- Academic Editor
†These authors contributed equally.
Background: Inflammation and oxidative stress are thought to play an
important role in the pathophysiology of heart failure with preserved ejection
fraction (HFpEF) through the development of endothelial dysfunction.
Myeloperoxidase (MPO) functions as a link between oxidative stress and
inflammation and is an interesting therapeutic target. The objective of this
observational cohort study was to compare MPO levels between HFpEF and old
controls, to define clinical characteristics associated with high levels of MPO
and to assess the relation between MPO levels and vascular function.
Methods: Patients with HFpEF (N = 55) and controls
Heart failure with preserved ejection fraction (HFpEF) is characterized by signs
and symptoms of heart failure, including peripheral oedema, dyspnea and exercise
intolerance, in the absence of a reduced left ventricular ejection fraction (LVEF
Oxidative stress and inflammation are closely interconnected. Transcription
factors that regulate the expression of pro inflammatory cytokines are activated
under oxidative stress conditions and in turn, induce the generation of ROS, thus
creating a vicious cycle of oxidation and inflammation [3]. Myeloperoxidase
(MPO), a leukocyte-derived enzyme, functions as a link between oxidative stress
and inflammation. During inflammation, MPO is released and uses H
Studies suggest that plasma MPO levels are elevated in patients with HF compared to controls and that increasing levels of MPO are associated with restrictive diastolic stage, right ventricular systolic dysfunction and tricuspid regurgitation in HFrEF [4]. Furthermore, MPO may be involved in the pathophysiology of atrial fibrillation through atrial accumulation of MPO and consequent increase in fibrosis [5]. These results imply that MPO may be important also for the development of HFpEF where diastolic dysfunction, atrial fibrillation and fibrosis are major components. Indeed, a recent study showed that HFpEF patients displayed higher plasma concentration of MPO compared to healthy controls [6]. Furthermore, since oxidative stress and microvascular endothelial dysfunction are suggested as fundamental parts of the pathophysiology and development of HFpEF, MPO inhibition appears as an interesting therapeutic approach and a clinical trial investigating MPO inhibitor “AZD4831” (ENDEAVOR NCT04986202 and NCT03611153) is currently ongoing. Heterogeneity among patients with HFpEF has been singled out to explain the difficulty to find treatments improving prognosis in this population. Hence, identifying characteristics associated with high levels of MPO could be interesting to target subgroups of patients most likely to benefit from treatment with MPO inhibitors.
In this context, the objective of our study was to reinforce data about MPO elevation in HFpEF, to assess the relation between MPO levels and clinical parameters including vascular function and to determine patient characteristics associated with high levels of MPO.
Patients with HFpEF encountered in our division of cardiology between May 2019
and May 2021 were prospectively screened for inclusion in the study. HFpEF was
diagnosed according to the guidelines of the European society of cardiology [7, 8]. Briefly, patients had to be symptomatic (New York Heart Association (NYHA)
functional class
The local ethics committee approved the study, and all subjects gave written informed consent before study enrolment (Clinical trial NCT03197350). The investigation conforms to the principles outlined in Declaration of Helsinki. Patients and controls were interrogated about symptoms, medical history and treatment and were thoroughly examined. Other information was retrieved from medical files and from review of hospital records.
Standardized complete transthoracic echocardiography (TTE) exams were acquired according to established guidelines [9] using iE33 ultrasound systems (Philips Medical Systems, Andover, Massachusetts) equipped with a 3.5/1.75-MHz phased-array transducer and stored on a XCELERA 2.1 PACS server (Philips Medical Systems, Andover, Massachusetts). Annular e’ velocity, average E/e’ ratio, LA volume index and peak TR velocities were measured to evaluate LV diastolic function [10].
