- Academic Editors
Background: In patients undergoing percutaneous coronary intervention (PCI), drug eluting stents (DES) are currently the standard of care. Stent design and alloy composition, biocompatibility of the drug-eluting polymer coating, the antiproliferative agent properties and release are the three main characteristics that affects DES performance. Cre8 (Alvimedica, Istanbul, Turkey) is a polymer-free amphilimus-eluting stents (PF-AES). In this study, we aimed to investigate the clinical efficacy and safety of Cre8 DES in daily cardiology practice. Methods: Patients presenting with chronic coronary syndrome (CCS) or acute coronary syndrome (ACS) including unstable angina pectoris (USAP), myocardial infarction with and without ST-segment elevation and treated with PCI using Cre8 DES between December 2015 and 2016 were retrospectively analyzed in this study. Results: Between December 2015 and 2016, 808 lesions of 664 patients treated with Cre8 DES in a single center were included in this retrospective analysis. The mean age of study group was 60 years (between 33 and 93 years) and were predominantly consisting of male patients (79.4%). The median follow-up duration was 487 days (min: 30 days, max: 919 days) and two-thirds of all patients presented with ACS. The culprit lesion was on left anterior descending artery (LAD) (40.5%) and right coronary artery (RCA) (25.9%) in most of the patients. The procedural success rate was 97.3%. Most of the lesions were type B1 (40.6%) according to American College of Cardiology/American Heart Association (ACC/AHA) coronary lesion classification. The device oriented primary end-point defined as target lesion failure (TLF) occurred in 52 (6.4%) of 808 lesions. The primary safety end-point was cardiac death in 20 patients (3.0%) and target vessel myocardial infarction in 2 patients (0.3%). Target vessel revascularization (TVR) occurred in 29 patients (4.4%) as primary safety endpoint. Multivariable logistic regression analysis revealed diabetes mellitus and ejection fraction as the predictors of mortality and device oriented primary end-point. Conclusions: This trial revealed clinical efficacy and safety of Cre8 stents in real world practice. Device oriented primary end points were similar with previous studies which are randomized, open label in nature and showed the efficacy and safety of Cre8 stent towards latest generation DES.
Drug eluting stents (DES) are the current standard of care in patients undergoing percutaneous coronary intervention (PCI). The restenosis rate of DES is lower than bare metal stents (BMS), as antiproliferative agents are released by DES platforms [1]. Stent design and alloy composition, biocompatibility of the drug-eluting polymer coating, the antiproliferative agent properties and release are the three main characteristics that affect DES performance.
Increased risk of late stent thrombosis is major problem for DES as permanent polymer content causes incomplete stent strut endothelialization due to impaired arterial healing especially in patients with comorbidities and complex lesions [2]. Contrarily, late restenosis rate of polymer-free amphilimus-eluting stents (PF-AES) is less than permanent polymer paclitaxel-eluting stent [3]. Cre8 (Alvimedica, Istanbul, Turkey), a PF-AES, was non-inferior to latest generation permanent-polymer zotarolimus-eluting stents (PP-ZES) regarding target lesion failure at 12 months in Randomized All-Comers Evaluation of a Permanent Polymer Zotarolimus-Eluting Stent Versus a Polymer-Free Amphilimus-Eluting Stent: a Multicenter, Noninferiority Trial (ReCre8) [4].
The use of Cre8 in acute coronary syndrome (ACS) patients is poorly investigated, especially in the myocardial infarction with ST segment elevation (STEMI) scenario. In this study, we aimed to investigate the clinical efficacy and safety of Cre8 DES in daily cardiology practice.
This is a retrospective, observational, single center study. Patients presenting with chronic coronary syndrome (CCS) or ACS including unstable angina pectoris (USAP), myocardial infarction with and without ST-segment elevation and were treated with Cre8 DES between December 2015 and 2016 at Istanbul University Institute of Cardiology were retrospectively analyzed in this study. All lesion treated with Cre8 and classified as American College of Cardiology/American Heart Association (ACC/AHA) class A, B1, B2 and C according to lesion complexity were included in the final analysis. There was no restriction for lesion types, lengths, or number of treated lesions. The duration of dual antiplatelet therapy (DAPT) was in accordance with current guideline.
