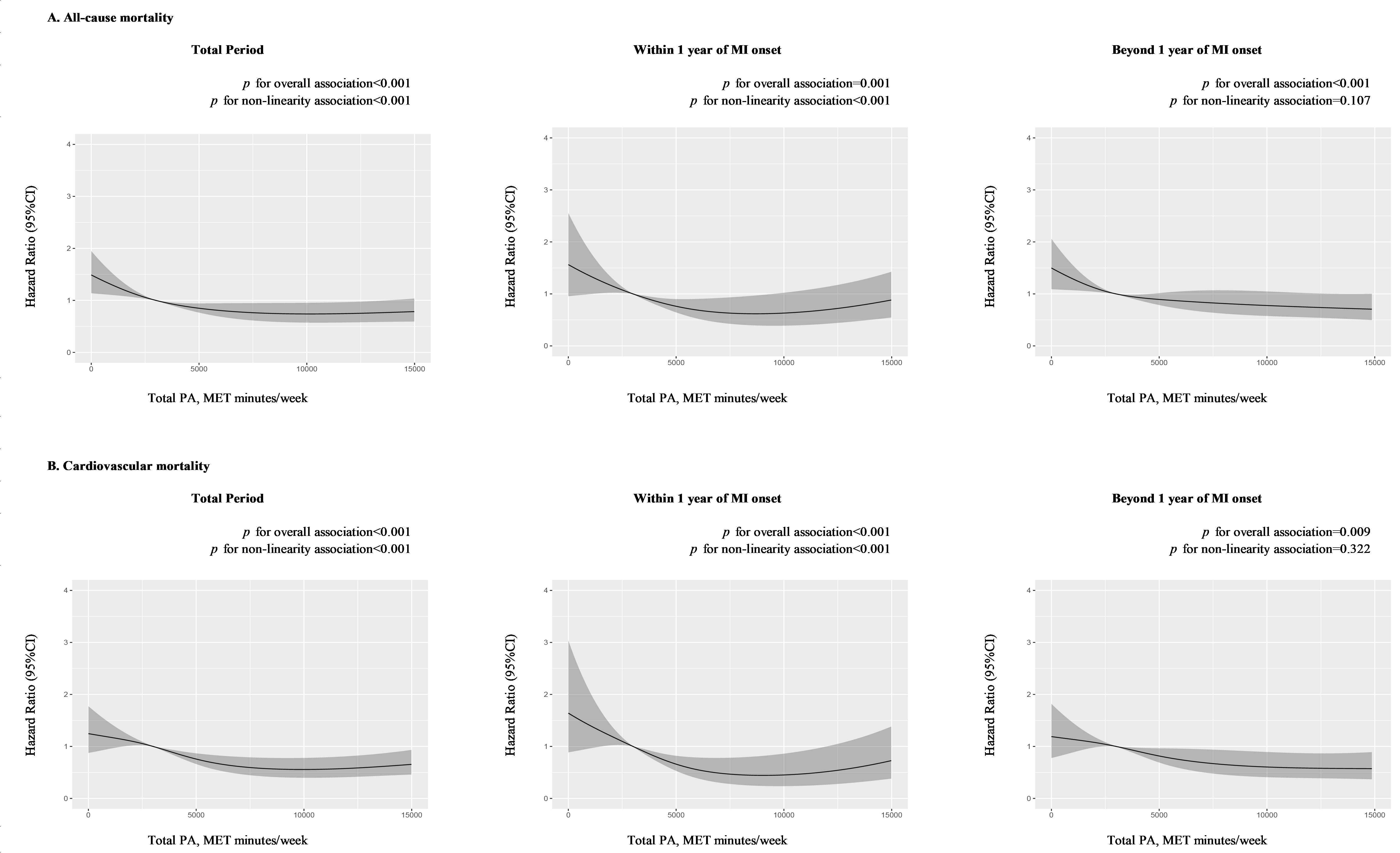 Fig. 2.
Fig. 2.Dose-response association between PA and mortality at different post-MI periods. (A) Dose-response association between total PA and all-cause mortality at total, within-1-year, and beyond-1-year period. (B) Dose-response association between total PA and cardiovascular mortality at total, within-1-year, and beyond-1-year period. Hazard ratios were adjusted for age, sex, household income, occupation type, education level, high blood pressure, high blood glucose, high total cholesterol, high body mass index, high alcohol consumption, current smoking, unhealthy diet, history of heart failure, history of chronic kidney disease, and medication use including angiotensin-converting enzyme inhibitors or angiotensin receptor blockers, beta-blockers, statins, and aspirin. MET, metabolic equivalent of task; MI, myocardial infarction; PA, physical activity.