Blood samples were collected from the cubital vein. Samples were immediately centrifuged and aliquots of plasma and serum were stored in microcentrifuge tubes at –80 ℃ until analysis. Plasma MPO concentration was determined by an enzyme-linked immunosorbent assay (ELISA) method (#DMYE00B, R&D Systems) according to the manufacturer’s instructions.
Effective arterial elastance (Ea) was calculated as described in the literature
[11]: end-systolic pressure divided by stroke volume. End-systolic pressure was
estimated as systolic pressure times 0.9, as previously validated [12]. Digital
hyperemia response was measured at finger (index) tips using an EndoPat2000
device (Rev 3, Itamar Medical, Caesara, Israël) (Fig. 1). Briefly, pulse
wave amplitude (PWA) changes were assessed as beat-to-beat plethysmographic
signals in the index finger by high-sensitive pneumatic probes (EndoPAT, Itamar).
The signals were measured at basal state during 5 minutes from each fingertip.
Then brachial blood flow was interrupted for 5 minutes by inflation of a
sphyngomanometer cuff placed on one proximal forearm, and signals were recorded
during occlusion (5 minutes) and after restoration of blood flow (5 minutes).
Data were digitized and computed automatically by EndoPat2000 software; the
reactive hyperemia index (RHI) was defined as the ratio of mean post-deflation
signal (in the 90 to 120-second post-deflation interval) to baseline signal in
hyperemic finger normalized by the same ratio in the contra-lateral finger and
multiplied by a baseline correction factor (K = 0.523976
 Fig. 1.
Fig. 1.Calculation of the reactive hyperemia index (RHI) and the augmentation index (Aix) by the EndoPAT2000 software (Itamar Medical).
Statistical analyses were performed using SPSS version 25 (SPSS Corp., Somers,
NY, USA). All tests were 2-sided and p-value
The characteristics of all 55 patients with HFpEF are presented in Table 1.
Patients were 80
| All patients (N = 55) | MPO below median (N = 28) | MPO above median (N = 27) | p-value | ||
| Age (years) | 80 |
79 |
80 |
0.72 | |
| Female (n, %) | 36 (65%) | 22 (79%) | 14 (52%) | 0.037 | |
| Body mass index (kg/m |
28.2 |
28.3 |
28.3 |
0.99 | |
| Systolic blood pressure (mmHg) | 134 |
138 |
129 |
0.099 | |
| Diastolic blood pressure (mmHg) | 74 |
75 |
72 |
0.45 | |
| Heart rate at inclusion (bpm) | 72 |
74 |
70 |
0.26 | |
| NYHA III – IV (n, %) | 20 (36%) | 10 (36%) | 10 (37%) | 0.92 | |
| Diabetes (n, %) | 18 (33%) | 5 (18%) | 13 (48%) | 0.017 | |
| HbA1C (in diabetic patients) (%) | 6.7 [6.35; 8.23] | 7.8 [6.40; 9.90] | 6.6 [6.15; 7.85] | 0.34 | |
| Atrial fibrillation (n, %) | 42 (76%) | 23 (85%) | 19 (68%) | 0.13 | |