Procedural success was defined as less than a
For statistical analysis, Number Cruncher Statistical System (NCSS)
2007 (Kaysville, UT, USA) was used. Kolmogorov Smirnov test is the preferred
method to evaluate the distribution of variables. The variables were expressed as
mean
Between December 2015 and 2016, 808 lesions of 664 patients treated with Cre8 DES in a single center were included in this retrospective analysis. The mean age of study group was 60 years (between 33 and 93 years). All patients were treated with at least one Cre8 DES. The median follow-up duration was 487 days (min: 30 days, max: 919 days). The study group predominantly consisted of male patients (79.4%) and two-thirds of all patients presented with ACS. Baseline characteristics are shown in Table 1.
| Overall (n = 664) | ||
| Clinical characteristics | ||
| Age (years) | 60.2 | |
| Male sex (n, %) | 527 (79.4) | |
| Diabetes mellitus (n, %) | 246 (37.0) | |
| Hypertension (n, %) | 338 (50.9) | |
| Hypercholesterolemia (n, %) | 90 (13.6) | |
| Coronary artery disease (n, %) | 329 (49.5) | |
| Current smoker (n, %) | 171 (25.8) | |
| Clinical presentation (n, %) | ||
| Chronic coronary syndrome | 279 (42.0) | |
| Acute coronary syndrome | 385 (58.0) | |
| Unstable angina | 101 (15.2) | |
| NSTEMI | 119 (17.9) | |
| STEMI | 165 (24.1) | |
| Coronary anatomy (n, %) | ||
| Left main | 8 (1.0) | |
| Left anterior descending artery | 342 (42.4) | |
| Left circumflex artery | 204 (25.2) | |
| Right coronary artery | 220 (27.2) | |
| Bypass graft | 34 (4.2) | |
| Abbreviations: NSTEMI, Myocardial infarction without ST segment elevation; STEMI, Myocardial infarction with ST segment elevation. | ||
The indication was ACS in 57.7% of patients and CCS in the rest of patients. The culprit lesion was on left anterior descending artery (LAD) (40.5%) and right coronary artery (RCA) (25.9%) in most of the patients. While direct stenting was the treatment of choice in 28.5% of lesions, most of the lesions were predilated with balloon before stenting. The mean stent diameter and length were 2.8 mm (min: 2.25 mm, max: 3.5 mm), 21.2 mm (min: 15 mm, max: 38 mm) respectively. The procedural success rate was 97.3%. Most of the lesions were type B1 (40.6%) according to ACC/AHA classification of coronary lesion. It was followed by type B2, type A, and type C 23.0%, 22.3%, and 14.1% respectively. Lesion and procedural characteristics are listed in Table 2.
| Overall (808 Lesions) | ||
| Procedural characteristics | ||
| No of stents, per lesion | 1.2 | |
| No of stents, per patients | 1.4 | |
| Total stent length, mm | 21.2 | |
| Stent diameter, mm | 2.8 | |
| Pre-dilatation (n, %) | 578 (71.5) | |
| Post-dilatation (n, %) | 214 (26.5) | |
| Lesion complexity (n, %) | ||
| ACC/AHA Class A | 151 (18.9) | |
| ACC/AHA Class B1 | 330 (40.8) | |
| ACC/AHA Class B2 | 212 (26.2) | |
| ACC/AHA Class C | 115 (14.2) | |
| Procedural success (n, %) | 785 (97.3) | |
| Abbreviations: ACC, American College of Cardiology; AHA, American Heart Association. | ||
The device oriented primary end-point defined as TLF occurred in 52 (6.4%) of 808 lesions. The primary safety end-point was cardiac death in 20 patients (3.0%) and target vessel myocardial infarction in 2 patients (0.3%). Target vessel revascularization (TVR) occurred in 29 patients (4.4%) as primary safety end-point. Diabetes mellitus (DM), lesion complexity (type B2 and C lesion) and ejection fraction (EF) were the predictors for the device oriented primary end-point. There was no relation between stent diameter, stent length, final diameter and event-free survival (Table 3). Multivariable logistic regression analysis revealed diabetes mellitus and ejection fraction as the predictors of both mortality and device oriented primary end-point (Table 4). Kaplan Meier Survival analysis showed no significant survival difference between patient with and without complex coronary lesion (Fig. 1).