Among the survivors beyond 1 year of MI onset, moderate PA was not significantly associated with lower mortality risk after adjusting for covariates (unadjusted: 0.79, 0.62 to 0.99, p = 0.042; adjusted: 0.83, 0.66 to 1.05, p = 0.118), while HRs of high PA were significant both before (0.70, 0.58 to 0.85) and after (0.69, 0.56 to 0.86) adjusting for covariates. The dose-response association (Fig. 2) between total PA and mortality risk was linear in the beyond-1-year group (p for non-linearity = 0.107).
No significant interaction was found between different volumes and post-MI periods (p = 0.515 for interaction). Similar findings were also observed between different volumes of PA and cardiovascular mortality. No substantial changes in the results were observed in the sensitivity analyses using the inverse probability weighting method (Supplementary Table 4).
Overall, sufficient PA, i.e., leisure and non-leisure patterns, was associated with significantly lower risk of mortality compared with those with insufficient PA at each post-MI period.
In the unadjusted models (Supplementary Table 5), both leisure and non-leisure patterns were associated with significantly lower HRs for all-cause mortality in within-1-year (leisure: 0.46, 0.26 to 0.82; non-leisure: 0.74, 0.56 to 0.98) and beyond-1-year (leisure: 0.61, 0.46 to 0.83; non-leisure: 0.77, 0.64 to 0.92) groups. In the fully adjusted models (Fig. 1, Supplementary Table 5), among survivors within 1 year of MI onset, leisure (0.52, 0.29 to 0.94) and non-leisure (0.64, 0.46 to 0.88) patterns were both associated with significantly lower risk of mortality. A similar association was observed among survivors beyond 1 year of MI onset (leisure: 0.64, 0.48 to 0.87; nonleisure: 0.79, 0.65 to 0.97). However, no significant interaction was found between different patterns of PA and post-MI periods (p = 0.775 for interaction). The results were consistent for cardiovascular mortality, and did not change substantially in the sensitivity analyses using the inverse probability weighting method (Supplementary Table 6).
Our study found that sufficient total PA was associated with lower risk of mortality among MI survivors. The dose-response association of total PA and mortality was curvilinear within 1 year and linear after 1 year of MI onset. The reduction in mortality risk among MI survivors appeared to be apparent with moderate PA within 1 year after MI, and those with high PA beyond 1 year after MI. Both leisure and non-leisure patterns were associated with lower mortality risk, independent of the post-MI periods.
This study adds to the prior literature in several ways. First, we provided information regarding the association between total PA and mortality risk among MI survivors and found a difference between the within-1-year and beyond-1-year groups. Guidelines for cardiac rehabilitation recommend regular moderate-intensity aerobic PA lasting 30–60 minutes on each day and 3–5 days/week for stage 2 rehabilitation (within 1 year after discharge), and an individual home-based training program for stage 3 rehabilitation (beyond 1 year after discharge) [14]. One possible reason for the different recommendations is the potential heterogeneity in cardiac structure and function [3, 4], and mortality risk [5] between two post-MI periods. In this study, a significant difference in the distribution of PA was observed among MI survivors within and beyond 1 year of MI onset. Within 1 year, MI survivors tended to perform less leisure time PA, compared with those beyond 1 year. It is a reasonable assumption that MI survivors within 1 year tended to be reluctant to perform leisure time PA for fear of adverse events following their acute MI. However, although they did not actively select leisure time PA, they performed more non-leisure time PA and total PA, and had a lower proportion of insufficient PA. Apart from the possible different cardiac structures and functions, we speculate that the difference in the association between PA and mortality risk at two post-MI periods might be partly due to the distribution of PA habits.
Second, we report the association between different volumes of PA and the risk
of mortality at different post-MI periods. Previous studies of graded effects of
total PA were limited to the general population and patients with CVD [22, 33].
Although the Global Burden of Disease study recommends 3000–4500 MET
minutes/week of total PA [28] based on a meta-analysis quantifying the
dose-response associations in five chronic diseases (including ischemic heart
disease events) [34], it is still unclear whether a similar volume could also
work in MI survivors at different post-MI periods. In our study,
Third, our study filled the gap in the association between different patterns of sufficient PA and mortality risk. We found that sufficient total PA, either leisure (higher proportion of leisure time PA) or non-leisure (higher proportion of non-leisure time PA) pattern, was associated with lower risk of mortality, compared with insufficient total PA. In our study, only a few MI patients had leisure pattern. This is consistent with previous studies which observed that PA in China mainly involved occupation and housework, with less contribution from leisure time PA [21]. Prior evidence on leisure time PA [33, 38] and non-leisure time PA [22, 33] was reported in the general population and patients with CVD, among which the evidence was inconsistent on non-leisure time PA. And in the research where non-leisure time PA was categorized into the domains of household, occupational, and transport, the findings were still inconsistent [39, 40, 41, 42]. Leisure time PA is usually a short period PA with a long recovery time, which may improve cardiovascular risk factors (such as insulin resistance, hypertension, dyslipidemia, and obesity) [40, 43]. However, non-leisure time PA, such as occupational PA, is of low intensity or long duration and may not improve cardiovascular health. Occupational PA could raise 24-hour heart rate and blood pressure, and increase the levels of inflammation [41]. While the potential mechanisms of household and transport PA still need further study. The possible influential factors are that some housework is also an aerobic PA with sufficient recovery time, while transport PA may be affected by traffic and harmful exposure (e.g., gases on roads) as well as active or passive commuting types [42].