| Paroxysmal (n, %) | 10 (18%) | 6 (21%) | 6 (22%) | ||
| Permanent (n, %) | 32 (58%) | 17 (61%) | 13 (48%) | ||
| Ischemic cardiomyopathy (n, %) | 21 (38%) | 8 (29%) | 13 (48%) | 0.14 | |
| Smoking (n, %) | 18 (18%) | 9 (32%) | 9 (33%) | 0.93 | |
| Hypertension (n, %) | 52 (95%) | 26 (93%) | 26 (93%) | 1 | |
| Hypercholesterolemia (n, %) | 39 (71%) | 17 (61%) | 22 (81%) | 0.09 | |
| Sleep apneas (n, %) | 6 (11%) | 3 (11%) | 3 (11%) | 1 | |
| COPD (n, %) | 6 (11%) | 4 (14%) | 2 (7%) | 0.67 | |
| Medication | |||||
| Loopdiuretics (n, %) | 42 (76%) | 19 (68%) | 23 (85%) | 0.13 | |
| MRA (n, %) | 18 (33%) | 11 (39%) | 7 (26%) | 0.19 | |
| Beta blockers (n, %) | 34 (62%) | 21 (75%) | 22 (81%) | 0.56 | |
| ACE inhibitors/ARB (n, %) | 43 (78%) | 16 (57%) | 18 (67%) | 0.47 | |
| Statins (n, %) | 35 (64%) | 15 (54%) | 20 (74%) | 0.11 | |
| Biology | |||||
| eGFR (mL/min/1.73 m |
49.4 |
51.4 |
47.4 |
0.42 | |
| Hemoglobin (g/dL) | 12.0 |
12.3 |
11.7 |
0.19 | |
| NT-proBNP (pg/mL) | 1302 [498; 2435] | 1015 [361; 2251] | 1668 [824; 3386] | 0.044 | |
| Troponin (pg/mL) | 21 [11; 40] | 16 [10; 40] | 32 [16; 41] | 0.47 | |
| CRP (mg/L) | 3.1 [1.2; 8.4] | 2.1 [1.2; 4.2] | 4.7 [1.4; 10.2] | 0.045 | |
| Myeloperoxidase (ng/mL) | 34.7 [22.7; 44.0] | 23.9 [18.4; 32.0] | 44.0 [37.8; 78.5] | By design | |
| Uric acid (mg/dL) | 7.3 |
6.6 |
8.0 |
0.06 | |
| Neutrophiles | 4.3 |
4.3 |
4.2 |
0.98 | |
| Lymphocytes | 1.6 |
1.7 |
1.5 |
0.45 | |
| Monocytes | 0.68 |
0.65 |
0.71 |
0.56 | |
| Neutrophile to lymphocyte ratio | 3.2 |
3.0 |
3.3 |
0.63 | |
| Echocardiography | |||||
| Indexed LA volume (mL/m |
37.6 |
36.1 |
39.2 |
0.32 | |
| LV ejection fraction (%) | 57.7 |
59.5 |
55.8 |
0.007 | |
| E wave velocity (mm/s) | 108.5 |
105.6 |
111.4 |
0.49 | |
| E/e’ ratio | 16.3 |
14.4 |
18.2 |
0.012 | |
| TAPSE (mm) | 19.2 |
19.9 |
18.7 |
0.53 | |
| eSPAP (mmHg) | 50.7 |
49.8 |
51.7 |
0.66 | |
| Vascular function | |||||
| Effective arterial elastance (mmHg/mL) | 2.24 |
2.43 |
2.06 |
0.065 | |
| EndoPAT | (n = 45) | (n = 22) | (n = 23) | 0.55 | |
| Reactive hyperemia index (RHI) | 1.67 [1.33; 2.02] | 1.66 [1.32; 1.95] | 1.82 [1.34; 2.30] | ||
| Augmentation Index (AIx) | 17.81 [2.64; 31.24] | 19.9 [10.5; 33.4] | 11.1 [0.1; 30.7] | 0.018 | |
| NYHA, New York heart association; COPD, chronic obstructive pulmonary disease;
MRA, mineralocorticoid receptor antagonist; ACE, angiotensin-converting enzyme;
ARB, angiotensin II receptor blocker; eGRF, estimated glomerular filtration rate;
NT-proBNP, N-terminal of brain natriuretic peptide; CRP, C-reactive protein; LA,
left atrium; LV, left ventricle; TAPSE, tricuspid annular plane systolic
excursion; eSPAP, estimated systolic pulmonary artery pressure.