| Parameter | TLF (+) (52, 6.4%) | TLF (–) (756, 93.6%) | p value |
|---|---|---|---|
| Age (years) | 60.0 |
60.2 |
0.986 |
| Sex (male) (%) | 70.8 | 80.0 | 0.147 |
| DM (%) | 60.4 | 35.4 | 0.001 |
| HT (%) | 62.5 | 50.3 | 0.102 |
| HL (%) | 22.9 | 12.9 | 0.071 |
| Smoking (%) | 62.5 | 80.9 | 0.194 |
| CAD (%) | 52.1 | 49.5 | 0.731 |
| Lesion type (B2 and C) (%) | 44.3 | 39.1 | 0.017 |
| EF (%) | 49.4 |
57 |
0.020 |
| Stent diameter (mm) | 2.8 |
2.8 |
0.788 |
| Stent length (mm) | 20.3 |
21.2 |
0.497 |
| Final diameter (mm) | 3.2 |
3.2 |
0.494 |
| Abbreviations: CAD, Coronary artery disease; DM, Diabetes mellitus; EF, Ejection fraction; HT, Hypertension; HL, Hyperlipidemia. | |||
| Model | Variables | B | S. Error | Wald | p |
|---|---|---|---|---|---|
| 1 | Constant | –5.790 | 1.902 | 9.270 | 0.002** |
| HT | 0.299 | 0.556 | 0.290 | 0.590 | |
| IHD | 0.023 | 0.506 | 0.002 | 0.964 | |
| Age | 0.043 | 0.026 | 2.769 | 0.096 | |
| HL | –0.274 | 0.615 | 0.199 | 0.656 | |
| DM | –1.549 | 0.575 | 7.267 | 0.007** | |
| Sex | 0.421 | 0.614 | 0.470 | 0.493 | |
| R | |||||
| Model | Variables | B | S. Error | Wald | p |
| 1 | Constant | –6.088 | 2.617 | 5.411 | 0.02* |
| Stent Diameter | 1.190 | 0.919 | 1.675 | 0.196 | |
| EF | –0.019 | 0.009 | 4.741 | 0.029* | |
| R | |||||
| Abbreviations: DM, Diabetes mellitus; EF, Ejection fraction; HT, Hypertension;
HL, Hyperlipidemia; IHD, Ischemic heart disease. * p | |||||
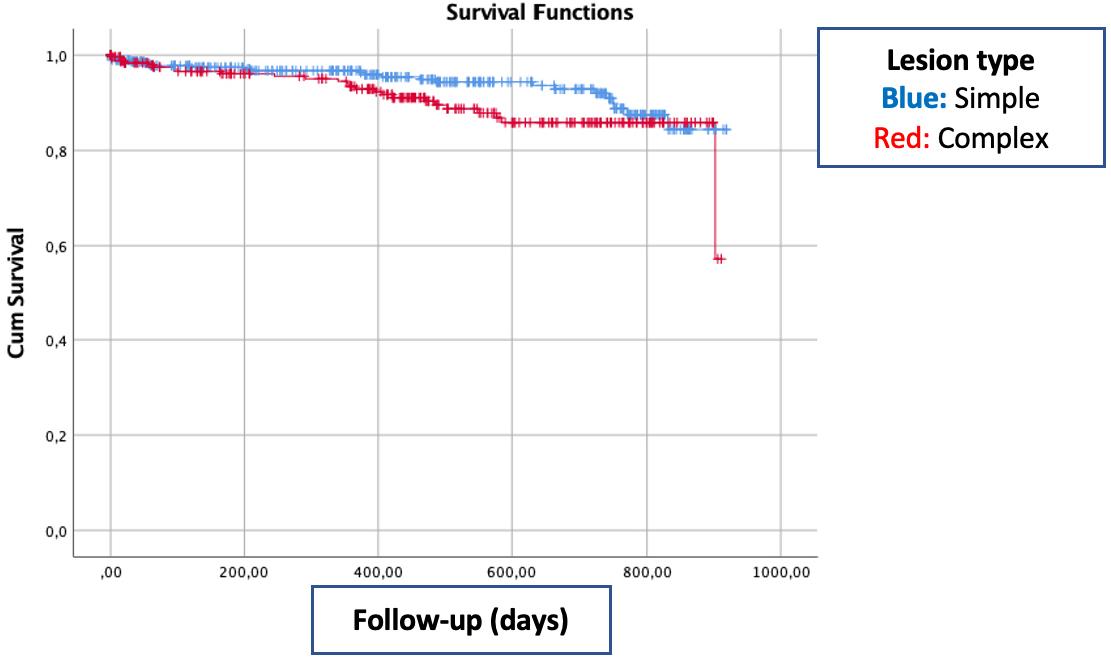 Fig. 1.