Our findings have several important implications. First, appropriately high PA after MI indicates lower risk of mortality. This finding serves as empirical support for making scientifically warranted secondary prevention recommendations for the post-MI population to promote sufficient PA. Second, the evidence on proper volumes and patterns of PA for patients at different post-MI periods could facilitate more tailored health advice for PA after MI. Third, for MI survivors within 1 year of their MI onset, as non-leisure time PA is usually performed on a daily basis, we suggest selecting an adequate volume of leisure time PA after fully considering their non-leisure time PA to reach a total level of approximately at least 3000 MET minutes/week within 1 year of MI onset, and no less than 4500 MET minutes/week beyond 1 year of MI onset. Nevertheless, patients should be cautious about their choice in the proper volume of PA. The volumes well above the PA recommendations were reported to be associated with increased mortality risk in CVD patients [44, 45]. More importantly, MI patients should consult with their physicians before adjusting for their PA volume.
Our study has several limitations. First, MI history and PA information were self-reported, which may bring recall bias and may overestimate PA volume [46]. However, questionnaires were collected by trained professionals following a standardized process, the self-reported medical information was based on physician diagnosis, and the questions of PA information had been used and validated in other cohort studies [24, 25, 26]. We assumed that the recall bias is relatively small in a large-scale population. Second, we used the recommended total PA level of the Global Burden of Disease study rather than the widely used World Health Organization guideline [47]. In Chinese adults, PA mainly involves occupation and housework, without much input from leisure time PA [21], so the studies in China usually referred to the World Health Organization standard for leisure time PA rather than total PA [48]. Third, the study lacks data on important clinical risk factors, such as exercise capacity, cardiac function, and disease severity, which are also potential confounders. Nevertheless, we adjusted for history of heart failure, history of chronic kidney disease, metabolic factors, lifestyle factors, and medication use to reduce the confounding effect when analyzing the association between PA and mortality. Fourth, a risk of reverse causality is present, so participants with less severity of MI may have a higher PA and a better prognosis. To address the concerns related to reverse causality, we excluded the participants who reported problems in walking, self-care, and daily activities in the EQ-5D questionnaire. In addition, we did sensitivity analyses using the inverse probability weighting method, and the results did not substantially change our main analyses. However, reverse causality may be unavoidable in observational studies, establishing the optimal volume and pattern of PA in each clinical setting would require randomized head-to-head comparisons.
Our findings demonstrated that sufficient total PA was associated with lower risk of mortality compared with insufficient PA among MI survivors. The dose-response association varied by the different post-MI periods. Both leisure and non-leisure patterns were associated with lower mortality risk, independent of the post-MI periods. These findings could facilitate tailored recommendations on PA in secondary prevention after MI. Further studies are needed to confirm the causal effect of different volumes and patterns of PA on mortality risk at different periods after MI.
CVD, cardiovascular disease; China PEACE MPP, China Patient-centered Evaluative Assessment of Cardiac Events Million Persons Project; CI, confidence interval; HR, hazard ratio; ICD-10, International Classification of Diseases, 10th edition; IQR, interquartile range; MI, myocardial infarction; MET, metabolic equivalent of task; PA, physical activity; SD, standard deviation.
The data that support the findings of this study are available from China Patient-centered Evaluative Assessment of Cardiac Events Million Persons Project (China PEACE MPP) but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of China PEACE MPP. China PEACE MPP only provides conditional data access for qualified researchers with legitimate requests; a formal application and research proposal is required. Please contact cvd-project@nccd.org.cn to seek approval for data access.
XZ, XL and TL designed the research study. XL conceived of the China Patient-centered Evaluative Assessment of Cardiac Events Million Persons Project (China PEACE MPP) and takes responsibility for all aspects of it. XiaYZ, YY, JLC, WX, LJS, HY, WYH and YZ participated in the project operation. TL and XinYZ analyzed the data. TL wrote the manuscript, with further contributions from XZ, XL, XinYZ, XLW, JLS, CQW, AXT, XiaYZ, YY, JLC, WX, LJS, HY, WYH, and YZ. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript.
The project protocol was approved by the central ethics committee at Fuwai Hospital, Beijing, China (Approval No. 2014-574). Written informed consent was obtained from all enrolled participants.
We appreciate the multiple contributions made by study teams at the National Center for Cardiovascular Diseases, and the local sites in the collaborative network in the realms of study design and operations.
This research was funded by the CAMS Innovation Fund for Medical Sciences (CIFMS), grant number 2021-I2M-1-011; and National High Level Hospital Clinical Research Funding, grant number 2022-GSP-GG-4.
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