p values are for differences of characteristics between the groups MPO above median vs MPO below median and are derived from independent sample t-test, Mann Whitney U test, Chi-square test or Fisher exact test when appropriate). All tests were 2-sided and p-value | |||||
Besides expected differences in NT-proBNP levels and echocardiographic
parameters, patients with HFpEF had higher levels of CRP (3.1 mg/L [1.2; 8.4] vs
1.2 mg/L [1.0; 1.75], p = 0.001), uric acid (7.3
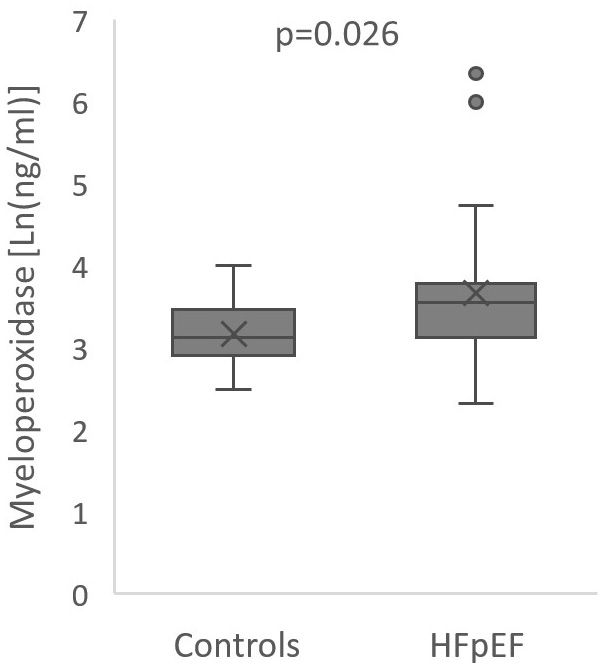 Fig. 2.
Fig. 2.Boxplot of myeloperoxidase levels in heart failure and preserved
ejection fraction patients and controls. Center line: median; box limits: upper
and lower quartiles; whiskers: 1.5
The AUC of the ROC curves for myeloperoxidase was 0.72 (0.59; 0.84) p
= 0.006 indicating moderate diagnostic value for HFpEF. Expectedly, NT-proBNP
levels had a very good diagnostic value of 0.94 (0.89; 1.00) p
Among HFpEF patients, MPO levels were correlated with markers of inflammation; CRP (R = 0.46, p = 0.001) and neutrophile to lymphocyte ratio (R = 0.36, p = 0.031) and with signs of LV remodelling and elevated filling pressures, namely NT-proBNP levels (R = 0.32, p = 0.019), decreased LV ejection fraction (LVEF, R = –0.36, p = 0.008) and E/e’ ratio (R = 0.35, p = 0.011) (Fig. 3). There was no correlation with age (R = 0.12, p = 0.41), body mass index (R = 0.09, p = 0.54), nor renal function (glomerular filtration rate estimated by Chronic Kidney Disease Epidemiology Collaboration CKD-EPI equation) (R = –0.13, p = 0.34) [14].