Fig. 1.Kaplan Meier graph of event free survival according to lesion complexity.
Our study showed the clinical efficacy and safety of Cre8 stent in real-life practice. At median 16-month follow-up, the event-free survival rate was 93.6%. Although two thirds of patients presented with ACS, the mortality rate at follow-up was 3.0%. Also the procedural success rate was high (97.3%) despite that one third of patients had complex lesion (type B2 and C lesion according to ACC/AHA classification of coronary lesion classification). Diabetes mellitus, EF and lesion complexity were predictors of outcome in univariable analysis. After multivariable logistic regression analysis, DM and EF were constant predictors of outcome.
The ReCre8 study showed that Cre8 polymer-free amphilimus-eluting stents (PF-AES) (Alvimedica, Istanbul, Turkey) is clinically non-inferior to latest generation DES regarding TLF at 12 months [4]. As in our study, most of patients presented with ACS and reflected true all-comers population. Half of patients had complex lesion (type B2 and C) and the procedural success rate was 99.3% in Cre8 group. Although device oriented primary end point was lower in ReCre8 trial, (5.6% and 6.4%), our study consisted of more patients with DM (20.4% and 30.4%) and the mortality rate was similar between the two studies (2.4% and 3.0%). Also the median follow-up duration was 12-month in ReCre8 trial. Recently van Hemert et al. [6], presented 3-year clinical outcomes of ReCre 8 study showing PF-AES are clinically noninferior to PP-ZES regarding TLF between 1 and 3 years.
Although target lesion failure (TLF) was slightly higher than in previous studies [4, 6, 7, 8], TVR rate (4.4%) was lower in our study. Also the proportion of patients with target-vessel myocardial infarction (0.3%) was distinctly lower than that reported in previous studies (2%–6%) [4, 7, 9, 10, 11].
Despite advance in stent technology, the clinical outcomes in diabetic patients is still worse than in non-diabetics. The rate of in-stent restenosis and TLR is reaching up to 13.5% in diabetic population [12]. The amphilimus formulation consists of a mixture of sirolimus and long-chained fatty acids used in polymer free amphilimus eluting-stents and this enhances the uptake of antiproliferative agents. This property may be associated with higher anti-restenosis potency in diabetics [13]. Previous clinical studies have revealed encouraging results on PF-AES in DM [3, 14]. Patients with DM almost had two-fold increased risk for device oriented primary end point (60.4% and 35.4%) in our study. After logistic regression analysis, DM is one of the predicting factors for device oriented cardiac events.
Our study has several limitations. First of all, it was a single center retrospective study. Patients treated with Cre8 in a year period were included in this analysis. It was open label and non-randomized study. Second, there was no other group of DES as comparator. So we were not able to compare efficacy and safety of different DES.
This trial reveals clinical efficacy and safety of Cre8 stents in real world practice. Device oriented primary end points are similar to previous studies which are randomized, open label in nature and showed the efficacy and safety of Cre8 stent towards latest generation DES.
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
UYS has been designed and coordinated the study. The manuscript also has been prepared by UYS. He is also the contributing author. ES and BKM were responsible for data collection and analysis. AAO was the supervisor and checked the final draft before submit.
We have ethics approval from Istanbul University-Cerrahpasa Institute of Cardiology Ethics Committee (No.74555795-050.01.04-) and also we have patients consent to be included in this study.
Not applicable.
This research received no external funding.
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