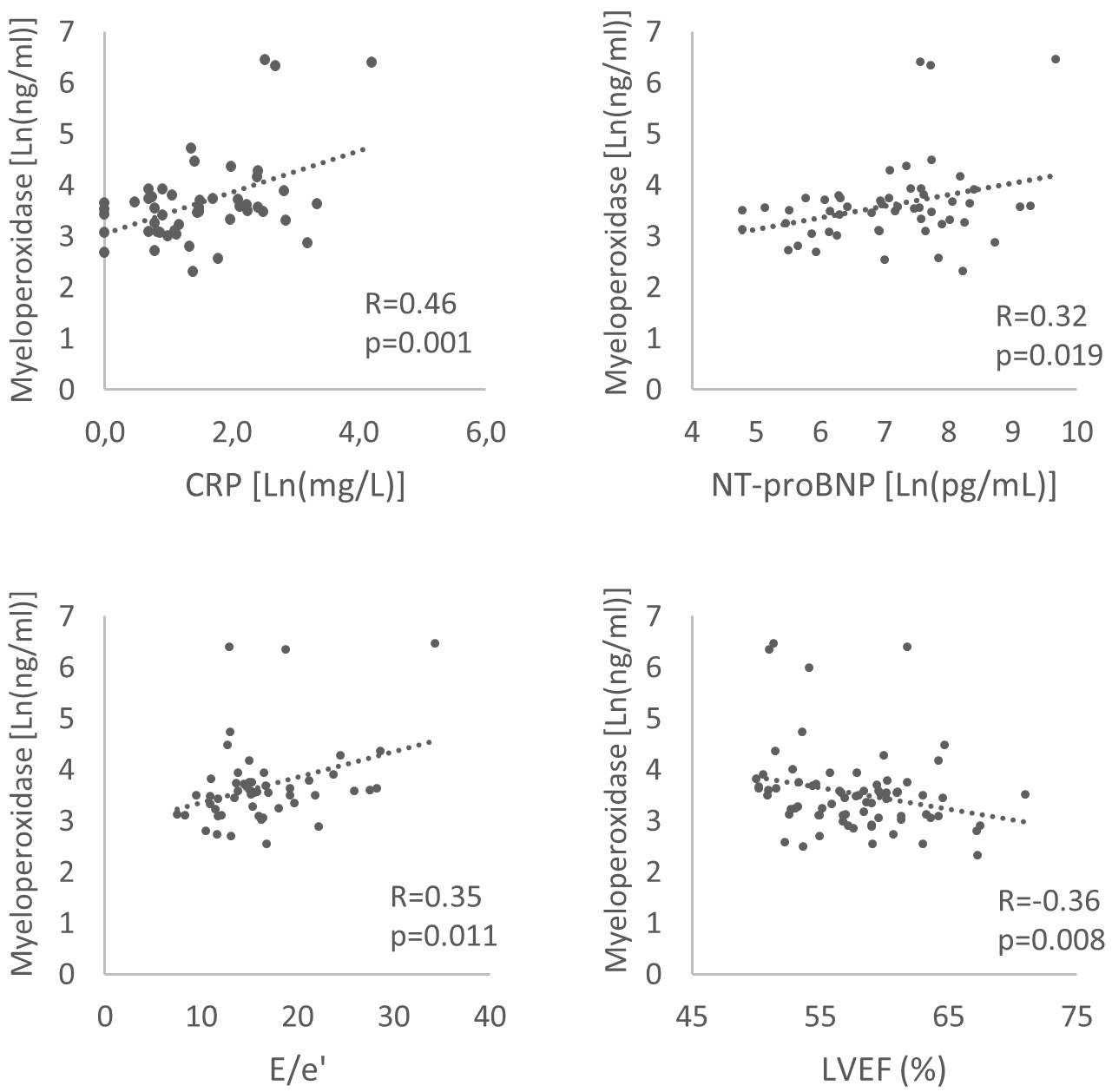 Fig. 3.
Fig. 3.Correlations between myeloperoxidase and C-reactive protein (CRP), NT-proBNP, E/e’ ratio, left ventricular ejection fraction (LVEF) in heart failure and preserved ejection fraction patients.
Patients with MPO levels above the median consistently had higher levels of CRP
and NT-proBNP levels. They also showed lower LVEF (55.8
In multivariable logistic regression, diabetic status remained predictive of high levels of myeloperoxidase after adjustment for age and sex (OR = 4.7, 95% CI 1.15–19.19, p = 0.031). Fig. 4 illustrates the proportion of patients with MPO levels above or below median according to sex and diabetic status. Interestingly, all men suffering from diabetes (9, 100%) had MPO levels above the median, while in women (both with or without diabetes) and in men without diabetes the proportion was similar, around 40%.
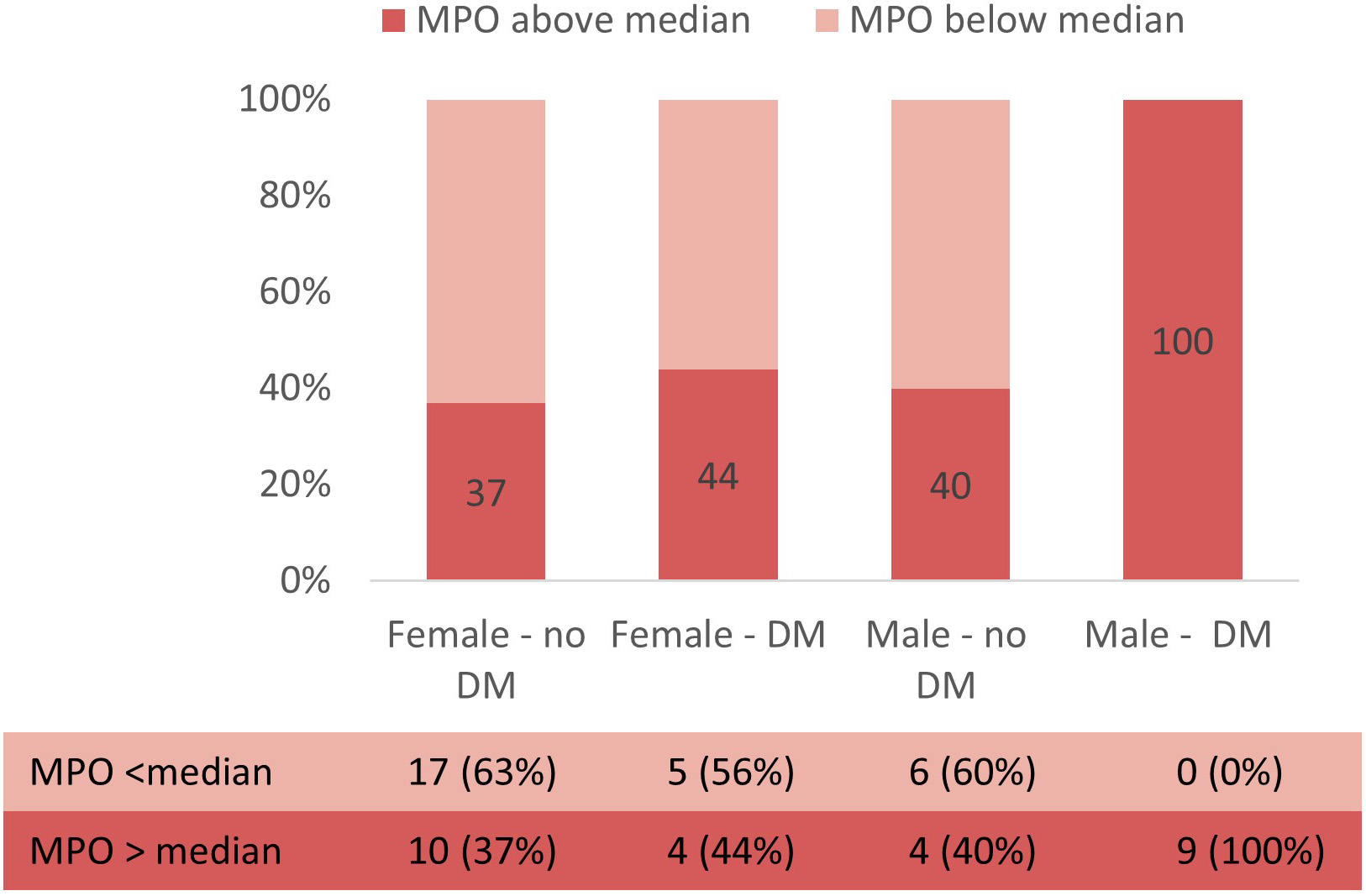 Fig. 4.
Fig. 4.Proportion of patients with MPO levels above or below median according to sex and diabetic status.
Intriguingly, patients with higher levels of MPO showed lower augmentation index
(11.1 [0.1; 30.7] vs 19.9 [10.5; 33.4], p = 0.018) and a trend towards
lower effective arterial elastance (2.06
The findings of this study are as follows: patients with HFpEF have higher levels of MPO than controls, MPO levels in HFpEF are positively correlated with inflammation (CRP levels), diastolic dysfunction (E/e’) and congestion (NT-proBNP) and negatively with left ventricular ejection fraction. Patients with MPO levels above the median suffer more often from diabetes, are more often males but tend to show less vascular stiffness (lower AIx) than patients with MPO levels below the median.
Several studies have shown a strong correlation between MPO and cardiovascular
disease (CVD) including acute coronary syndrome, atherosclerosis, hypertension,
and stroke [4, 15]. Consistently, recent studies that target MPO in animal models
of CVD have demonstrated favourable outcomes with regard to disease progression
[16]. However, data in HFpEF are limited to the study by Hage and collegues [6].
Our study corroborates their finding that MPO is elevated in HFpEF patients
compared to controls and demonstrates that this applies also when the control
group is older (74
MPO-mediated oxidative stress may be one of the mechanistic link between
comorbidities, inflammation and endothelial dysfunction at the source of HFpEF
[3]. Comorbidities, namely obesity, diabetes, and ageing generate inflammation
[17, 18, 19, 20], during which MPO is released and uses H
High MPO levels were associated with diabetic status. This is not surprising since diabetes is known to promote a systemic pro-inflammatory state [24, 25]. Furthermore, MPO was shown to be predictive of insulin resistance in a population of obese patients [26]. Since the proportion of patients with diabetes was higher in patients with MPO levels above the median, and since the difference in pathophysiology between diabetic and non-diabetic HFpEF patients is a topic of interest [27, 28], we further investigated this association with logistic regression adjusted for age and sex. Interestingly, the combination of male sex and diabetic status seem particularly associated with higher levels of MPO among patients with HFpEF. Indeed, all men suffering from diabetes had MPO levels above the median, while the proportion was limited to 40% in the other subgroups (Fig. 4). This finding is consistent with the sex-specific proteomic profile of patients with HFpEF in the PROMIS study [29], where they demonstrated that inflammation-related pathways predominated in men.
On the other hand, we found no association between vascular stiffness or
endothelial function and MPO levels. Even more surprising, vascular stiffness
seemed less important in the patients with higher MPO levels (lower AIx, lower
Ea). The augmentation index (AIx) is calculated from pulse waveforms as the ratio
of the difference between the early and late systolic peaks of the waveform
relative to the early peak (Fig. 1) and represents the relative importance of the
reflected wave [30]. Multiple small reflections travel back to the proximal aorta
and merge into a “net” reflected wave whose magnitude and timing depend on
vascular stiffness. In older subjects, systolic wave reflections mediate late
systolic load, with an important impact on LV relaxation [31, 32]. The
augmentation index is not simply a measure of arterial stiffness and wave
reflection, but was also shown to be elevated in conditions of increased LV
contractility and may reflect overall ventricular-vascular coupling [33]. In
HFpEF, high AIx was associated with abnormal LV diastolic responses to exercise,
particularly in women, suggesting that arterial stiffness may contribute to the
pathophysiology of HFpEF more commonly in women than in men [34]. The finding
that patients with MPO levels above median do not display more endothelial
dysfunction, nor vascular stiffness might be an indication that the sequence:
comorbidities, inflammation, oxidative stress, endothelial dysfunction,
myocardial remodelling is not straightforward. Rather, different mechanisms are
probably involved in the development of myocardial remodeling and impaired
vascular function, while both condition can ultimately lead to HFpEF. Recent data
from phenomapping point towards the same direction. Indeed, although studies
identify slightly different clusters depending on available variables [35, 36, 37, 38, 39],
two clusters seem to be commonly differentiated: one with older patients with
stiff arteries, small highly contractile LVs and high rates of electrical
remodelling (atrial fibrillation) and the other with high rates of metabolic
comorbidities, mainly diabetes, marked LV remodelling and advanced diastolic
dysfunction. Inflammation and oxidative stress may play a more prominent role in
the latter, hence the elevation of MPO (Fig. 5). Accordingly, there were more men
and more patients suffering from diabetes in the group of patients with MPO
levels above the median and they displayed lower (although
 Fig. 5.
Fig. 5.Illustration of patients’ characteristics associated with levels of myeloperoxidase below or above the median. Patients with heart failure and preserved ejection fraction and myeloperoxidase above the median are more often men, suffer more often form diabetes, show subtle left ventricular dysfunction and pronounced diastolic dysfunction (high E/e’) while patients with myeloperoxidase below the median are more often women with elevated vascular stiffness and high left ventricular ejection fraction.
We acknowledge this single centre study has several limitations. Maybe the most important arising from the small sample size. Unfortunately, restrictions related to the COVID pandemic interrupted the recruitment for several months. Furthermore, due to limitations of the EndoPAT technique, we could not obtain RHI and AIx for all patients. Despite our best effort to include controls of similar age and sex, both groups are not accurately matched for these characteristics. However, our groups are more alike than the only other published study demonstrating higher MPO levels in HFpEF [6]. The presence of a control group of similar age and sex is important since there are no validated reference values of MPO. On the contrary, there is a wide range of concentrations reported in the literature [40, 41, 42], hence standardisation will be necessary before routine use of MPO measurements.
In the context of the development of treatment with MPO inhibitor “AZD4831” (NCT03611153) it is interesting to note that not all patients might respond homogeneously. The results of our study suggest that patients with metabolic comorbidities, particularly diabetes, subtle LV dysfunction and evident diastolic dysfunction might benefit more from treatment targeting MPO while patients with predominant arterial stiffness (mostly females) and hyper contractile LV might be less responsive. Hence, while this study should be considered exploratory and hypothesis generating, it adds relevant information to existing literature. Future studies should aim at exploring the sex specific interplay between vascular inflammation and stiffness in this population, with special interest in features of metabolic stress such as obesity and diabetes.
Myeloperoxidase levels are elevated in HFpEF compared to controls, reflecting leukocyte activation and oxidative stress. Patients with levels of MPO above the median are more often males and suffer more often from diabetes. MPO levels in HFpEF are positively correlated with diastolic dysfunction and congestion and negatively with left ventricular ejection fraction. The association between oxidative stress and vascular stiffness, on the other hand could not be demonstrated and deserves future attention.
Data available on reasonable request.
SL recruited patients and controls, analysed echocardiographic studies, interpreted data and wrote the first version of the manuscript. AG performed the ELISA assay for myeloperoxidase. NM made substantial contributions to acquisition of data. DV, AP and BLG participated in data analysis and extensively revised the manuscript. SH and CB made substantial contribution to study design, acquisition of funding and extensively revised the manuscript. ACP designed the study protocol, acquired funding, was involved in data acquisition and interpretation and revised the manuscript. All authors read and approved the final manuscript.
The local ethics committee approved the study (approval number 2012/23AVR/199), and all subjects gave written informed consent before study enrolment (Clinical trial NCT03197350). The investigation conforms to the principles outlined in Declaration of Helsinki.
The authors wish to thank Florence Sinnaeve for the blood punctures as well as all the enrolled subjects for agreeing to participate in the study. Fig. 5 was created with https://biorender.com/.
This work was funded by a grant of the Fondation Nationale de la Recherche Scientifique of the Belgian Government (FRSM CDR 23597851). Dr Pouleur is supported by the FNRS as Clinical Researcher (40000507). Dr Lejeune is supported by Fondation Damman and Fondation Saint Luc for her fellowship.
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
